Ionic Liquid as an Efficient Medium for the Synthesis of Quinoline Derivatives via α-Chymotrypsin-Catalyzed Friedländer Condensation
Abstract
:1. Introduction
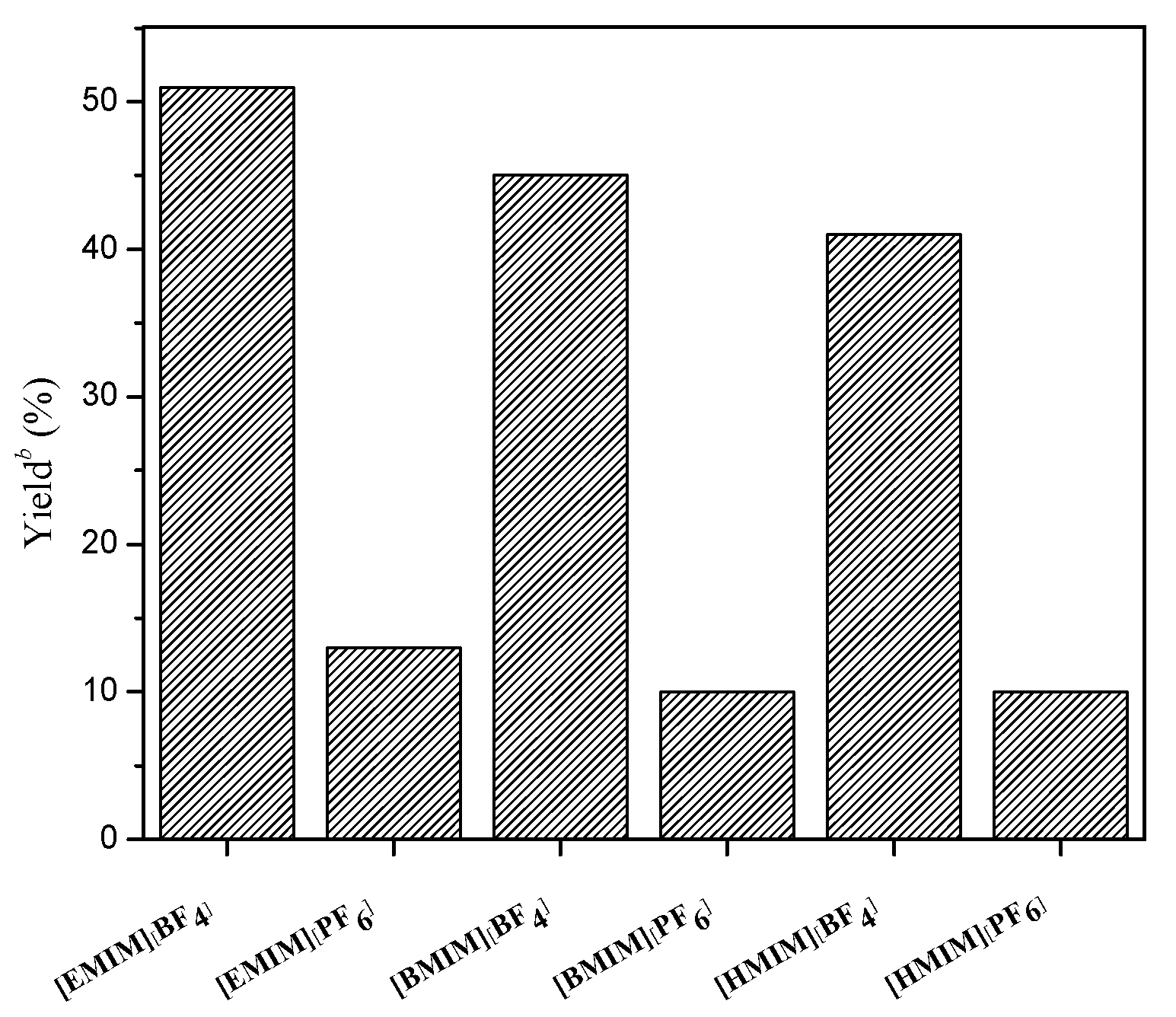
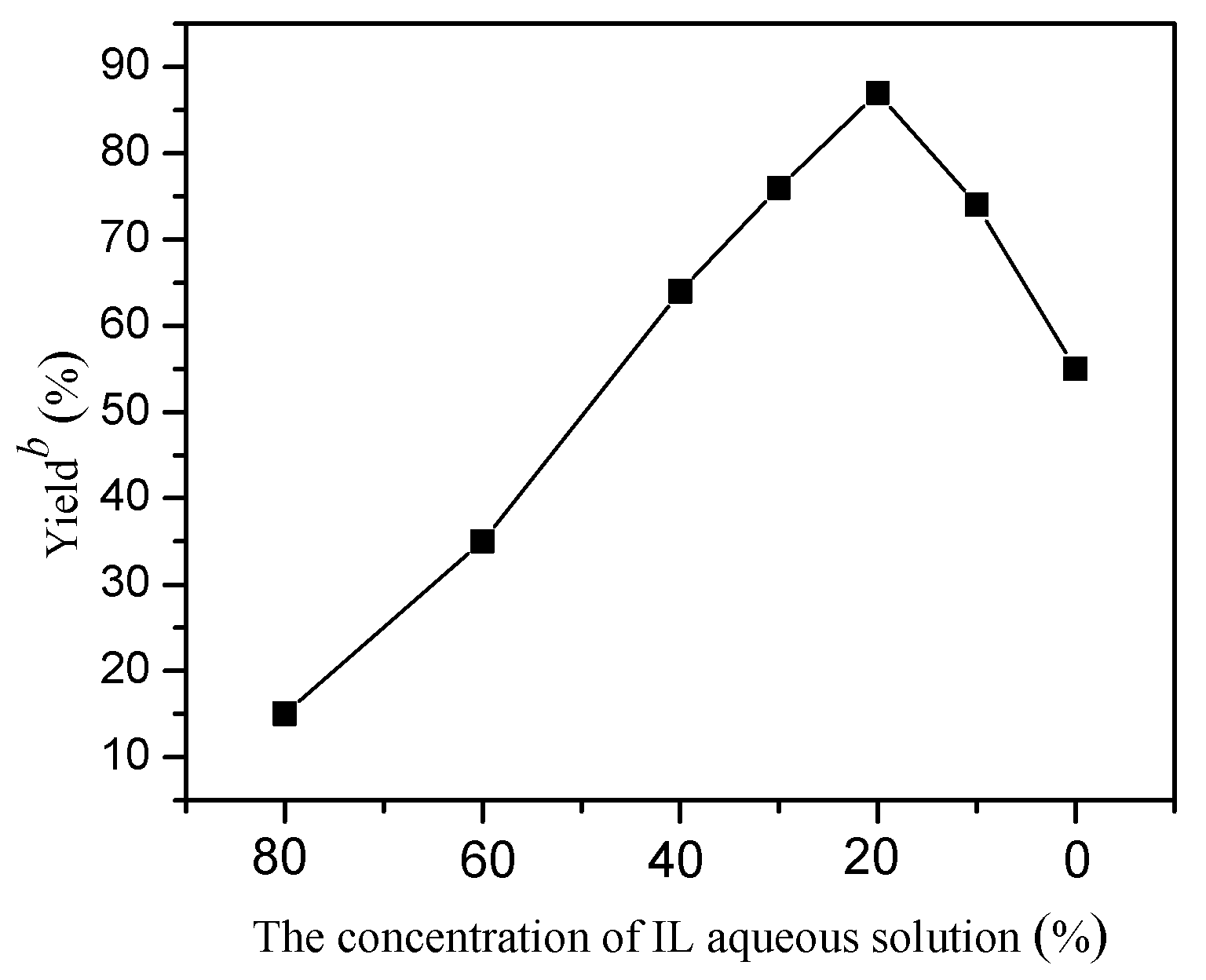
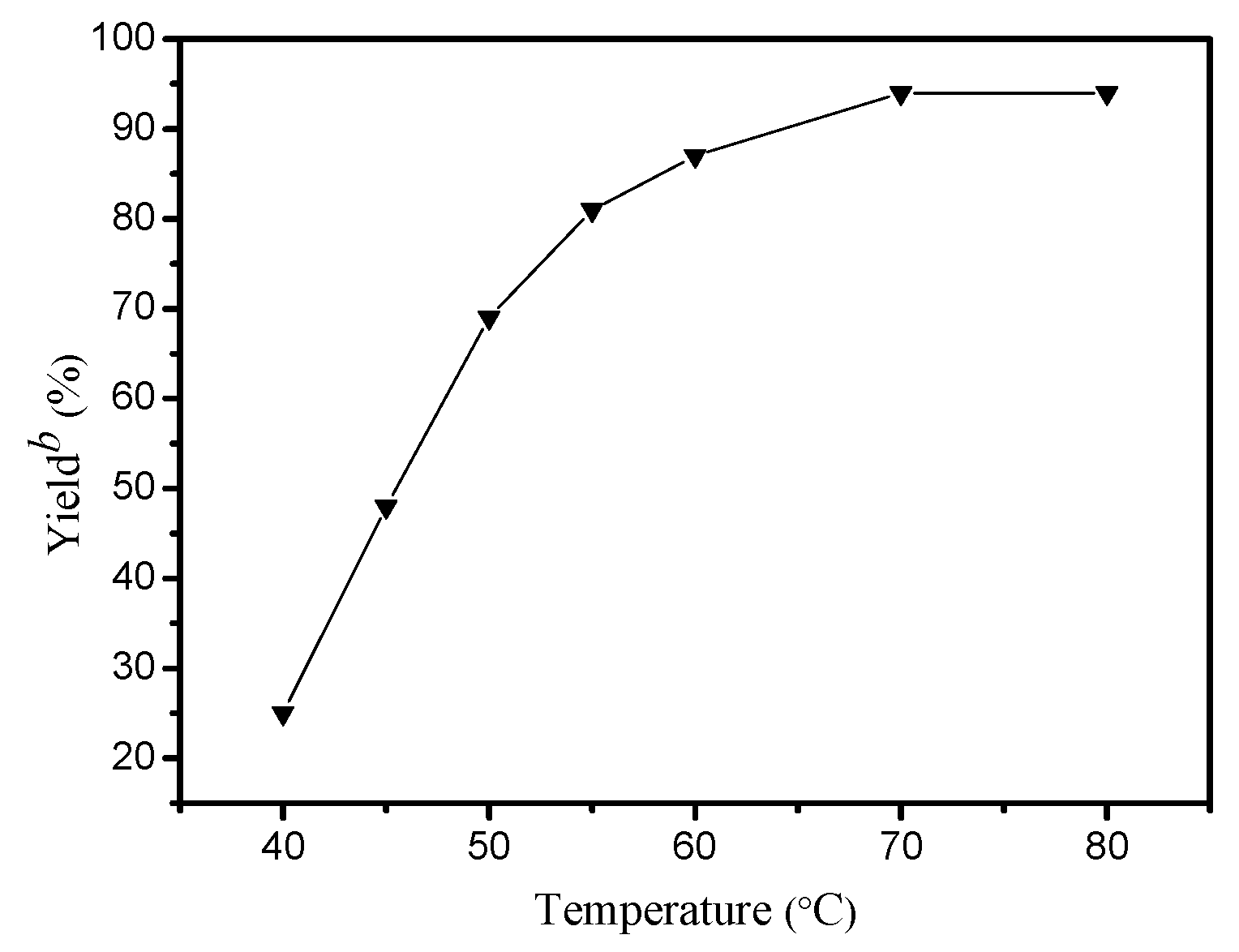
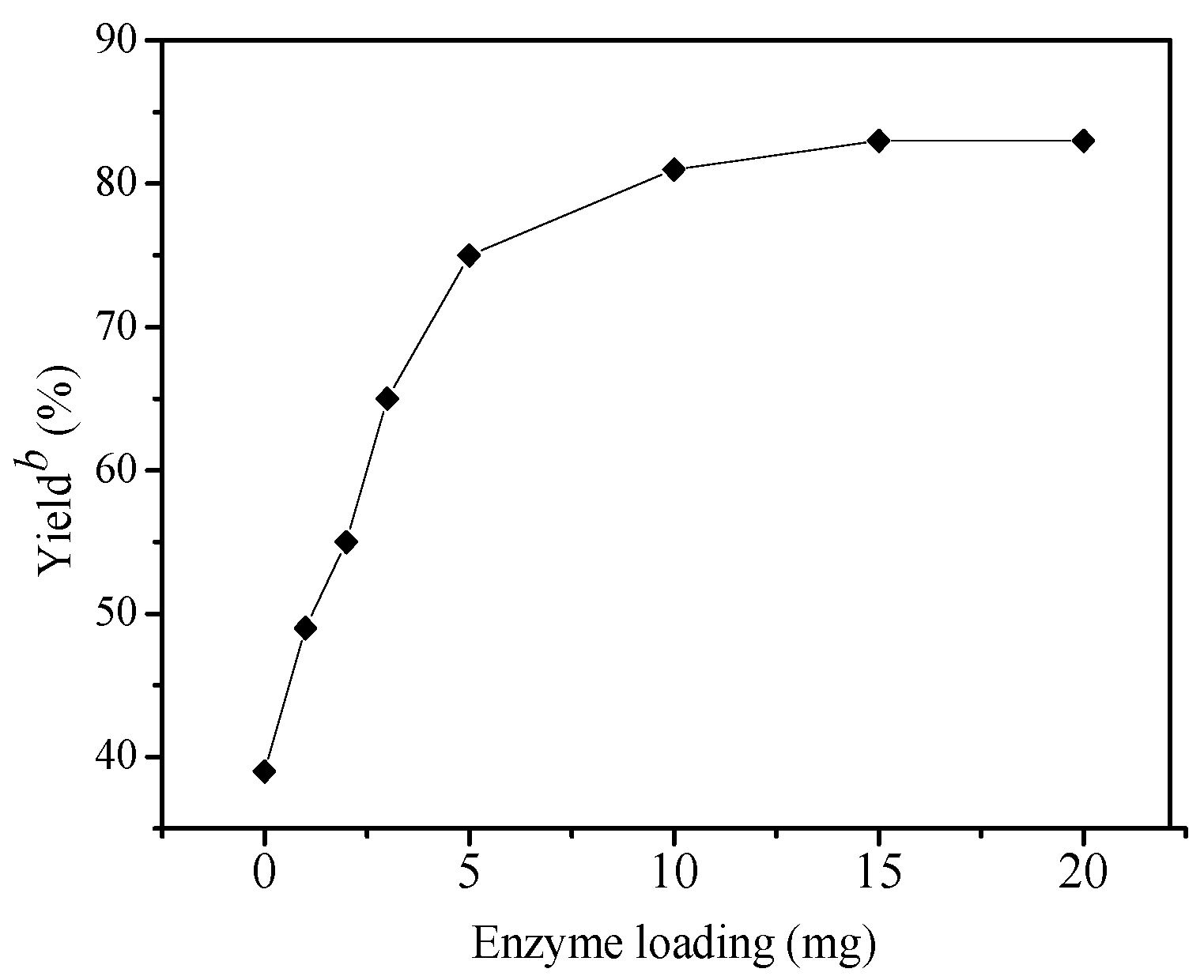
2. Results and Discussion
3. Experimental
3.1. General Information
3.2. General Procedure for the Synthesis of Bis(Indolyl)Methane
4 Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Y.-L.; Fang, K.-C.; Sheu, J.-Y.; Hsu, S.-L.; Tzeng, C.-C. Synthesis and antibacterial evaluation of certain quinolone derivatives. J. Med. Chem. 2001, 44, 2374–2377. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.-H.; Lee, K.K.-H.; Gambari, R.; Kok, S.H.-L.; Kok, T.-W.; Chan, A.S.-C.; Bian, Z.-X.; Wong, W.-Y.; Wong, R.S.-M.; Lau, F.-Y. Anti-tumour and pharmacokinetics study of 2-Formyl-8-hydroxy-quinolinium chloride as Galipea longiflora alkaloid analogue. Phytomedicine 2014, 21, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Talamas, F.X.; Abbot, S.C.; Anand, S.; Brameld, K.A.; Carter, D.S.; Chen, J.; Davis, D.; de Vicente, J.; Fung, A.D.; Gong, L. Discovery of N-[4-[6-tert-Butyl-5-methoxy-8-(6-methoxy-2-oxo-1H-pyridin-3-yl)-3-quinolyl]phenyl]methanesulfonamide (RG7109), a Potent Inhibitor of the Hepatitis C Virus NS5B Polymerase. J. Med. Chem. 2013, 57, 1914–1931. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.-H.; Gambari, R.; Lee, K.K.-H.; Chen, Y.-X.; Kok, S.H.-L.; Wong, R.S.-M.; Lau, F.-Y.; Cheng, C.-H.; Wong, W.-Y.; Bian, Z.-X. Preparation of 8-hydroxyquinoline derivatives as potential antibiotics against Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2014, 24, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Vandekerckhove, S.; Tran, H.G.; Desmet, T.; D’hooghe, M. Evaluation of (4-aminobutyloxy) quinolines as a novel class of antifungal agents. Bioorg. Med. Chem. Lett. 2013, 23, 4641–4643. [Google Scholar] [CrossRef] [PubMed]
- Ratheesh, M.; Sindhu, G.; Helen, A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm. Res. 2013, 62, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-J.; Chang, Y.-L.; Teng, C.-M.; Su, C.-C.; Chen, I.-S. Quinoline alkaloids and anti-platelet aggregation constituents from the leaves of Melicope semecarpifolia. Planta Med. 2002, 68, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.K.; Jenekhe, S.A. New conjugated polyanthrazolines containing thiophene moieties in the main chain. Macromolecules 1991, 24, 6806–6808. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Lu, L.; Alam, M.M. New Conjugated Polymers with Donor−Acceptor Architectures: Synthesis and Photophysics of Carbazole−Quinoline and Phenothiazine−Quinoline Copolymers and Oligomers Exhibiting Large Intramolecular Charge Transfer. Macromolecules. 2001, 34, 7315–7324. [Google Scholar] [CrossRef]
