Ontogenetic Variation of Individual and Total Capsaicinoids in Malagueta Peppers (Capsicum frutescens) during Fruit Maturation
Abstract
:1. Introduction
2. Results and Discussion
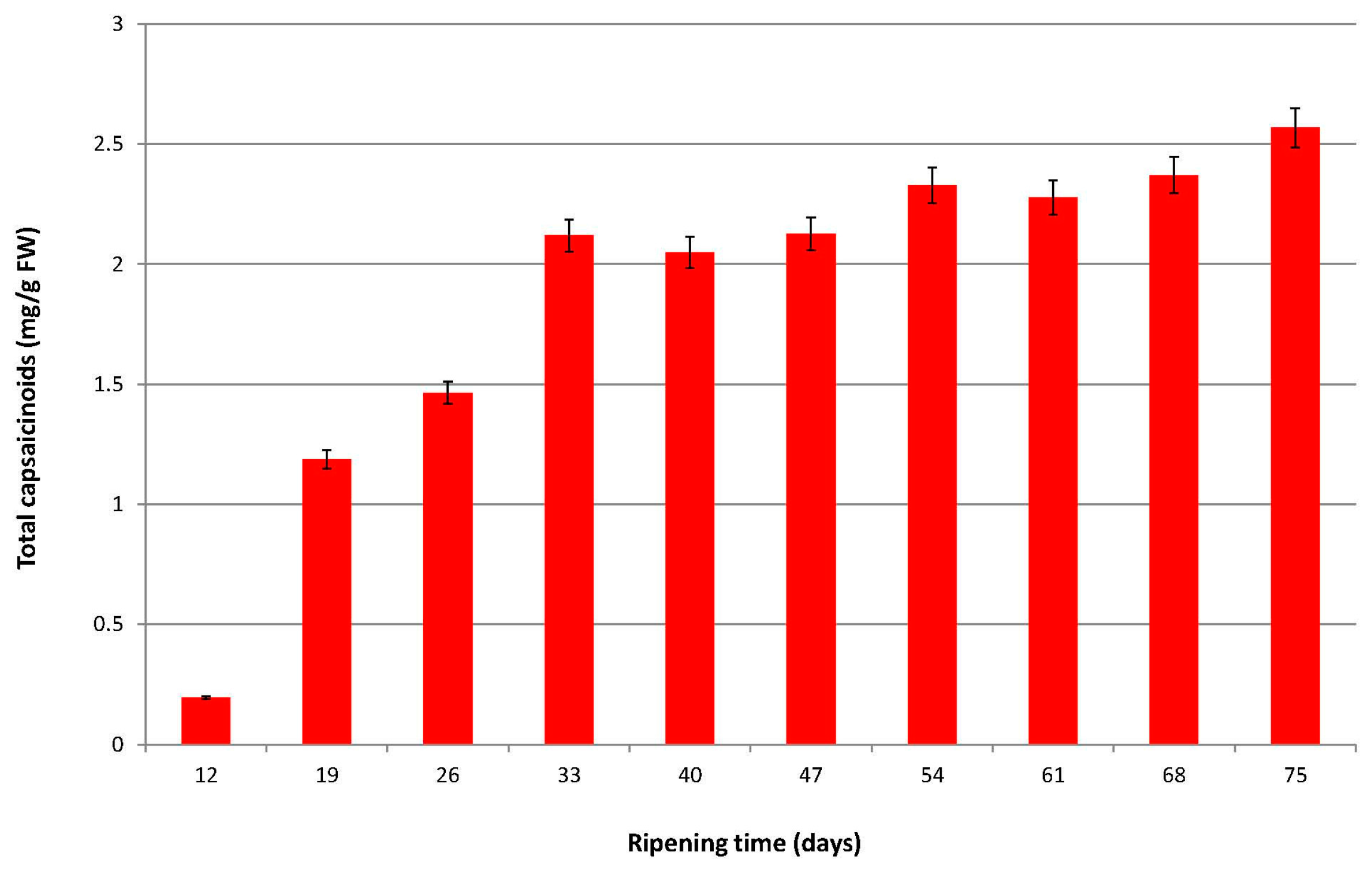
2.1. Evolution of the Total Capsaicinoid Content in Malagueta pepper
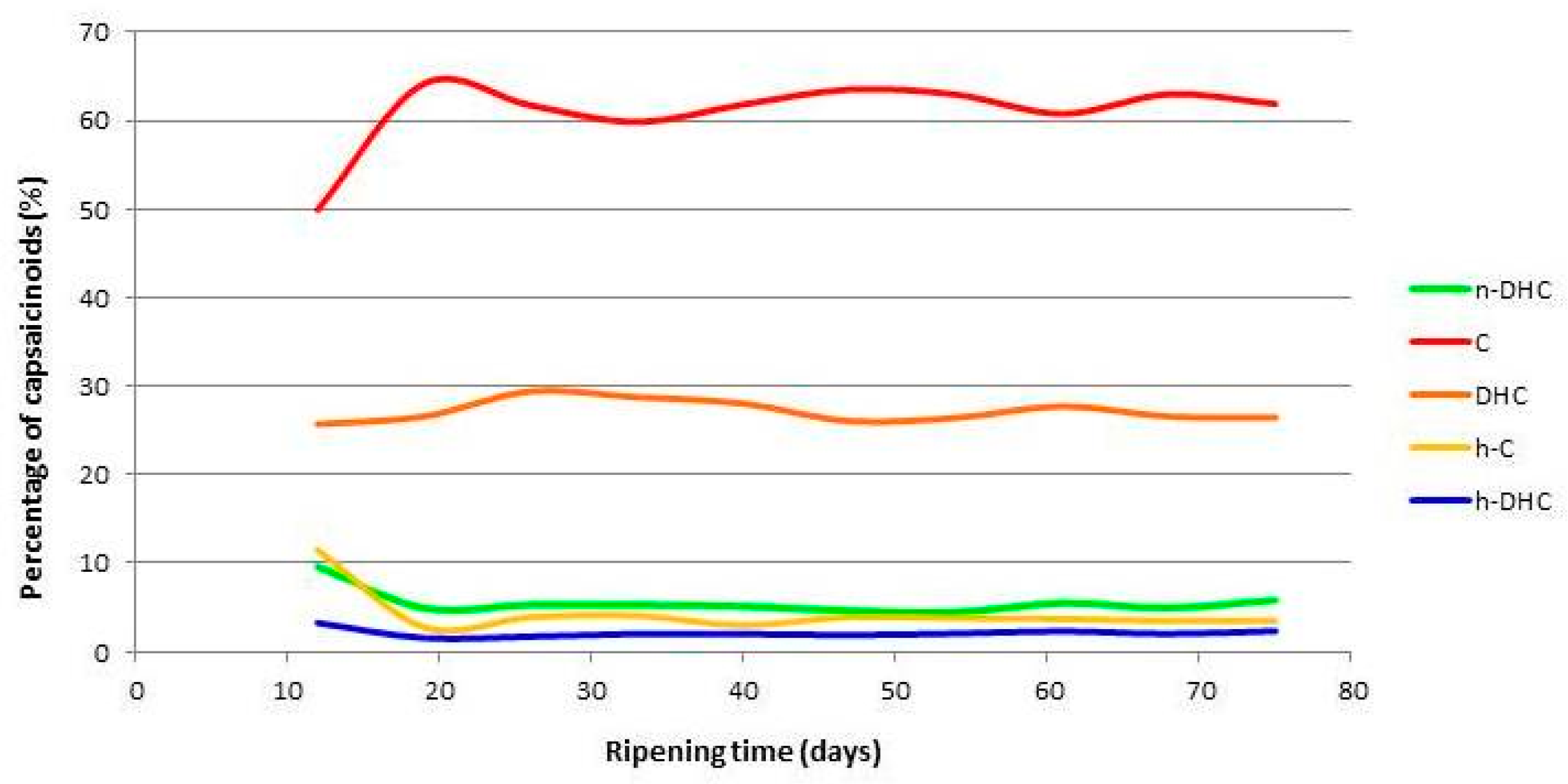
2.2. Evolution of the Individual Contents of Capsaicinoids in Malagueta pepper
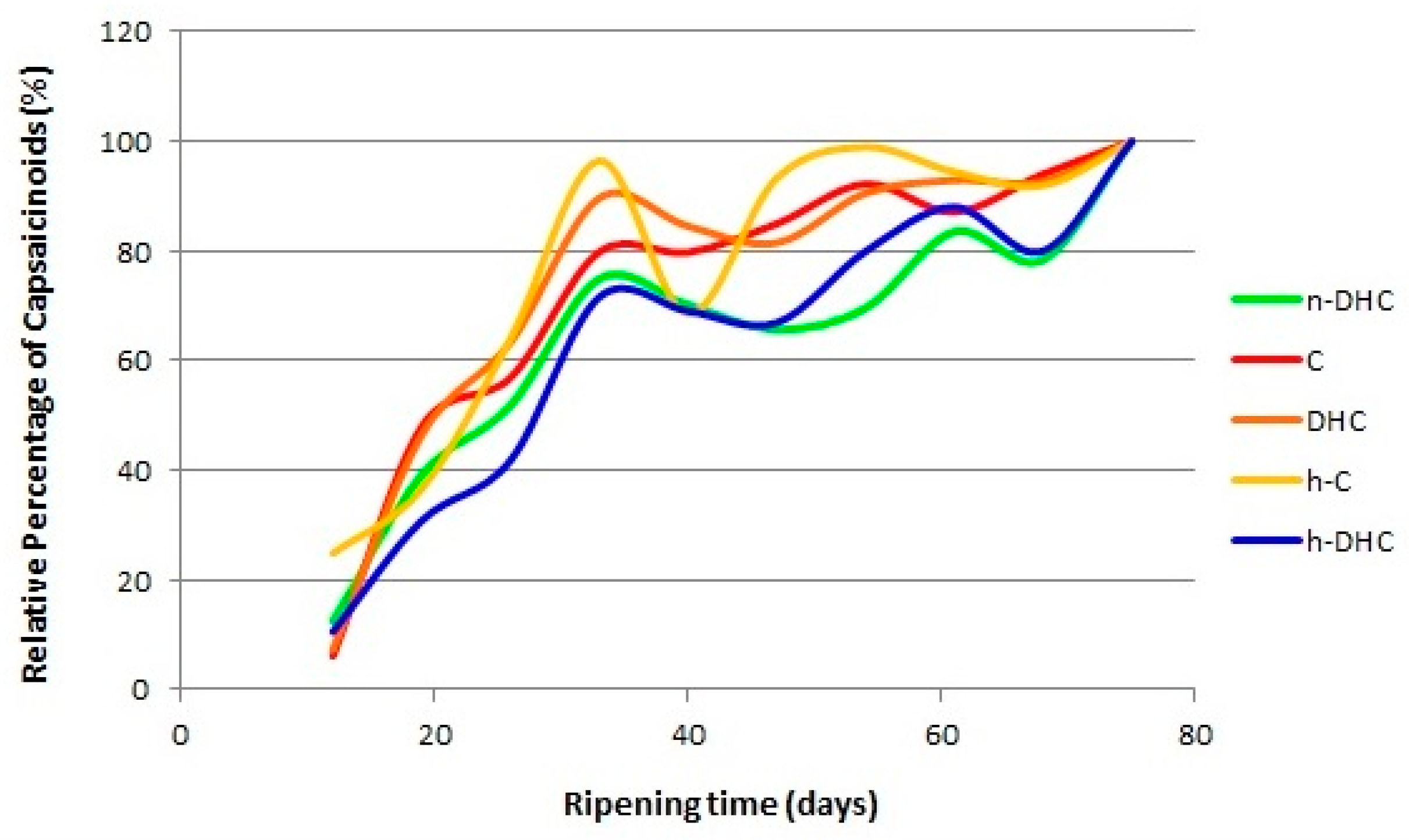
2.3. Evolution of the Standardized Values of Capsaicinoids in Malagueta Pepper
3. Materials and Methods
3.1. Chemicals
3.2. Pepper Crops
3.3. Fertilization of the Plants
3.4. Monitoring of the Ripening and Harvesting of Peppers
3.5. Plant Material
3.6. Extraction Procedure
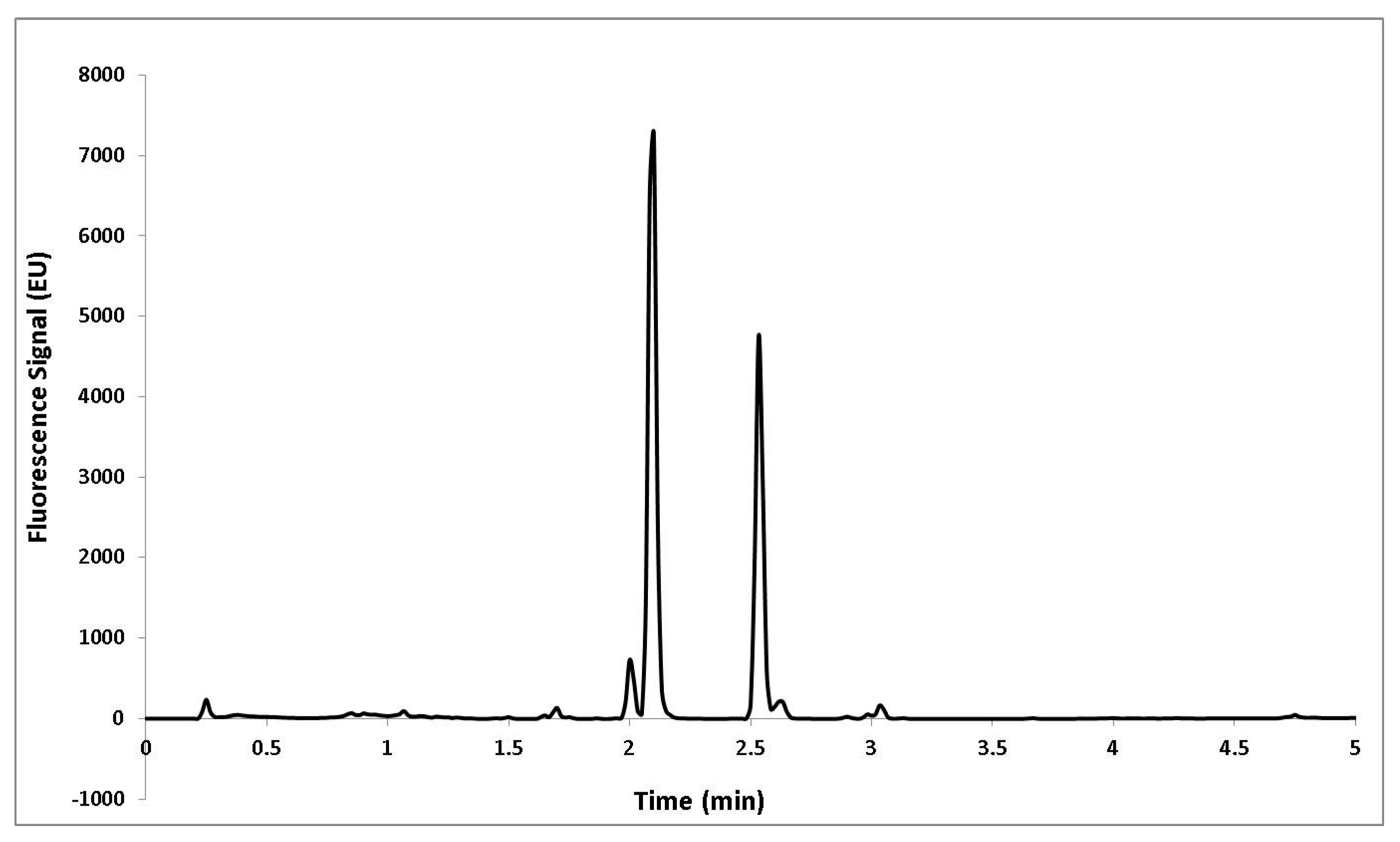
3.7. UHPLC-Fluorescence Analysis
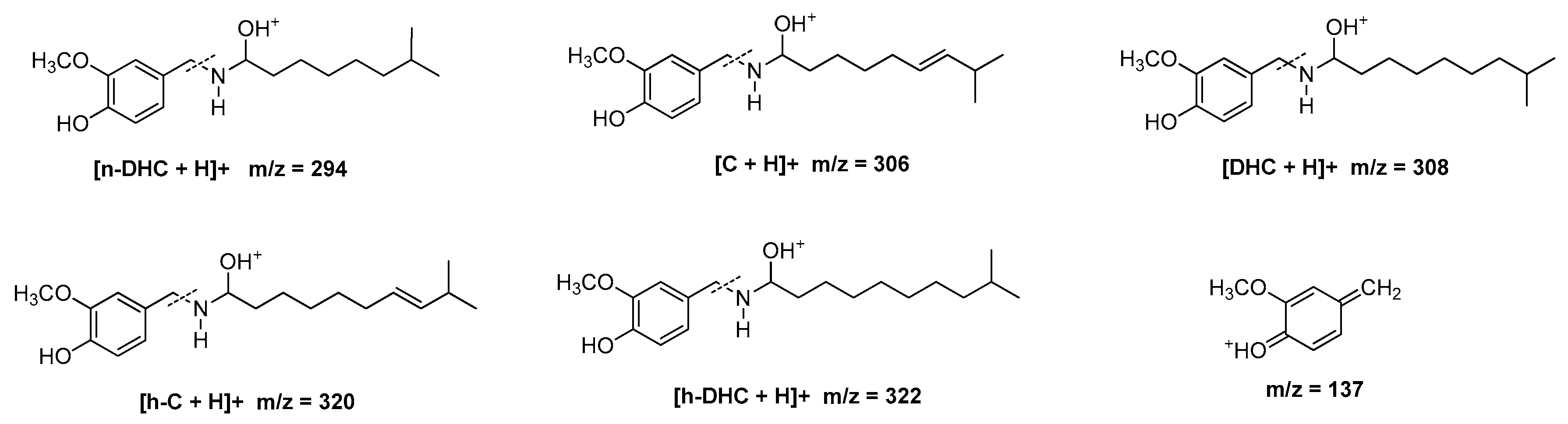
3.8. Identification of Capsaicinoids by Liquid Chromatography Coupled to Mass Spectrometry
3.9. UHPLC Calibration
3.10. Quantification of the Capsaicinoids Present in Malagueta Peppers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barbero, G.F.; Liazid, A.; Azaroual, L.; Palma, M.; Barroso, C.G. Capsaicinoid Contents in Peppers and Pepper-Related Spicy Foods. Int. J. Food Prop. 2016, 19, 485–493. [Google Scholar] [CrossRef]
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Evaluation of carotenoid and capsaicinoid contents in powder of red chili peppers during one year of storage. Food Res. Int. 2014, 65, 163–170. [Google Scholar] [CrossRef]
- Díaz, J.; Pomar, F.; Bernal, Á.; Merino, F. Peroxidases and the metabolism of capsaicin in Capsicum annuum L. Phytochem. Rev. 2004, 3, 141–157. [Google Scholar] [CrossRef]
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chem. 2013, 140, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Laskaridou-Monnerville, A. Determination of capsaicin and dihydrocapsaicin by micellar electrokinetic capillary chromatography and its application to various species of Capsicum, Solanaceae. J. Chromatogr. A 1999, 838, 293–302. [Google Scholar] [CrossRef]
