Design, Synthesis and Antifungal Activity of Coumarin Ring-Opening Derivatives
Abstract
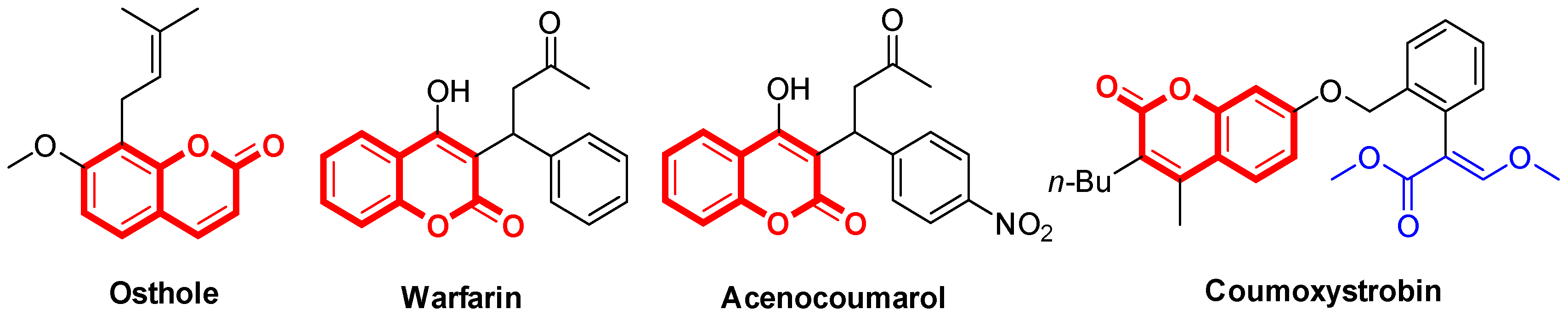
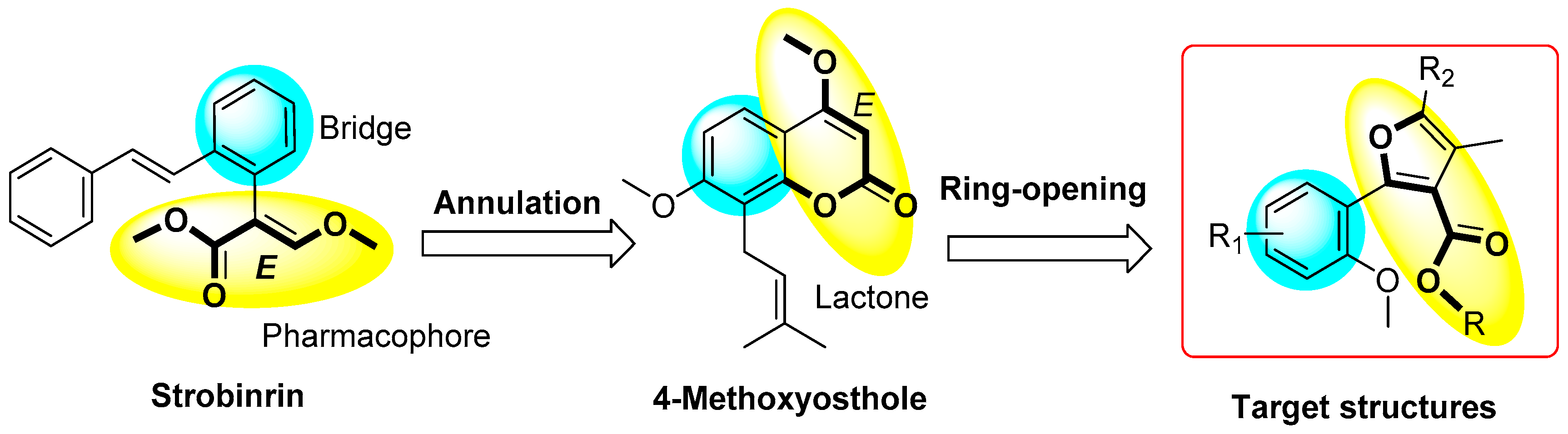
:1. Introduction
2. Results and Discussion
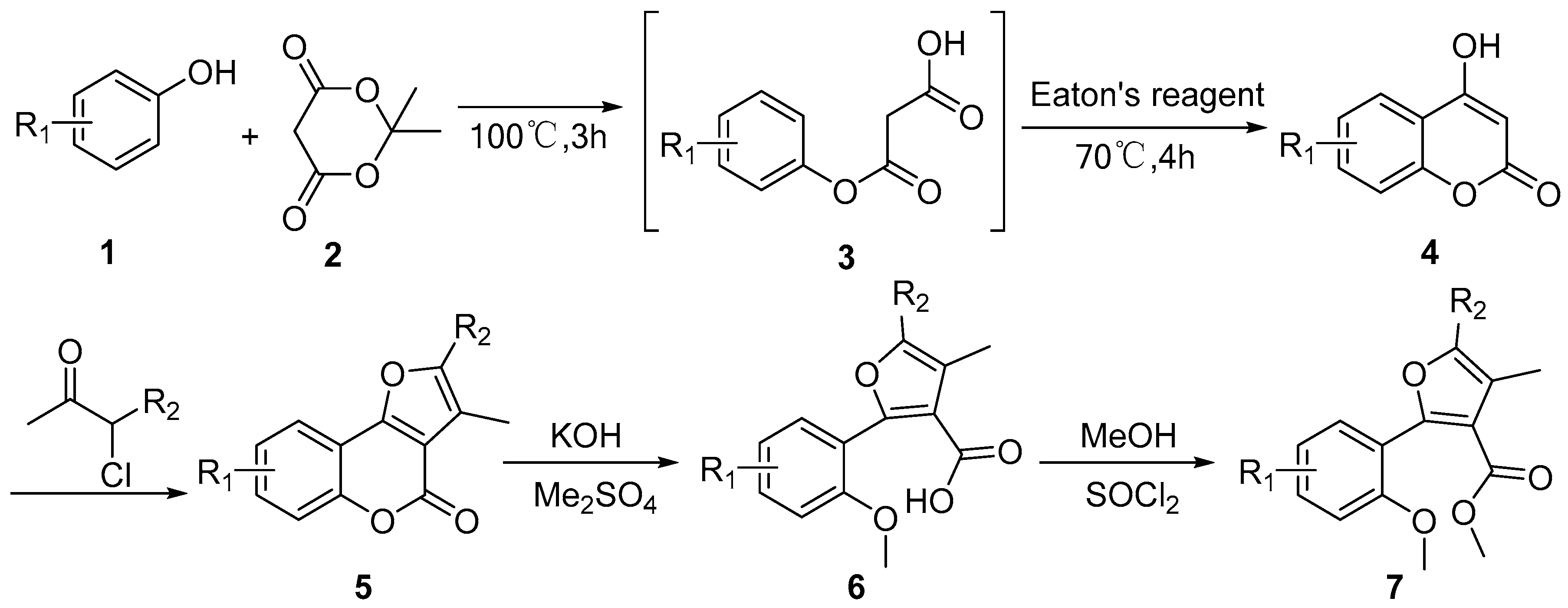
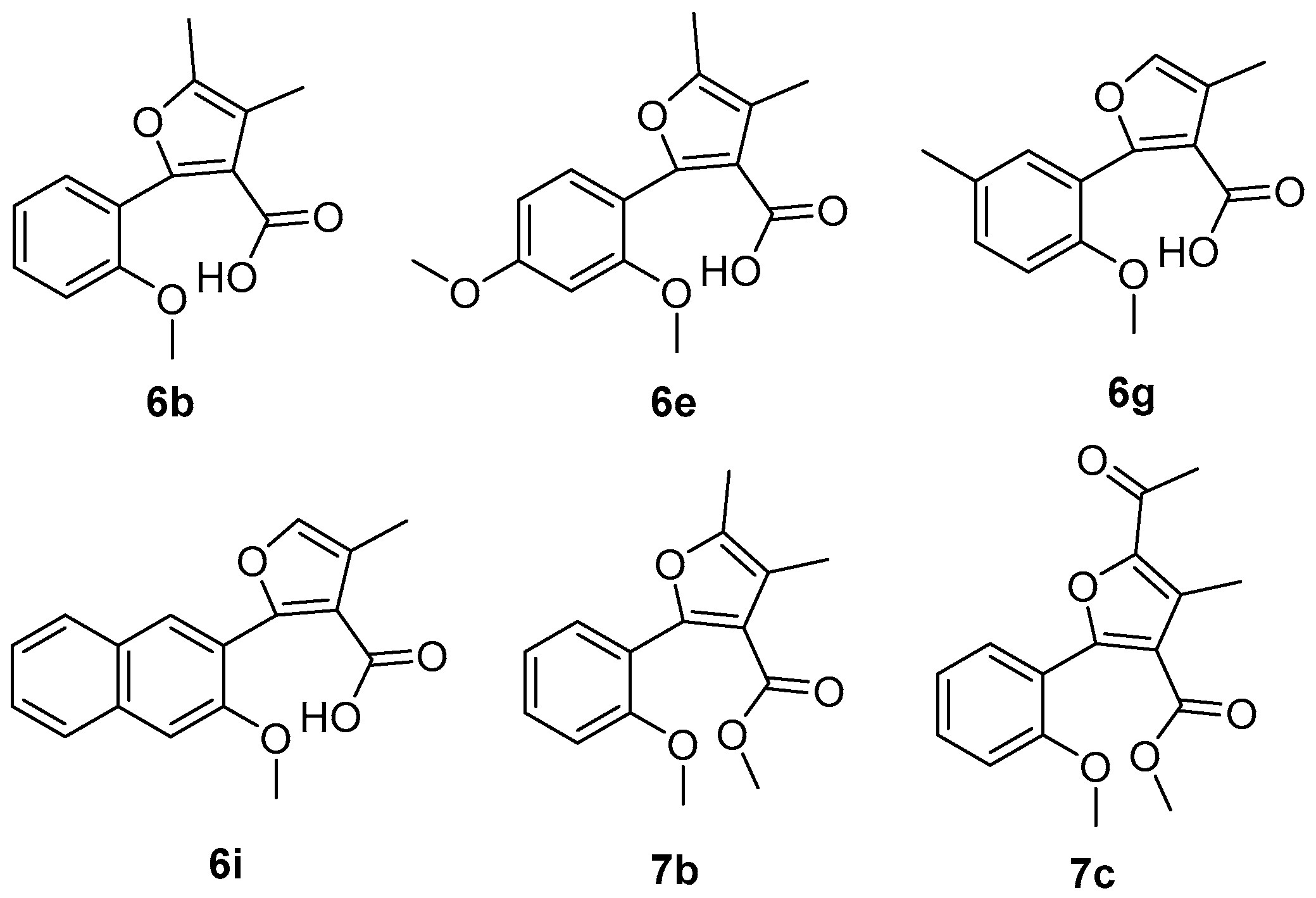
2.1. Synthetic Chemistry
2.2. Antifungal Activity and the Structure-Activity Relationships (SAR)
3. Materials and Methods
3.1. Chemicals and Methods
3.1.1. General Procedure for the Synthesis of Compound 4 (Scheme 1)
3.1.2. General Procedure for the Synthesis of Compound 5 (Scheme 1)
3.1.3. General Procedure for the Synthesis of Target Compounds 6 and 7 (Scheme 1)
3.2. Biological Assays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bovicelli, P.; Sanetti, A.; Bernini, R.; Lupattelli, P. Oxyfunctionalisation of activated methylenes by dimethyldioxirane: An easy conversion of isochromans into isocoumarins. Tetrahedron 1997, 53, 9755–9760. [Google Scholar] [CrossRef]
- Bovicelli, P.; Lupattelli, P.; Crescenzi, B.; Sanetti, A.; Bernini, R. Oxidation of 3-arylisochromans by dimethyldioxirane. An easy route to substituted 3-arylisocoumarins. Tetrahedron 1999, 55, 14719–14728. [Google Scholar]
- Tsay, S.C.; Lin, S.Y.; Huang, W.C.; Hsu, M.H.; Hwang, K.C.; Lin, C.C.; Horng, J.C.; Chen, I.C.; Hwu, J.R.; Shieh, F.K.; et al. Synthesis and Structure-activity relationships of imidazole-coumarin conjugates against hepatitis C virus. Molecules 2016, 21, 228. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liang, Z.; Xu, H.; Mou, Y.; Guo, C. Design, synthesis and cytotoxicity of novel dihydroartemisinin-coumarin hybrids via click chemistry. Molecules 2016, 21, 758. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.G.; Marrero, J.G.; Macias-Alonso, M.; Gonzalez, M.C.; Cordova-Guerrero, I.; Teissier Garcia, A.G.; Osegueda-Robles, S. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Dadashpour, S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015, 102, 611–630. [Google Scholar] [CrossRef] [PubMed]
- Vogl, S.; Zehl, M.; Picker, P.; Urban, E.; Wawrosch, C.; Reznicek, G.; Saukel, J.; Kopp, B. Identification and quantification of coumarins in Peucedanum ostruthium (L.) Koch by HPLC-DAD and HPLC-DAD-MS. J. Agric. Food Chem. 2011, 59, 4371–4377. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, T.; Ito, Y. Preparative isolation of osthol and xanthotoxol from Common Cnidium Fruit (Chinese traditional herb) using stepwise elution by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1033, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wang, F.; Zhou, W.; Zhang, P.; Fan, Y.J. Application of osthol induces a resistance response against powdery mildew in pumpkin leaves. Int. J. Mol. Sci. 2007, 8, 1001–1012. [Google Scholar] [CrossRef]
- Holbrook, A.M.; Pereira, J.A.; Labiris, R.; McDonald, H.; Douketis, J.D.; Crowther, M.; Wells, P.S. Systematic overview of warfarin and its drug and food interactions. Arch. Intern. Med. 2005, 165, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Cesar, J.M.; Garcia-Avello, A.; Navarro, J.L.; Herraez, M.V. Aging and oral anticoagulant therapy using acenocoumarol. Blood Coagul. Fibrinolysis 2004, 15, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Ufer, M. Comparative pharmacokinetics of vitamin K antagonists: Warfarin, phenprocoumon and acenocoumarol. Clin. Pharmacokinet. 2005, 44, 1227–1246. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.Y.; Liu, C.L.; Li, M.; Zhang, H.; Li, Z.N.; Li, Z.M. Design, synthesis and structure-activity relationship of novel coumarin derivatives. Pest. Manag. Sci. 2011, 67, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guan, A.; Yang, J.; Chai, B.; Li, M.; Li, H.; Yang, J.; Xie, Y. Efficient approach to discover novel agrochemical candidates: Intermediate derivatization method. J. Agric. Food Chem. 2016, 64, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.; Liu, C.; Yang, X.; Dekeyser, M. Application of the intermediate derivatization approach in agrochemical discovery. Chem. Rev. 2014, 114, 7079–7107. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Yin, W.; Liu, P.; Liu, J.; Hu, M.C.; Zhang, W. Palladium-catalyzed synthesis of 6-allylcoumarins using organotin reagents as multicoupling organometallic nucleophiles. Appl. Organomet. Chem. 2014, 28, 747–749. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Z.; Yin, W.; Liu, P.; Zhang, W. Microwave-assisted synthesis and antifungal activities of polysubstituted furo[3,2-c]chromen-4-ones and 7,8,9,10-tetrahydro-6H-benzofuro[3,2-c]chromen-6-ones. Synth. Comm. 2014, 44, 3257–3263. [Google Scholar] [CrossRef]
- Yin, Q.; Yan, H.; Zhang, Y.; Wang, Y.; Zhang, G.; He, Y.; Zhang, W. Palladium-catalyzed synthesis of 8-allyl or 8-prenylcoumarins by using organotin reagents as multicoupling nucleophiles. Appl. Organomet. Chem. 2013, 27, 85–88. [Google Scholar] [CrossRef]
- Gao, W.T.; Hou, W.D.; Zheng, M.R.; Tang, L.J. Clean and convenient one-pot synthesis of 4-hydroxycoumarin and 4-hydroxy-2-quinolinone derivatives. Synth. Comm. 2010, 40, 732–738. [Google Scholar] [CrossRef]