- Zhang, X.; Campo, M.A.; Yao, T.; Larock, R.C. Synthesis of substituted quinolines by electrophilic cyclization of N-(2-alkynyl) anilines. Org. Lett. 2005, 7, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsov, V.V.; Méndez, L.Y.V.; Gómez, C.M.M. Recent progress in the synthesis of quinolines. Curr. Org. Chem. 2005, 9, 141–161. [Google Scholar] [CrossRef]
- Sloop, J.C. Quinoline formation via a modified Combes reaction: Examination of kinetics, substituent effects, and mechanistic pathways. J. Phys. Organ. Chem. 2009, 22, 110–117. [Google Scholar] [CrossRef]
- Yadav, J.; Rao, P.P.; Sreenu, D.; Rao, R.S.; Kumar, V.N.; Nagaiah, K.; Prasad, A. Sulfamic acid: An efficient, cost-effective and recyclable solid acid catalyst for the Friedlander quinoline synthesis. Tetrahedron Lett. 2005, 46, 7249–7253. [Google Scholar] [CrossRef]
- Ghassamipour, S.; Sardarian, A. Friedländer synthesis of poly-substituted quinolines in the presence of dodecylphosphonic acid (DPA) as a highly efficient, recyclable and novel catalyst in aqueous media and solvent-free conditions. Tetrahedron Lett. 2009, 50, 514–519. [Google Scholar] [CrossRef]