- Stewart, C.; Mazourek, M.; Stellari, G.M.; O’Connell, M.; Jahn, M. Genetic control of pungency in C. chinense via the Pun1 locus. J. Exp. Bot. 2007, 58, 979–991. [Google Scholar] [PubMed]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chapa-Oliver, A.M.; Mejía-Teniente, L. Capsaicin: From plants to a cancer-suppressing agent. Molecules 2016, 21, 931. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Hohmann, M.; Rossaneis, A.; Pinho-Ribeiro, F.; Verri, W. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Chopan, M.; Littenberg, B. The Association of Hot Red Chili Pepper Consumption and Mortality: A Large Population-Based Cohort Study. PLoS ONE 2017, 12, e0169876. [Google Scholar] [CrossRef] [PubMed]
- Fujiwake, H.; Suzuki, T.; Iwai, K. Intracellular localization of capsaicin and its analogues in Capsicum fruit II. The vacuole as the intracellular accumulation site of capsaicinoid in the protoplast of Capsicum fruit. Plant Cell Physiol. 1980, 21, 1023–1030. [Google Scholar]
- Iwai, K.; Suzuki, T.; Fujiwake, H. Formation and accumulation of pungent principle of hot pepper fruits, capsaicin and its analogues, in Capsicum annuun var. Annuun cv. karayatsubusa at different growth stages after flowering. Agric. Biol. Chem. 1979, 43, 2493–2498. [Google Scholar]
- Barbero, G.F.; Ruiz, A.G.; Liazid, A.; Palma, M.; Vera, J.C.; Barroso, C.G. Evolution of total and individual capsaicinoids in peppers during ripening of the Cayenne pepper plant (Capsicum annuum L.). Food Chem. 2014, 153, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Barrera, J.A.; Hernández, M.S.; Melgarejo, L.M.; Martínez, O.; Fernández-Trujillo, J.P. Physiological behavior and quality traits during fruit growth and ripening of four Amazonic hot pepper accessions. J. Sci. Food Agric. 2008, 88, 847–857. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Yahia, E.M. Changes in capsaicinoids during development, maturation, and senescence of chile peppers and relation with peroxidase activity. J. Agric. Food Chem. 1998, 46, 2075–2079. [Google Scholar] [CrossRef]