- Risitano, F.; Grassi, G.; Foti, F.; Bilardo, C. A convenient synthesis of furo[3,2-c]coumarins by a tandem alkylation/intramolecular aldolisation reaction. Tetrahedron Lett. 2001, 42, 3503–3505. [Google Scholar] [CrossRef]
- Dong, Y.; Shi, Q.; Liu, Y.N.; Wang, X.; Bastow, K.F.; Lee, K.H. Antitumor agents. 266. Design, synthesis, and biological evaluation of novel 2-(furan-2-yl)naphthalen-1-ol derivatives as potent and selective antibreast cancer agents. J. Med. Chem. 2009, 52, 3586–3590. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Pasqualetti, M.; Provenzano, G.; Tempesta, S. Ecofriendly synthesis of halogenated flavonoids and evaluation of their antifungal activity. New J. Chem. 2015, 39, 2980–2987. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Chen, Q.; Mulholland, N.; Beattie, D.; Irwin, D.; Gu, Y.C.; Yang, G.F.; Clough, J. Synthesis and fungicidal activity of novel pimprinine analogues. Eur. J. Med. Chem. 2012, 53, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Ng, T.B.; Zhang, J.; Zhou, M.; Song, F.; Lu, F.; Liu, Y. Bacisubin, an antifungal protein with ribonuclease and hemagglutinating activities from Bacillus subtilis strain B-916. Peptides 2007, 28, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the all target compounds are available from the authors.
| Compound | Species a | |||||
|---|---|---|---|---|---|---|
| BOT | ALS | GIB | RHI | RHI | ALM | |
| 50 Rate (μM) | 50 Rate (μM) | 50 Rate (μM) | 50 Rate (μM) | 50 Rate (μM) | 50 Rate (μM) | |
| 6a | 60.3 b | 38.0 | 16.7 | 31.2 | − c | 7.8 |
| 6b | 81.0 | 24.0 | 28.3 | 42.2 | 0 | 9.8 |
| 6c | 41.3 | 12.0 | 0 | 6.2 | 0 | − |
| 6d | 49.2 | 30.0 | 5.0 | 23.4 | 12.1 | 19.6 |
| 6e | 74.1 | 7.3 | 7.1 | 25.9 | 18.2 | 14.8 |
