2D-QSAR and 3D-QSAR/CoMSIA Studies on a Series of (R)-2-((2-(1H-Indol-2-yl)ethyl)amino)-1-Phenylethan-1-ol with Human β3-Adrenergic Activity
Abstract
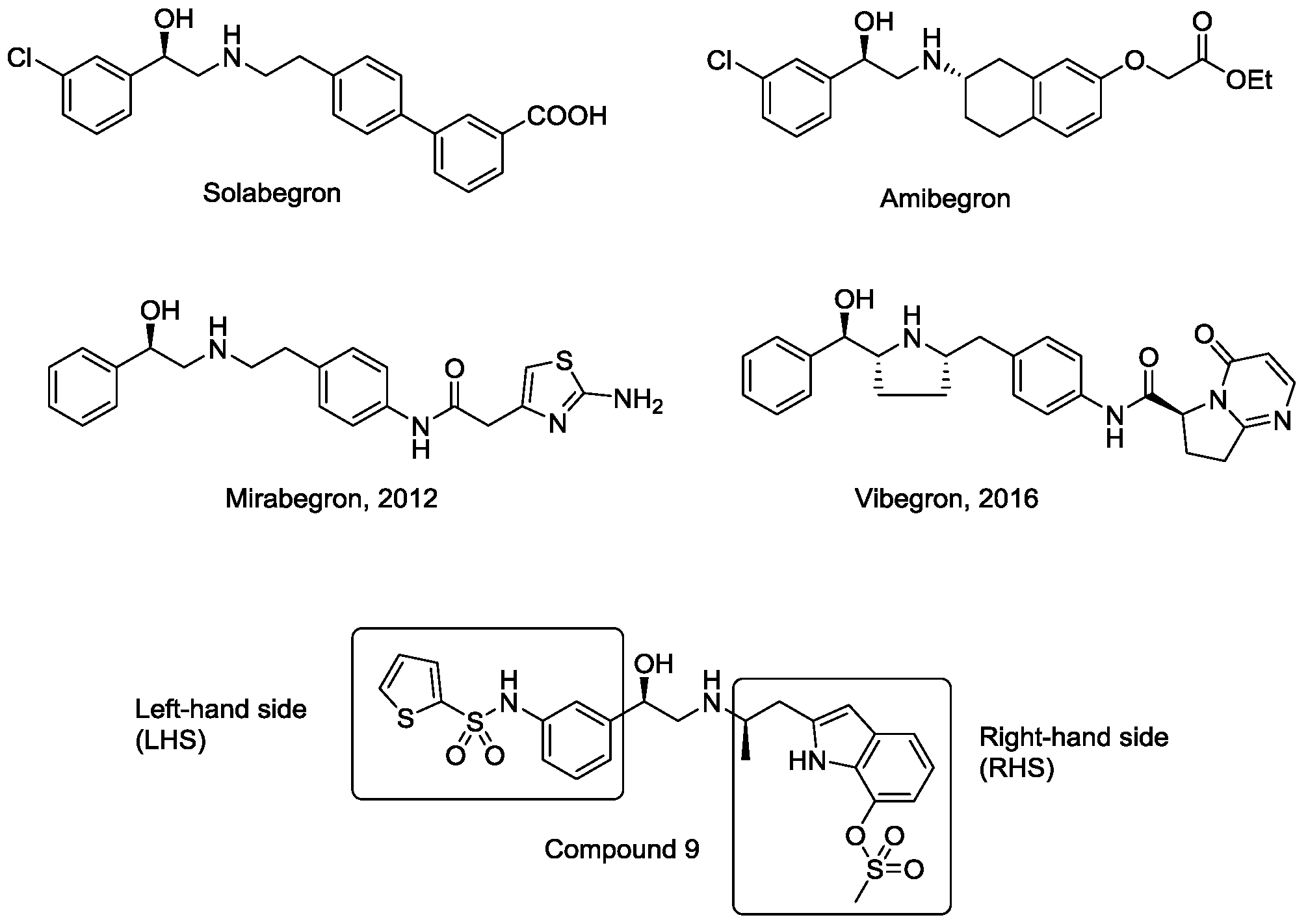