- Estrada, B.; Bernal, M.A.; Díaz, J.; Pomar, F.; Merino, F. Fruit development in Capsicum annuum: Changes in capsaicin, lignin, free phenolics, and peroxidase patterns. J. Agric. Food Chem. 2000, 48, 6234–6239. [Google Scholar] [CrossRef] [PubMed]
- Estrada, B.; Bernal, M.A.; Díaz, J.; Pomar, F.; Merino, F. Capsaicinoids in vegetative organs of Capsicum annuum L. in Relation to fruiting. J. Agric. Food Chem. 2002, 50, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Seitz, E.; Hiepler, C.; Petz, M. Chili pepper fruits: Content and pattern of capsaicinoids in single fruits of different ages. J. Agric. Food Chem. 2008, 56, 12114–12121. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.A.; Calderón, A.A.; Pedreño, M.A.; Muñoz, R.; Barceló, A.R.; de Cáceres, F.M. Dihydrocapsaicin Oxidation by Capsicum annuum (var. annuum) Peroxidase. J. Food Sci. 1993, 58, 611–613. [Google Scholar] [CrossRef]
- Bernal, M.A.; Calderón, A.A.; Pedreño, M.A.; Muñoz, R.; Barceló, A.R.; Merino De Cácerest, F. Capsaicin Oxidation by Peroxidase from Capsicum annuum (Var. annuum) Fruits. J. Agric. Food Chem. 1999, 41, 1041–1044. [Google Scholar] [CrossRef]
- Barbero, G.F.; de Aguiar, A.C.; Carrera, C.; Olachea, Á.; Ferreiro-González, M.; Martínez, J.; Palma, M.; Barroso, C.G. Evolution of Capsaicinoids in Peter Pepper (Capsicum annuum var. annuum) During Fruit Ripening. Chem. Biodivers. 2016, 13, 1068–1075. [Google Scholar] [PubMed]
- Harvell, K.P.; Bosland, P.W. The environment produces a significant effect on pungency of chiles. HortScience 1997, 32, 1292. [Google Scholar]