| 6f | 58.6 | 5.5 | 0 | 12.1 | 0 | − |
| 6g | 77.8 | 40.0 | 13.3 | 34.4 | − | 17.6 |
| 6h | 33.3 | 24.0 | 8.3 | 20.3 | 0 | − |
| 6i | 77.6 | 30.9 | 26.8 | 15.5 | 14.5 | 38.9 |
| 7a | 51.7 | 20.0 | 28.6 | 37.9 | 21.8 | 22.2 |
| 7b | 70.7 | 23.6 | 44.6 | 51.7 | 14.5 | 33.3 |
| 7c | 67.2 | 14.5 | 30.4 | 51.7 | 84.2 | 25.9 |
| 7d | 25.9 | 14.5 | 19.6 | 34.5 | 14.5 | 7.4 |
| 7e | 32.7 | 0 | 29.5 | 50.8 | 5.8 | 11.1 |
| 7f | 65.5 | 20.0 | 28.6 | 27.6 | 38.2 | 24.1 |
| 7g | − | 9.1 | 32.1 | 39.6 | 14.5 | 16.7 |
| 7h | 10.3 | − | 17.8 | 13.8 | 0 | 0 |
| 7i | 0 | 0 | 14.3 | 8.6 | 0 | 16.7 |
| Azoxystrobin | 50.4 | 31.3 | 58.2 | 60.7 | 89.9 | 44.5 |
| Pathogen | Compound | Toxic Regression | R | EC50 (µM) | 95% Confidence Interval |
|---|---|---|---|---|---|
| BOT | 6a | Y = 2.4538 + 1.7909x | 0.9905 | 0.1130 | 0.0966–0.1330 |
| BOT | 6b | Y = 3.3419 + 1.4690x | 0.9994 | 0.0544 | 0.0523–0.0566 |
| BOT | 6e | Y = 2.6307 + 1.7429x | 0.9932 | 0.0823 | 0.0724–0.0942 |
| BOT | 6g | Y = 2.1175 + 2.1486x | 0.9768 | 0.0889 | 0.0696–0.1130 |
| BOT | 6i | Y = 1.9225 + 2.1902x | 0.9779 | 0.0901 | 0.0699–0.1160 |
| BOT | 7b | Y = 2.0301 + 2.0457x | 0.9942 | 0.1090 | 0.0964–0.1230 |
| BOT | 7c | Y = 1.7715 + 2.0569x | 0.9980 | 0.1290 | 0.1200–0.1380 |
| BOT | 7f | Y = 0.9264 + 2.6215x | 0.9954 | 0.1120 | 0.1010–0.1250 |
| BOT | Azoxystrobin | Y = 3.5635 + 0.9256x | 0.9998 | 0.0884 | 0.0858–0.0910 |
| RHI | 7b | Y = 3.3881 + 1.0377x | 0.9994 | 0.1370 | 0.1320–0.1430 |
| RHI | 7c | Y = 2.0840 + 1.6967x | 0.9950 | 0.1820 | 0.1610–0.2050 |
| RHI | Azoxystrobin | Y = 3.4242 + 0.9321x | 0.9981 | 0.1220 | 0.1050–0.1410 |
| Compound | R1 | R2 | Yield | Compound | R1 | R2 | Yield |
|---|---|---|---|---|---|---|---|
