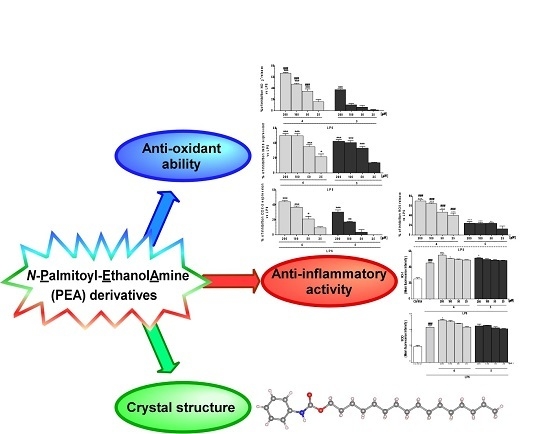
Anti-Inflammatory, Antioxidant and Crystallographic Studies of N-Palmitoyl-ethanol Amine (PEA) Derivatives
Abstract
:1. Introduction
2. Results and Discussion
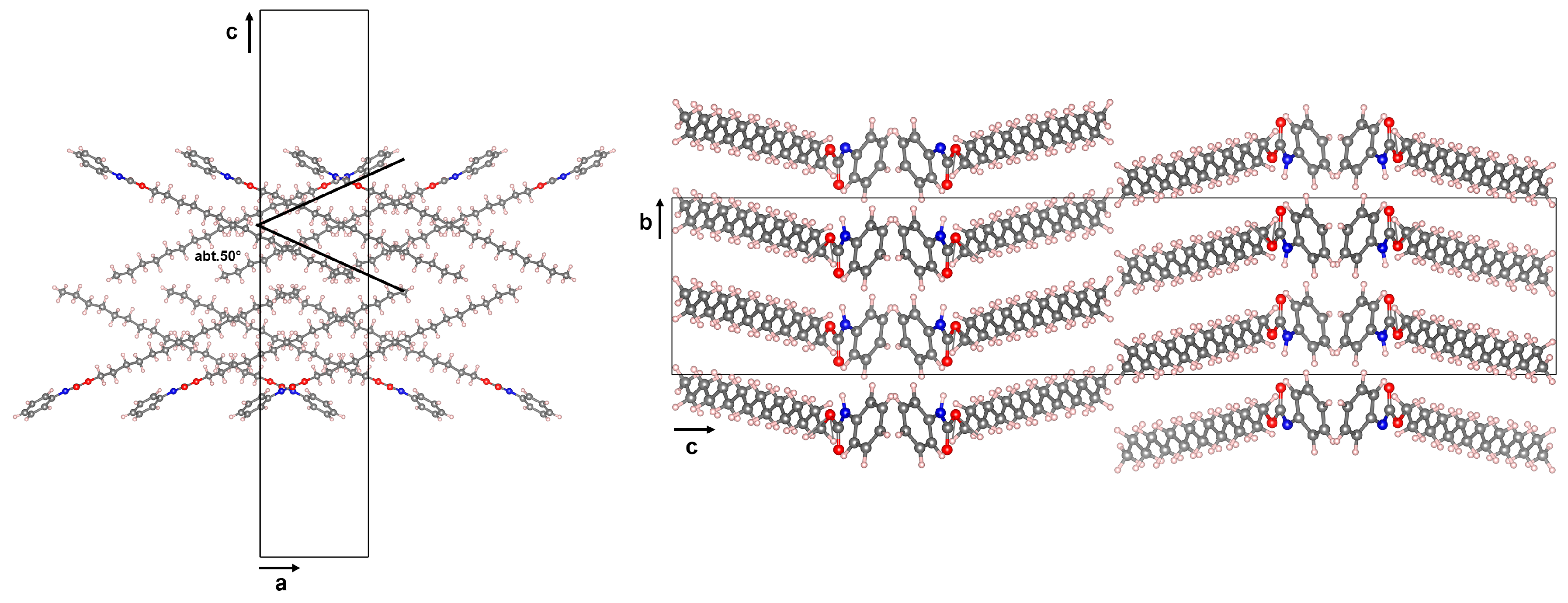
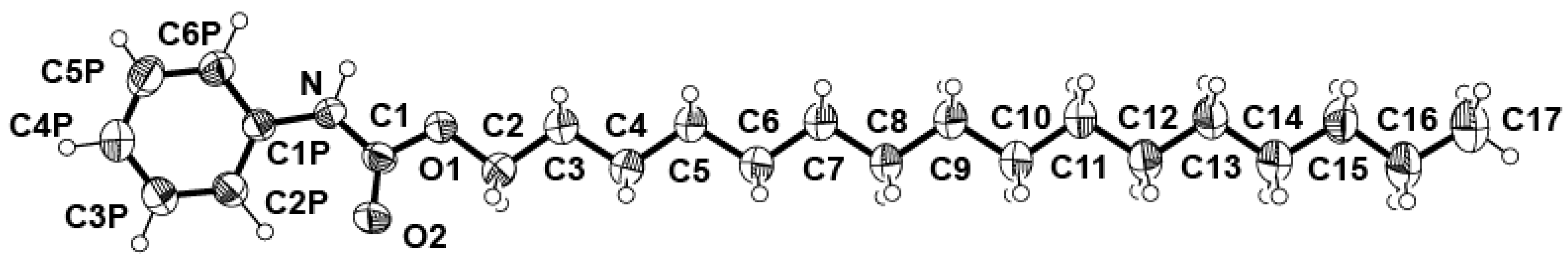
2.1. Crystal Structure Determination of 4
2.2. Compounds 4 and 5 Exert Macrophage Viability
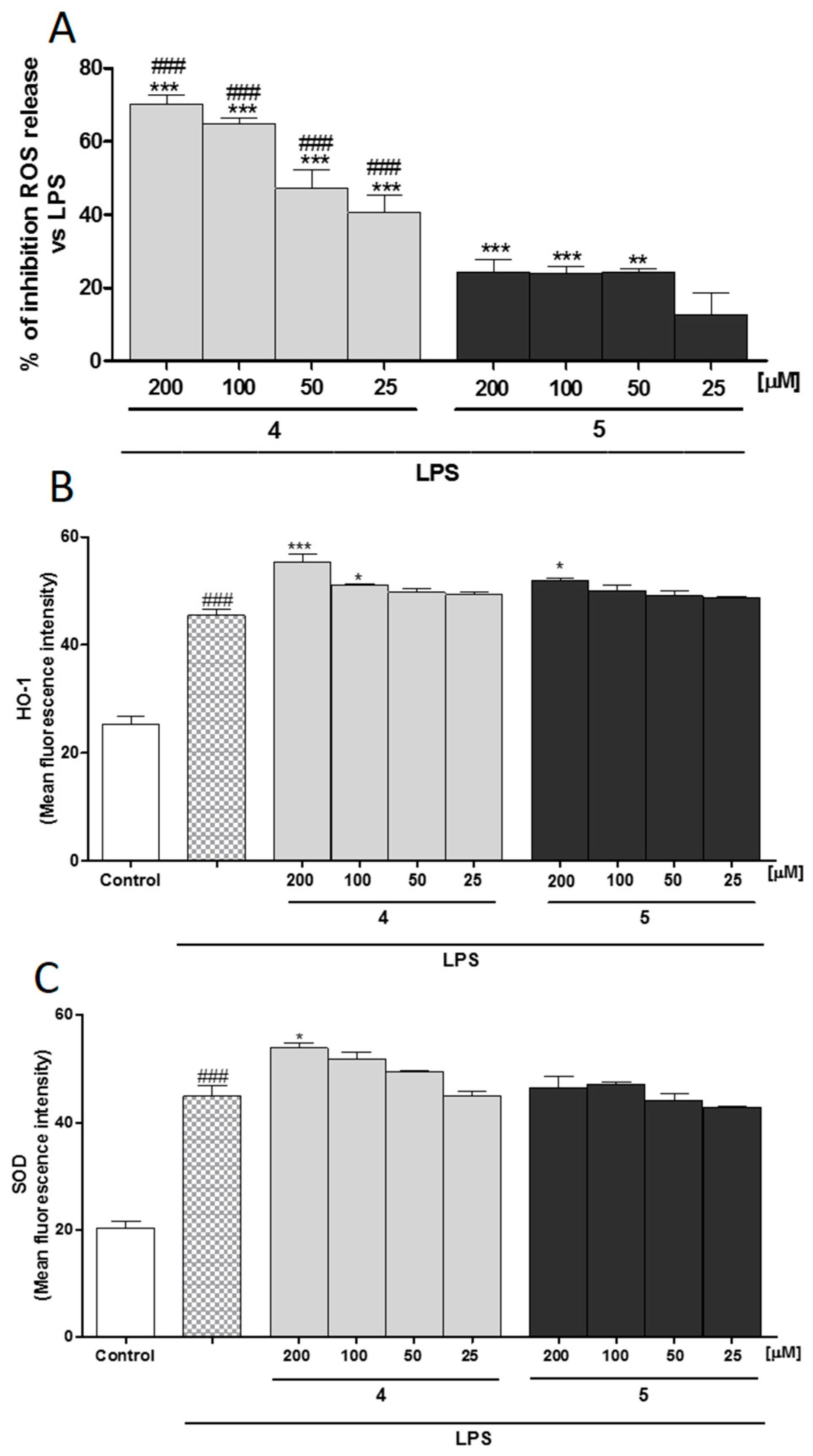
2.3. Compounds 4 and 5 Exert Anti-Inflammatory and Antioxidant Activity
3. Materials and Methods
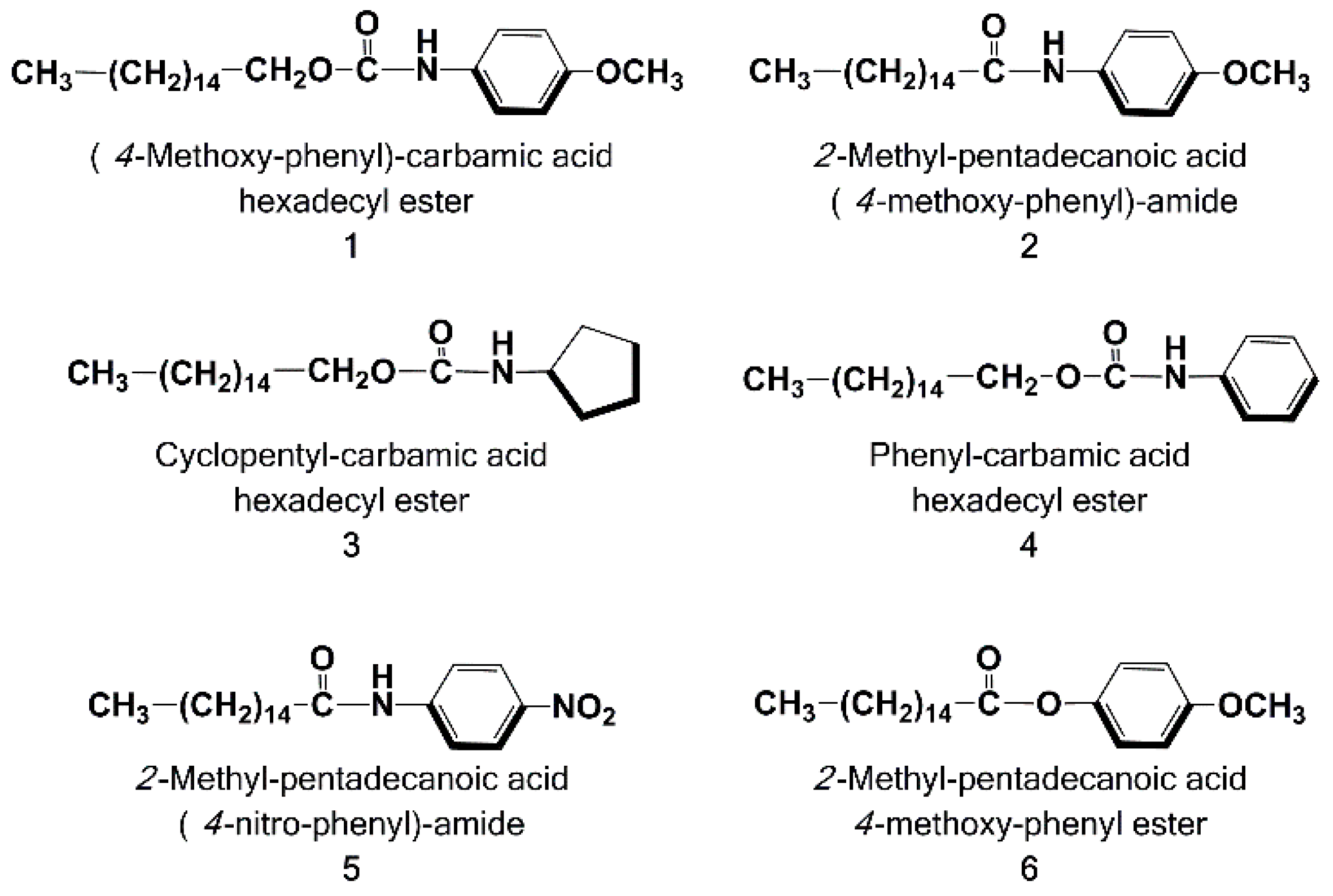
3.1. Chemistry
3.2. Cell Culture
3.3. Viability Assay
3.4. Nitrite Determination
3.5. Measurement of Intracellular ROS
3.6. Measurement of iNOS, COX-2, HO-1, and SOD Expression by Cytofluorimetry
3.7. Data Analysis
3.8. Crystal Structure Determination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bisogno, T.; Cascio, M.G.; Saha, B.; Mahadevan, A.; Urbani, P.; Minassi, A.; Appendino, G.; Saturnino, C.; Martin, B.; Razdan, R.; et al. Development of the first potent and specific inhibitors of endocannabinoid biosynthesis. Mol. Cell Biol. Lipids 2006, 1761, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Darmani, N.A.; Izzo, A.A.; Degenhardt, B.; Valenti, M.; Scaglione, G.; Capasso, R.; Sorrentini, I.; Di Marzo, V. Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: Review of the available pre-clinical data, and first human studies. Neuropharmacology 2005, 48, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Iuvone, T.; di Marzo, V. N-palmitoyl-ethanolamine: Biochemistry and new therapeutic opportunities. Biochimie 2010, 92, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Mattace Raso, G.; Russo, R.; Calignano, A.; Meli, R. Palmitoylethanolamide in CNS health and disease. Pharmacol. Res. 2014, 86, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.M.; Vandevoorde, S.; Jonsson, K.O.; Fowler, C. The palmitoylethanolamide family: A new class of anti-inflammatory agents? Curr. Med. Chem. 2002, 9, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Re, G.; Barbero, R.; Miolo, A.; di Marzo, V. Palmitoylethanolamide, endocannabinoids and related cannabimimetic compounds in protection against tissue inflammation and pain: Potential use in companion animals. Vet. J. 2007, 173, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Palazzo, E.; de Novellis, V.; Bisogno, T.; Rossi, F.S.; Maione, S.; Di Marzo, V. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology 2007, 52, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Autore, G.; Marzocco, S.; Palladino, C.; Saturnino, C.; Sinicropi, M.S.; Spagnuolo, A.; Vivacqua, E.; Capasso, A. New amides of arachidonic-acid as potential antiinflammatory drugs: Preliminary studies. Biomed. Res. 2010, 21, 328–332. [Google Scholar]
- Franklin, A.; Parmentier-Batteur, S.; Walter, L.; Greenberg, D.A.; Stella, N. Palmitoylethanolamide Increases after Focal Cerebral Ischemia and Potentiates Microglial Cell Motility. J. Neurosci. 2003, 23, 7767–7775. [Google Scholar] [PubMed]
- LoVerme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Malignano, A.; Pomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar]
- LoVerme, J.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The Search for the Palmitoylethanolamide Receptor. Life Sci. 2005, 77, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, R.; Wahli, W.; Michalik, L. PPARs in diseases: Control mechanisms of inflammation. Curr. Med. Chem. 2005, 12, 2995–3009. [Google Scholar] [CrossRef] [PubMed]
- Mazzari, S.; Canella, R.; Petrelli, L.; Marcolongo, G.; Leon, A. N-(2-hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur. J. Pharmacol. 1996, 300, 227–236. [Google Scholar] [CrossRef]