- Zewdie, Y.; Bosland, P.W. Evaluation of genotype, environment, and genotype-by-environment interaction for capsaicinoids in Capsicum annuum L. Euphytica 2000, 111, 185–190. [Google Scholar] [CrossRef]
- Estrada, B.; Pomar, F.; Díaz, J.; Merino, F.; Bernal, M.A. Effects of mineral fertilizer supplementation on fruit development and pungency in “Padron” peppers. J. Hortic. Sci. Biotechnol. 1998, 73, 493–497. [Google Scholar] [CrossRef]
- Gibbs, H.A.A.; O’Garro, L.W. Capsaicin content of West Indies hot pepper cultivars using colorimetric and chromatographic techniques. HortScience 2004, 39, 132–135. [Google Scholar]
- Tahboub, M.B.; Sanogo, S.; Bosland, P.W.; Murray, L. Heat level in chile pepper in relation to root and fruit infection by Phytophthora capsici. HortScience 2008, 43, 1846–1851. [Google Scholar]
- Kirschbaum-Titze, P.; Mueller-Seitz, E.; Petz, M. Pungency in paprika (Capsicum annuum). 2. Heterogeneity of capsaicinoid content in individual fruits from one plant. J. Agric. Food Chem. 2002, 50, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Zewdie, Y.; Bosland, P.W. Pungency of Chile (Capsicum annuum L.) fruit is affected by node position. HortScience 2000, 35, 1174. [Google Scholar]
- Thulin, M.; Moore, A.J.; El-Seedi, H.; Larsson, A.; Christin, P.A.; Edwards, E.J. Phylogeny and generic delimitation in molluginaceae, new pigment data in caryophyllales, and the new family corbichoniaceae. Taxon 2016, 65, 775–793. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Fast determination of capsaicinoids from peppers by high-performance liquid chromatography using a reversed phase monolithic column. Food Chem. 2008, 107, 1276–1282. [Google Scholar] [CrossRef]
- Garcés-Claver, A.; Gil-Ortega, R.; Álvarez-Fernández, A.; Arnedo-Andrés, M.S. Inheritance of capsaicin and dihydrocapsaicin, determined by HPLC-ESI/MS, in an intraspecific cross of Capsicum annuum L. J. Agric. Food Chem. 2007, 55, 6951–6957. [Google Scholar] [CrossRef] [PubMed]