| 5a | H | H | 79% | 5g | 8-Me | H | 67% |
| 5b | H | Me | 38% | 5h | 8-Me | Ac | 34% |
| 5c | H | Ac | 33% | 5i | 7,8-(CH)4 | H | 55% |
| 5d | 7-OMe | H | 79% | 5j | 7,8-(CH)4 | Me | 37% |
| 5e | 7-OMe | Me | 22% | 5k | 7,8-(CH)4 | Ac | 45% |
| 5f | 7-OMe | Ac | 36% |
| Compound | R1 | R2 | Yield | Compound | R1 | R2 | Yield |
|---|---|---|---|---|---|---|---|
| 6a | H | H | 65% | 7a | H | H | 75% |
| 6b | H | Me | 55% | 7b | H | Me | 65% |
| 6c | H | Ac | 55% | 7c | H | Ac | 68% |
| 6d | 4-OMe | H | 60% | 7d | 4-OMe | H | 74% |
| 6e | 4-OMe | Me | 56% | 7e | 4-OMe | Me | 71% |
| 6f | 4-OMe | Ac | 51% | 7f | 4-OMe | Ac | 73% |
| 6g | 5-Me | H | 71% | 7g | 5-Me | H | 69% |
| 6h | 5-Me | Me | 50% | 7h | 5-Me | Me | 62% |
| 6i | 4,5-(CH)4 | H | 48% | 7i | 4,5-(CH)4 | H | 58% |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.-Z.; Zhang, Y.; Wang, J.-Q.; Zhang, W.-H. Design, Synthesis and Antifungal Activity of Coumarin Ring-Opening Derivatives. Molecules 2016, 21, 1387. https://doi.org/10.3390/molecules21101387
Zhang M-Z, Zhang Y, Wang J-Q, Zhang W-H. Design, Synthesis and Antifungal Activity of Coumarin Ring-Opening Derivatives. Molecules. 2016; 21(10):1387. https://doi.org/10.3390/molecules21101387
Chicago/Turabian StyleZhang, Ming-Zhi, Yu Zhang, Jia-Qun Wang, and Wei-Hua Zhang. 2016. "Design, Synthesis and Antifungal Activity of Coumarin Ring-Opening Derivatives" Molecules 21, no. 10: 1387. https://doi.org/10.3390/molecules21101387
APA StyleZhang, M.-Z., Zhang, Y., Wang, J.-Q., & Zhang, W.-H. (2016). Design, Synthesis and Antifungal Activity of Coumarin Ring-Opening Derivatives. Molecules, 21(10), 1387. https://doi.org/10.3390/molecules21101387