- Aloe, L.; Leon, A.; Levi-Montalcini, R. A proposed autacoid mechanism controlling mastocyte behaviour. Agents Act. 1993, 39, C145–C147. [Google Scholar] [CrossRef]
- Conti, S.; Costa, B.; Colleoni, M.; Parolaro, D.; Giagnoni, G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br. J. Pharmacol. 2002, 135, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.H.; Vogel, Z.; Bisogno, T.; de Petrocellis, L.; di Marzo, V.; Mechoulam, R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef]
- Lambert, D.M.; Di Marzo, V. The Palmitoylethanolamide and Oleamide Enigmas: Are These Two Fatty Acid Amides Cannabimimetic? Curr. Med. Chem. 1999, 9, 757–773. [Google Scholar]
- Ho, W.S.; Barrett, D.A.; Randall, M.D. “Entourage” effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. J. Pharmacol. 2008, 155, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Urbani, P.; Cavallo, P.; Cascio, M.G.; Buonerba, M.; de Martino, G.; di Marzo, V.; Saturnino, C. New metabolically stable fatty acid amide ligands of cannabinoid receptors: Synthesis and receptor affinity studies. Bioorg. Med. Chem. Lett. 2006, 16, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Urbani, P.; Cascio, M.G.; Ramunno, A.; Bisogno, T.; Saturnino, C.; di Marzo, V. Novel sterically hindered cannabinoid CB1 receptor ligands. Bioorg. Med. Chem. 2008, 16, 7510–7515. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Lichtman, A.H. The Endogenous Cannabinoid System and Its Role in Nociceptive Behavior. Endocannabinoid Nociception 2004, 61, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Bisogno, T.; De Petrocellis, L.; Melck, D.; Martin, B.R. Cannabimimetic fatty acid derivatives: the anandamide family and other endocannabinoids. Curr. Med. Chem. 1999, 6, 721–744. [Google Scholar] [PubMed]