- Palimkar, S.S.; Siddiqui, S.A.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. Ionic liquid-promoted regiospecific friedlander annulation: Novel synthesis of quinolines and fused polycyclic quinolines. J. Org. Chem. 2003, 68, 9371–9378. [Google Scholar] [CrossRef] [PubMed]
- Pollard, D.J.; Woodley, J.M. Biocatalysis for pharmaceutical intermediates: The future is now. Trends Biotechnol. 2007, 25, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Busto, E.; Gotor-Fernández, V.; Gotor, V. Hydrolases: Catalytically promiscuous enzymes for non-conventional reactions in organic synthesis. Chem. Soc. Rev. 2010, 39, 4504–4523. [Google Scholar] [CrossRef] [PubMed]
- Branneby, C.; Carlqvist, P.; Magnusson, A.; Hult, K.; Brinck, T.; Berglund, P. Carbon−carbon bonds by hydrolytic enzymes. J. Am. Chem. Soc. 2003, 125, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Feng, X.-W.; Wang, N.; Zhou, Y.-J.; Yu, X.-Q. Biocatalytic promiscuity: The first lipase-catalysed asymmetric aldol reaction. Green Chem. 2008, 10, 616–618. [Google Scholar] [CrossRef]
- Li, K.; He, T.; Li, C.; Feng, X.-W.; Wang, N.; Yu, X.-Q. Lipase-catalysed direct Mannich reaction in water: Utilization of biocatalytic promiscuity for C–C bond formation in a “one-pot” synthesis. Green Chem. 2009, 11, 777–779. [Google Scholar] [CrossRef]