:1. Introduction
2. Results and Discussion
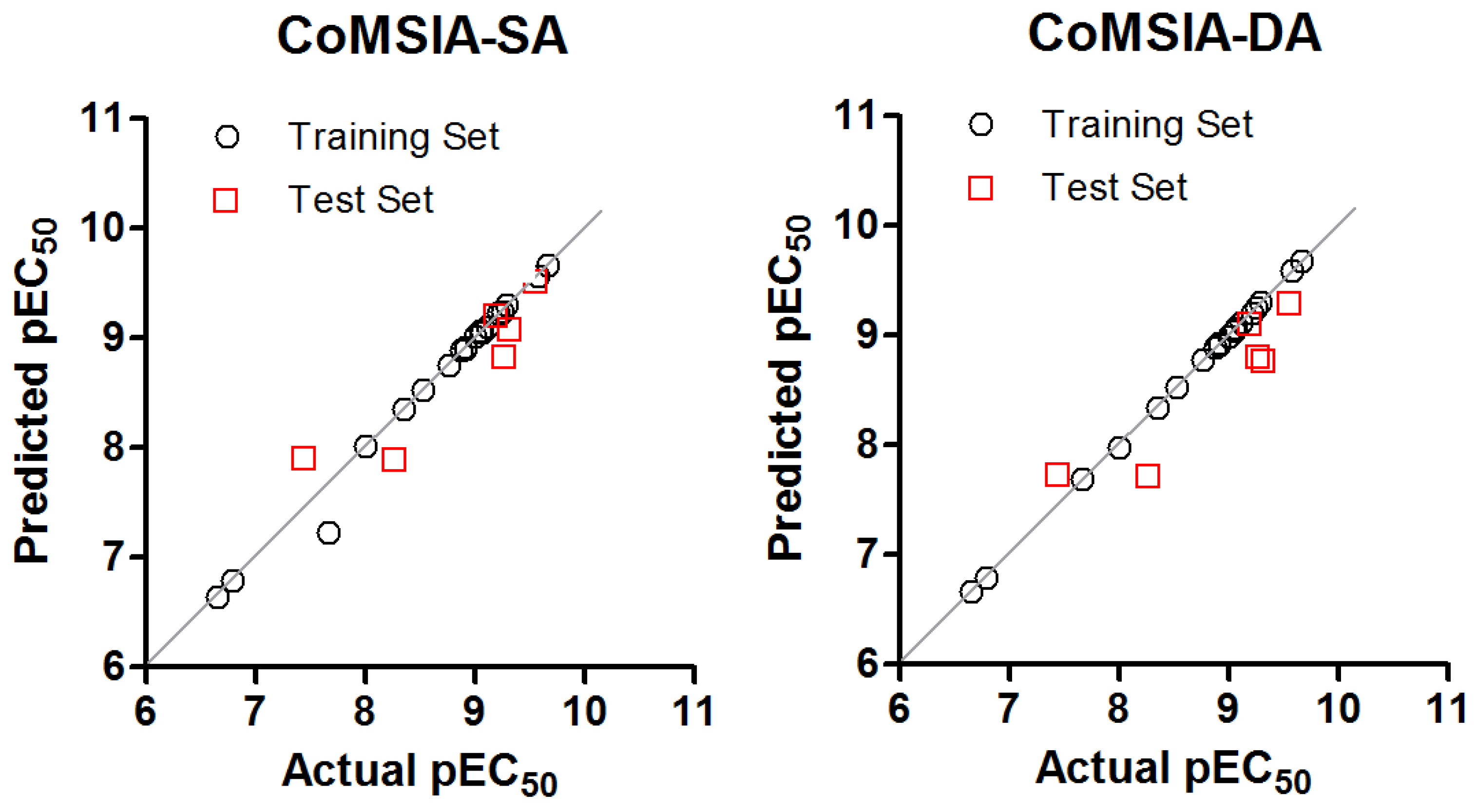