- Di Marzo, V.; De Petrocellis, L.; Fezza, F.; Ligresti, A.; Bisogno, T. Anandamide receptors. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Chlapanidas, T.; Perteghella, S.; Leoni, F.; Faragò, S.; Marazzi, M.; Rossi, D.; Martino, E.; Gaggeri, R.; Collina, S. TNF-α Blocker Effect of Naringenin-Loaded Sericin Microparticles that Are Potentially Useful in the Treatment of Psoriasis. Int. J. Mol. Sci. 2014, 15, 13624–13636. [Google Scholar] [CrossRef] [PubMed]
- Gaggeri, R.; Rossi, D.; Christodoulou, M.S.; Passarella, D.; Leoni, F.; Azzolina, O.; Collina, S. Chiral Flavanones from Amygdalus lycioides Spach: Structural Elucidation and Identification of TNFalpha Inhibitors by Bioactivity-guided Fractionation. Molecules 2012, 17, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, C.; Palladino, S.C.; De Martino, G.; Bisogno, T.; Di Marzo, V. Synthesis and biological evaluation of new potential inhibitors of N-acylethanolamine hydrolyzing acid amidase. Bioorg. Med. Chem. Lett. 2010, 20, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Cristino, L.; Karsak, M.; Gaffal, E.; Ueda, N.; Tuting, T.; Bisogno, T.; De Filippis, D.; D′Amico, A.; Saturnino, C.; et al. Protective role of palmitoylethanolamide in contact allergic dermatitis. Allergy 2010, 65, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Caruso, A.; Conforti, F.; Marrelli, M.; Kashef, H.E.; Lancelot Rault, J.-C.S.; Statti, G.A.; Menichini, F. Synthesis, inhibition of NO production and antiproliferative activities of some indole derivatives. J. Enzym. Inhib. Med. Chem. 2009, 24, 1148–1153. [Google Scholar]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [PubMed]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Sala, M.; Gomez-Monterey, I.; Musella, S.; Bertamino, A.; Caruso, A.; Sinicropi, M.S.; Sirianni, R.; Puoci, F.; Parisi, O.I.; et al. Biological activity of 3-chloro-azetidin-2-one derivatives having interesting antiproliferative activity on human breast cancer cell lines. Bioorg. Med. Chem. Lett. 2013, 23, 6401–6405. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Smith, D.L.; Zucker, S.D. Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology 2004, 40, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.S.; Wu, S.J.; Tsai, Y.H.; Lin, Y.H.; Chao, J.C. Lycium Barbarum and Rehmannia Glutinosa Inhibit Liver Inflammation and Fibrosis in Rats. Am. J. Chin. Med. 2011, 39, 1173–1191. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Sinicropi, M.S.; Lancelot, J.C.; El-Kashef, H.; Saturnino, C.; Aubert, G.; Belladonne, C.; Lesnard, A.; Cresteil, T.; Dallemagne, P.; et al. Synthesis and evaluation of cytotoxic activities of new guanidines derived from carbazoles. Bioorg. Med. Chem. Lett. 2014, 24, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Sirignano, E.; Saturnino, C.; Botta, A.; Sinicropi, M.S.; Caruso, A.; Lappano, R.; Maggiolini, M.; Longo, P. Synthesis, Characterization and Cytotoxic Activity on Breast Cancer Cells of New Half-Titanocene Derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 3458–3462. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Saturnino, C.; Iacopetta, D.; Mazzotta, R.; Caruso, A.; Plutino, M.R.; Mariconda, A.; Ramunno, A.; Sinicropi, M.S.; Pezzi, V.; et al. Inhibition of human topoisomerase I and II and anti-proliferative effects on MCF-7 cells by new titanocene complexes. Bioorg. Med. Chem. 2015, 23, 7302–7312. [Google Scholar] [CrossRef] [PubMed]