- Wang, J.-L.; Li, X.; Xie, H.-Y.; Liu, B.-K.; Lin, X.-F. Hydrolase-catalyzed fast Henry reaction of nitroalkanes and aldehydes in organic media. J. Biotechnol. 2010, 145, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Xie, Z.-B.; Ai, F.; Le, Z.-G. Synthesis of quinoline derivatives catalyzed by α-chymotrypsin. Chin. J. Org. Chem. 2016, 36, 2704–2708. [Google Scholar] [CrossRef]
- Erbeldinger, M.; Mesiano, A.J.; Russell, A.J. Enzymatic Catalysis of Formation of Z-Aspartame in Ionic Liquid−An Alternative to Enzymatic Catalysis in Organic Solvents. Biotechnol. Prog. 2000, 16, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Madeira Lau, R.; Van Rantwijk, F.; Seddon, K.; Sheldon, R. Lipase-catalyzed reactions in ionic liquids. Org. Lett. 2000, 2, 4189–4191. [Google Scholar] [CrossRef] [PubMed]
- Schöfer, S.H.; Kaftzik, N.; Wasserscheid, P.; Kragl, U. Enzyme catalysis in ionic liquids: Lipase catalysed kinetic resolution of 1-phenylethanol with improved enantioselectivity. Chem. Commun. 2001, 425–426. [Google Scholar]
- Heravi, M.R.P. An efficient synthesis of quinolines derivatives promoted by a room temperature ionic liquid at ambient conditions under ultrasound irradiation via the tandem addition/annulation reaction of o-aminoaryl ketones with α-methylene ketones. Ultrason. Sonochem. 2009, 16, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Pan, W. Ionic liquids: Green solvents for nonaqueous biocatalysis. Enzym. Microb. Technol. 2005, 37, 19–28. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the compounds are available from the authors. |