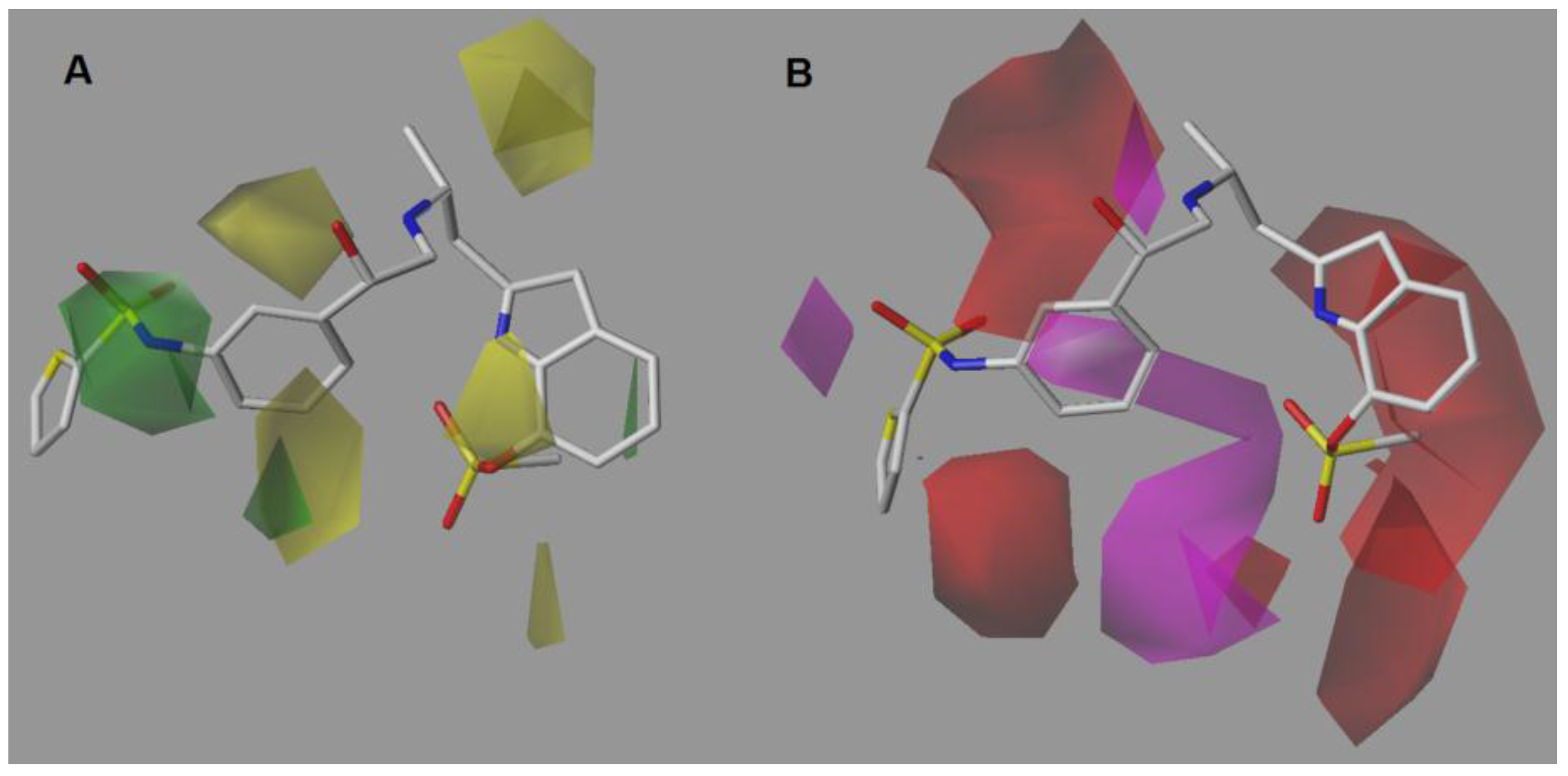
2.1. CoMSIA-SA (Model No.15)