- Autore, G.; Caruso, A.; Marzocco, S.; Nicolaus, B.; Palladino, C.; Pinto, A.; Popolo, A.; Sinicropi, M.S.; Tommonaro, G.; Saturnino, C. Acetamide Derivatives with Antioxidant Activity and Potential Anti-Inflammatory Activity. Molecules 2010, 15, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Fontanella, B.; Severino, L.; Quaroni, A.; Autore, G.; Marzocco, S. Nivalenol and Deoxynivalenol Affect Rat Intestinal Epithelial Cells: A Concentration Related Study. PLoS ONE 2012, 7, e52051. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Pagano, F.; Tenore, G.C.; Marzocco, S.; Manfra, M.; Calabrese, G.; Aquino, R.P.; Campiglia, P. UHPLC profiling and effects on LPS-stimulated J774A.1 macrophages of flavonoids from bergamot (Citrus bergamia) juice, an underestimated waste product with high anti-inflammatory potential. J. Funct. Foods 2014, 7, 641–649. [Google Scholar] [CrossRef]
- Paesano, N.; Marzocco, S.; Vicidomini, C.; Saturnino, C.; Autore, G.; de Martino, G.; Sbardella, G. Synthesis and biological evaluation of 3-benzyl-1-methyl- and 1-methyl-3-phenyl-isothioureas as potential inhibitors of iNOS. Bioorgan. Med. Chem. Lett. 2005, 15, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Sommella, E.; Manfra, M.; de Nisco, M.; Tenore, G.C.; Scopa, A.; Sofo, A.; Marzocco, S.; Adesso, S.; Novellino, T.; et al. Evaluation of anti-inflammatory activity and fast UHPLC–DAD–IT-TOF profiling of polyphenolic compounds extracted from green lettuce (Lactuca sativa L.; var. Maravilla de Verano). Food Chem. 2015, 167, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Pepe, G.; Sommella, E.; Manfra, M.; Scopa, A.; Sofo, A.; Tenore, G.C.; Russo, M.; Di Gaudio, F.; Autore, G.; et al. Anti-inflammatory and antioxidant activity of polyphenolic extracts from Lactuca sativa (var. Maravilla de Verano) under different farming methods. J. Sci. Food Agric. 2016, 12, 4194–4206. [Google Scholar]
- SAINT; Bruker AXS Inc.: Madison, WI, USA, 2002.