- González-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J.G.; Pérez-Morales, R.; Ortiz, J.C.R.; García-Hernández, J.L. Characterization of different capsicum varieties by evaluation of their capsaicinoids content by high performance liquid chromatography, determination of pungency and effect of high temperature. Molecules 2013, 18, 13471–13486. [Google Scholar] [CrossRef] [PubMed]
- Al Othman, Z.A.; Ahmed, Y.B.H.; Habila, M.A.; Ghafar, A.A. Determination of capsaicin and dihydrocapsaicin in Capsicum fruit samples using high performance liquid chromatography. Molecules 2011, 16, 8919–8929. [Google Scholar] [CrossRef] [PubMed]
- Alothman, Z.A.; Wabaidur, S.M.; Khan, M.R.; Ghafar, A.A.; Habila, M.A.; Ahmed, Y.B.H. Determination of capsaicinoids in Capsicum species using ultra performance liquid chromatography-mass spectrometry. J. Sep. Sci. 2012, 35, 2892–2896. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.G.; Rafii, M.Y.; Ismail, M.R.; Malek, M.A.; Latif, M.A. Capsaicin and dihydrocapsaicin determination in chili pepper genotypes using ultra-fast liquid chromatography. Molecules 2014, 19, 6474–6488. [Google Scholar] [CrossRef] [PubMed]
- Sganzerla, M.; Coutinho, J.P.; de Melo, A.M.T.; Godoy, H.T. Fast method for capsaicinoids analysis from Capsicum chinense fruits. Food Res. Int. 2014, 64, 718–725. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Ferreiro-González, M.; Palma, M.; Barroso, C.G. Fast Separation of Capsaicinoids from Peppers by Reversed Phase Ultra-Performance Liquid Chromatography: Comparation with Traditional High-Performance Liquid Chromatography Methods. Int. J. Food Prop. 2016, 19, 984–992. [Google Scholar] [CrossRef]
- Bogusz Junior, S.; Tavares, A.M.; Filho, J.T.; Zini, C.A.; Godoy, H.T. Analysis of the volatile compounds of Brazilian chilli peppers (Capsicum spp.) at two stages of maturity by solid phase micro-extraction and gas chromatography-mass spectrometry. Food Res. Int. 2012, 48, 98–107. [Google Scholar] [CrossRef]