| Entry | 2-Aminoaryl Ketone (1) | α-Methylene Ketone (2) | Product (3) | Yield b/% |
|---|---|---|---|---|
| 1 |  |  |  | 81 |
| 2 |  |  |  | 83 |
| 3 |  |  |  | 91 |
| 4 |  |  |  | 45 |
| 5 |  |  |  | 40 |
| 6 |  |  |  | 49 |
| 7 |  |  |  | 74 |
| 8 |  |  |  | 90 |
| 9 |  |  |  | 90 |
| 10 |  |  |  | 94 |
| 11 |  |  |  | 48 |
| 12 |  |  |  | 42 |
| 13 |  |  |  | 94 |
| 14 |  |  |  | 80 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, Z.-G.; Liang, M.; Chen, Z.-S.; Zhang, S.-H.; Xie, Z.-B. Ionic Liquid as an Efficient Medium for the Synthesis of Quinoline Derivatives via α-Chymotrypsin-Catalyzed Friedländer Condensation. Molecules 2017, 22, 762. https://doi.org/10.3390/molecules22050762
Le Z-G, Liang M, Chen Z-S, Zhang S-H, Xie Z-B. Ionic Liquid as an Efficient Medium for the Synthesis of Quinoline Derivatives via α-Chymotrypsin-Catalyzed Friedländer Condensation. Molecules. 2017; 22(5):762. https://doi.org/10.3390/molecules22050762
Chicago/Turabian StyleLe, Zhang-Gao, Meng Liang, Zhong-Sheng Chen, Sui-Hong Zhang, and Zong-Bo Xie. 2017. "Ionic Liquid as an Efficient Medium for the Synthesis of Quinoline Derivatives via α-Chymotrypsin-Catalyzed Friedländer Condensation" Molecules 22, no. 5: 762. https://doi.org/10.3390/molecules22050762