- SADABS; Bruker AXS Inc.: Madison, WI, USA, 2002.
- Altomare, A.; Burla, M.C.; Cavalli, M.; Cascarano, G.L.; Giacovazzo, C.; Gagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. Sir97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Burnett, M.N.; Johnson, C.K. ORTEP-III—Report ORNL-6895; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1996.
- Marra, A.; Rossi, D.; Pignataro, L.; Bigogno, C.; Canta, A.; Oggioni, N.; Malacrida, A.; Corbo, M.; Cavaletti, G.; Peviani, M.; et al. Toward the identification of neuroprotective agents: g-scale synthesis, pharmacokinetic evaluation and CNS distribution of (R)-RC-33, a promising SIGMA1 receptor agonist. Future Med. Chem. 2016, 8, 287–295. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–6 are available from the authors. |

| Empirical Formula | C23 H39 N O2 |
|---|---|
| Formula weight | 361.55 |
| Temperature | 293(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Orthorhombic |
| Space group | P b c a |
| Unit cell dimensions | a = 9.6110(10) Å |
| b = 9.7030(10) Å | |
| c = 48.575(6) Å | |
| Volume | 4529.9(9) Å3 |
| Z | 8 |
| Density (calculated) | 1.060 Mg/m3 |
| Absorption coefficient | 0.066 mm−1 |
| F(000) | 1600 |
| Theta range for data collection | 0.838 to 22.490° |
| Index ranges | −10 ≤ h ≤ 10, −10 ≤ k ≤ 10, -51 ≤ l ≤ 52 |
| Reflections collected | 24670 |
| Independent reflections | 2959 [R(int) = 0.0472] |
| Completeness to theta 22° | 99.6% |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2959/0/352 |
| Goodness-of-fit on F2 | 1.130 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0528, wR2 = 0.1370 |
| R indices (all data) | R1 = 0.0848, wR2 = 0.1609 |
| Largest diff. peak and hole | 0.188 and −0.195 e·Å−3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saturnino, C.; Popolo, A.; Ramunno, A.; Adesso, S.; Pecoraro, M.; Plutino, M.R.; Rizzato, S.; Albinati, A.; Marzocco, S.; Sala, M.; et al. Anti-Inflammatory, Antioxidant and Crystallographic Studies of N-Palmitoyl-ethanol Amine (PEA) Derivatives. Molecules 2017, 22, 616. https://doi.org/10.3390/molecules22040616
Saturnino C, Popolo A, Ramunno A, Adesso S, Pecoraro M, Plutino MR, Rizzato S, Albinati A, Marzocco S, Sala M, et al. Anti-Inflammatory, Antioxidant and Crystallographic Studies of N-Palmitoyl-ethanol Amine (PEA) Derivatives. Molecules. 2017; 22(4):616. https://doi.org/10.3390/molecules22040616
Chicago/Turabian StyleSaturnino, Carmela, Ada Popolo, Anna Ramunno, Simona Adesso, Michela Pecoraro, Maria Rosaria Plutino, Silvia Rizzato, Alberto Albinati, Stefania Marzocco, Marina Sala, and et al. 2017. "Anti-Inflammatory, Antioxidant and Crystallographic Studies of N-Palmitoyl-ethanol Amine (PEA) Derivatives" Molecules 22, no. 4: 616. https://doi.org/10.3390/molecules22040616