- Rêgo, E.R.; Finger, F.L.; Rêgo, M.M. Consumption of pepper in brazil and its implications on nutrition and health of humans and animals. In Peppers: Nutrition, Consumption and Health; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; Chapter 7; pp. 159–170. [Google Scholar]
- Santos, P.; Aguiar, A.C.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Supercritical carbon dioxide extraction of capsaicinoids from malagueta pepper (Capsicum frutescens L.) assisted by ultrasound. Ultrason. Sonochem. 2015, 22, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, P.L.A.; Nascimento, T.C.E.S.; Ramos, N.S.M.; Silva, G.R.; Gomes, J.E.G.; Falcão, R.E.A.; Moreira, K.A.; Porto, A.L.F.; Silva, T.M.S. Quantification, Antioxidant and Antimicrobial Activity of Phenolics Isolated from Different Extracts of Capsicum frutescens (Pimenta Malagueta). Molecules 2014, 19, 5434–5447. [Google Scholar] [CrossRef] [PubMed]
- Abud, H.F.; Araujo, E.F.; Araujo, R.F.; Araujo, A.V.; Pinto, C.M.F. Physiological quality of “malagueta” and “biquinho” pepper seeds during ontogeny. Pesqui. Agropecu. Bras. 2013, 48, 1546–1554. [Google Scholar] [CrossRef]
- Rebouças, T.N.; Valverde, R.M.; Teixeira, H.L. Bromatology of fresh and processed chili pepper. [Bromatologia da pimenta malagueta in natura e processada em conserva]. Hortic. Bras. 2013, 31, 163–165. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Sales, L.P.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Supercritical carbon dioxide extraction of Capsicum peppers: Global yield and capsaicinoid content. J. Supercrit. Fluids 2013, 81, 210–216. [Google Scholar] [CrossRef]
- Chinn, M.S.; Sharma-Shivappa, R.R.; Cotter, J.L. Solvent extraction and quantification of capsaicinoids from Capsicum chinense. Food Bioprod. Process. 2011, 89, 340–345. [Google Scholar] [CrossRef]
- Suzuki, T.; Iwai, K. Chapter 4 Constituents of Red Pepper Species: Chemistry, Biochemistry, Pharmacology, and food Science of the Pungent Principle of Capsicum Species. Alkaloids Chem. Pharmacol. 1984, 23, 227–299. [Google Scholar]
- Barbero, G.F.; Molinillo, J.M.G.; Varela, R.M.; Palma, M.; MacÍas, F.A.; Barroso, C.G. Application of hansch’s model to capsaicinoids and capsinoids: A study using the quantitative structure-activity relationship. A novel method for the synthesis of capsinoids. J. Agric. Food Chem. 2010, 58, 3342–3349. [Google Scholar] [CrossRef] [PubMed]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Han, C.; Zhou, X.; Zhu, Z.; Huang, F.; Shen, Y. Determination of three capsaicinoids in Capsicum annuum by pressurized liquid extraction combined with LC-MS/MS. J. Sep. Sci. 2013, 36, 857–862. [Google Scholar] [CrossRef] [PubMed]
- García Campaña, A.M.; Cuadros Rodríguez, L.; Alés Barrero, F.; Román Ceba, M.; Sierra Fernández, J.L. ALAMIN: A chemometric program to check analytical method performance and to assess the trueness by standard addition methodology. Trends Anal. Chem. 1997, 16, 381–385. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
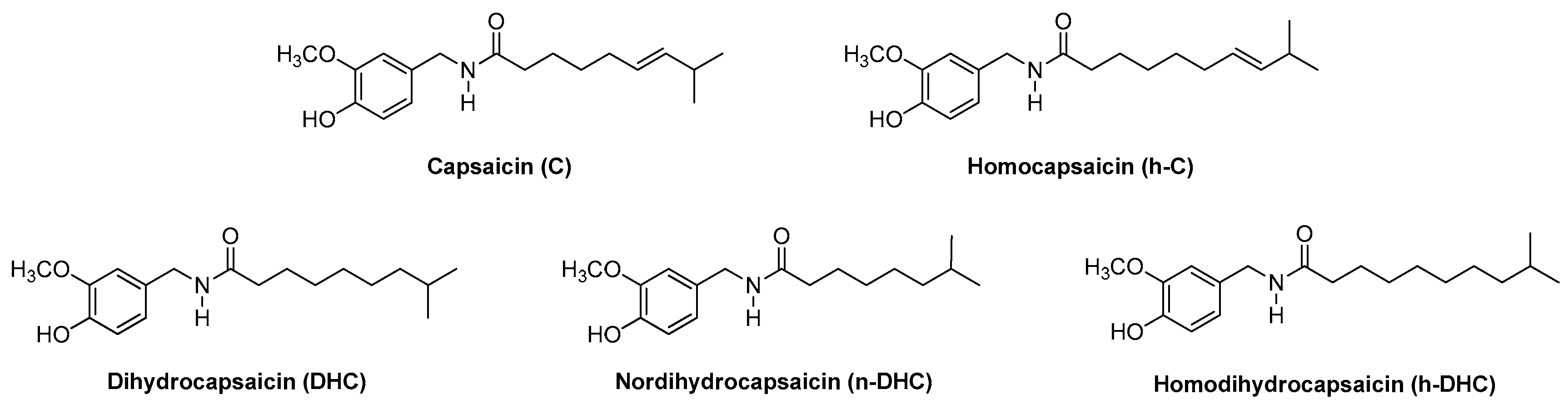
| Code | Start of Fruit Development | Days Till Harvest | Visual State at Harvest |
|---|---|---|---|
| S-1 | 18/09 | 12 | Green color |
| S-2 | 11/09 | 19 | Green color |
| S-3 | 04/09 | 26 | Green color |
| S-4 | 28/08 | 33 | Green color |
| S-5 | 21/08 | 40 | Green/red color |
| S-6 | 14/08 | 47 | Red color |
| S-7 | 07/08 | 54 | Red color |
| S-8 | 31/07 | 61 | Red color |
| S-9 | 24/07 | 68 | Red color |
| S-10 | 17/07 | 75 | Over-ripeness |
| Day | n-DHC | C | DHC | h-C | h-DHC |
|---|---|---|---|---|---|
| 12 | 0.035 ± 0.001 | 0.530 ± 0.021 | 0.229 ± 0.012 | 0.023 ± 0.001 | 0.013 ± 0.001 |
| 19 | 0.059 ± 0.002 | 0.761 ± 0.013 | 0.315 ± 0.017 | 0.033 ± 0.001 | 0.018 ± 0.001 |
| 26 | 0.078 ± 0.002 | 0.903 ± 0.043 | 0.430 ± 0.023 | 0.057 ± 0.002 | 0.025 ± 0.001 |
| 33 | 0.112 ± 0.006 | 1.268 ± 0.035 | 0.609 ± 0.022 | 0.086 ± 0.004 | 0.043 ± 0.002 |
| 40 | 0.105 ± 0.004 | 1.266 ± 0.065 | 0.574 ± 0.016 | 0.061 ± 0.003 | 0.041 ± 0.001 |
| 47 | 0.098 ± 0.003 | 1.350 ± 0.046 | 0.554 ± 0.019 | 0.084 ± 0.001 | 0.040 ± 0.002 |
| 54 | 0.104 ± 0.005 | 1.465 ± 0.021 | 0.615 ± 0.040 | 0.089 ± 0.003 | 0.048 ± 0.001 |
| 61 | 0.125 ± 0.006 | 1.384 ± 0.043 | 0.631 ± 0.032 | 0.085 ± 0.004 | 0.053 ± 0.002 |
| 68 | 0.117 ± 0.006 | 1.493 ± 0.054 | 0.630 ± 0.014 | 0.082 ± 0.004 | 0.048 ± 0.001 |
| 75 | 0.150 ± 0.009 | 1.589 ± 0.077 | 0.680 ± 0.030 | 0.090 ± 0.002 | 0.060 ± 0.005 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayos, O.; De Aguiar, A.C.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Garcés-Claver, A.; Martínez, J.; Mallor, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G.; et al. Ontogenetic Variation of Individual and Total Capsaicinoids in Malagueta Peppers (Capsicum frutescens) during Fruit Maturation. Molecules 2017, 22, 736. https://doi.org/10.3390/molecules22050736
Fayos O, De Aguiar AC, Jiménez-Cantizano A, Ferreiro-González M, Garcés-Claver A, Martínez J, Mallor C, Ruiz-Rodríguez A, Palma M, Barroso CG, et al. Ontogenetic Variation of Individual and Total Capsaicinoids in Malagueta Peppers (Capsicum frutescens) during Fruit Maturation. Molecules. 2017; 22(5):736. https://doi.org/10.3390/molecules22050736
Chicago/Turabian StyleFayos, Oreto, Ana Carolina De Aguiar, Ana Jiménez-Cantizano, Marta Ferreiro-González, Ana Garcés-Claver, Julián Martínez, Cristina Mallor, Ana Ruiz-Rodríguez, Miguel Palma, Carmelo G. Barroso, and et al. 2017. "Ontogenetic Variation of Individual and Total Capsaicinoids in Malagueta Peppers (Capsicum frutescens) during Fruit Maturation" Molecules 22, no. 5: 736. https://doi.org/10.3390/molecules22050736