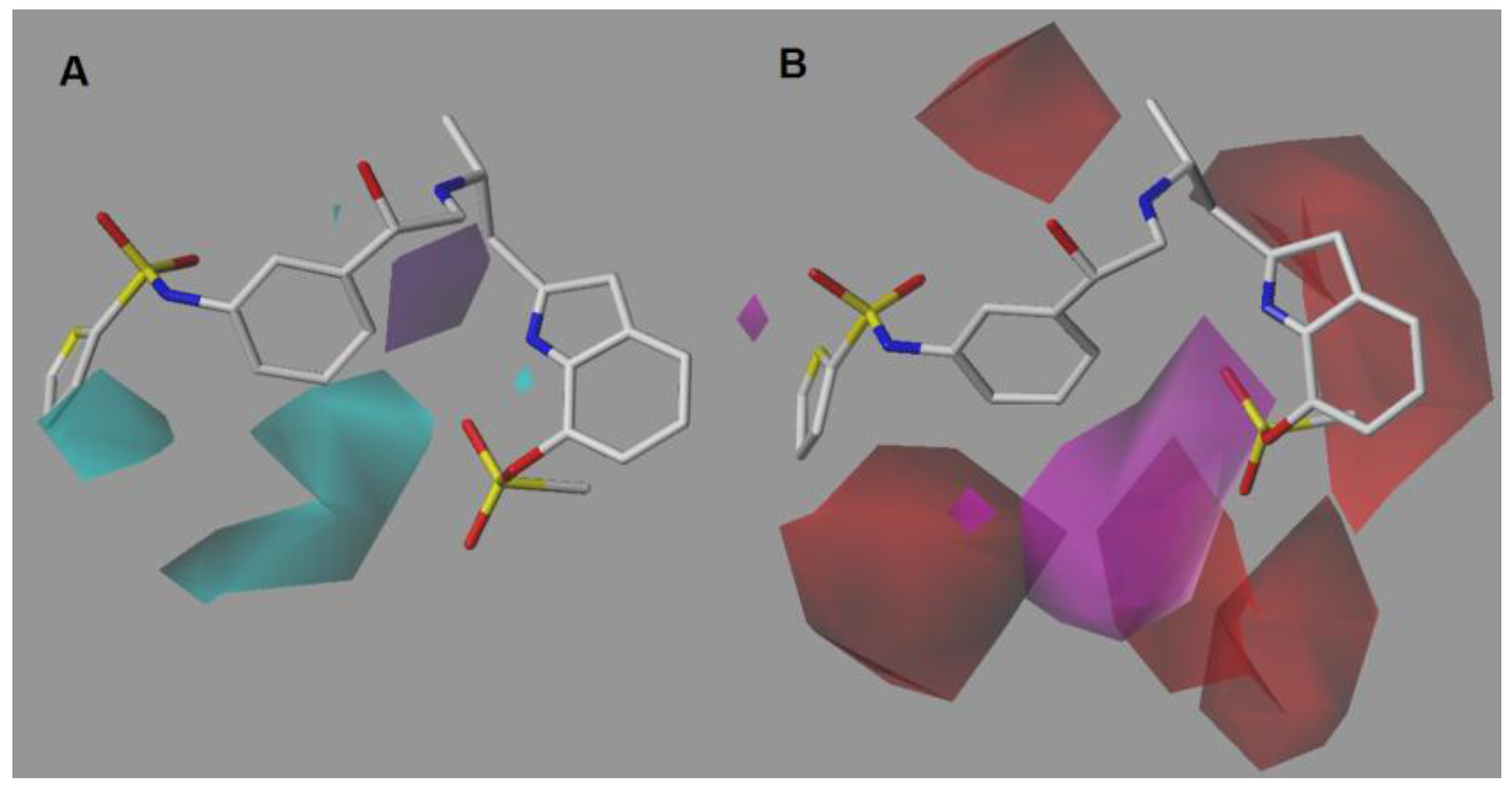
2.2. CoMSIA-DA (Model N°30)
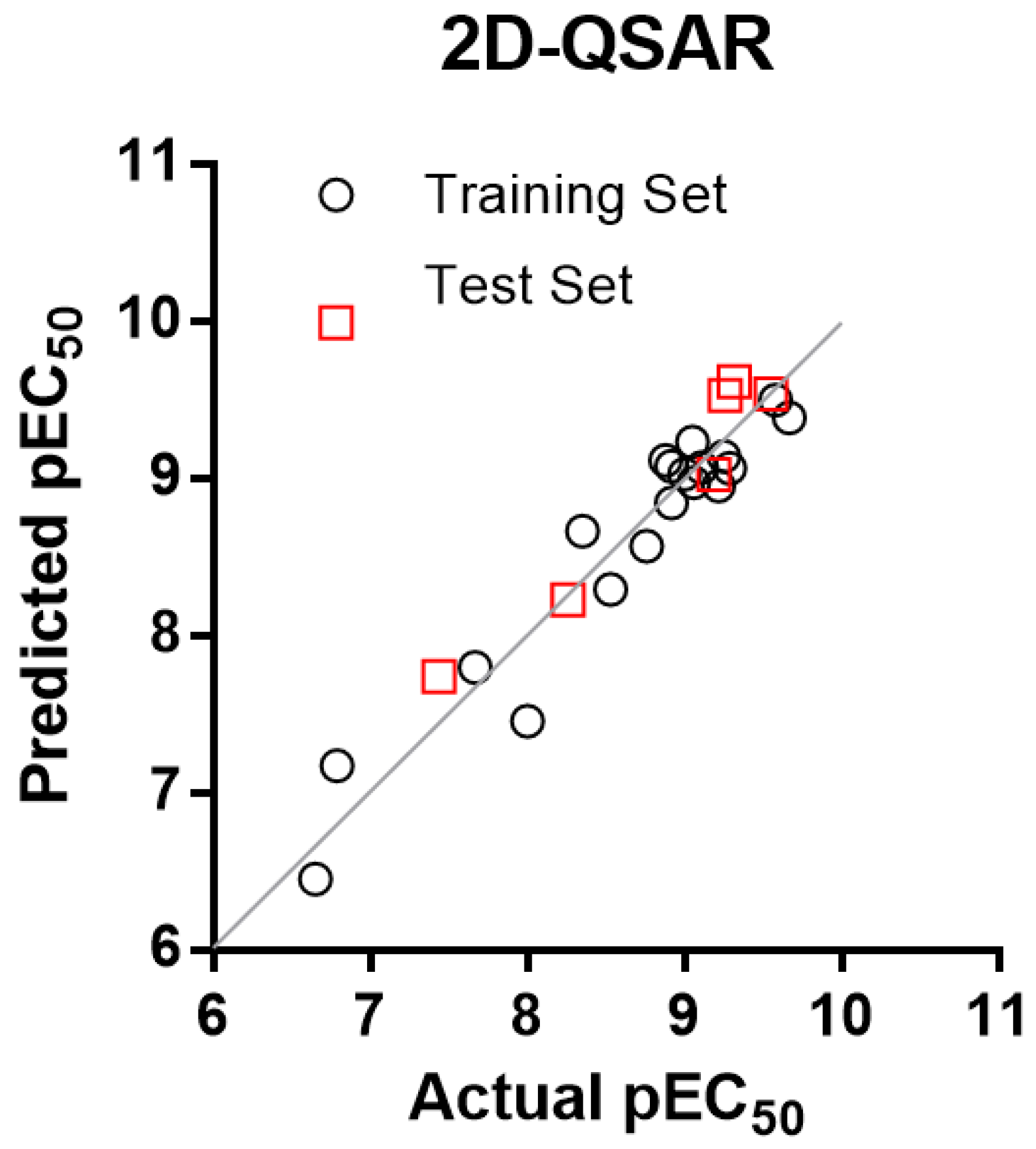
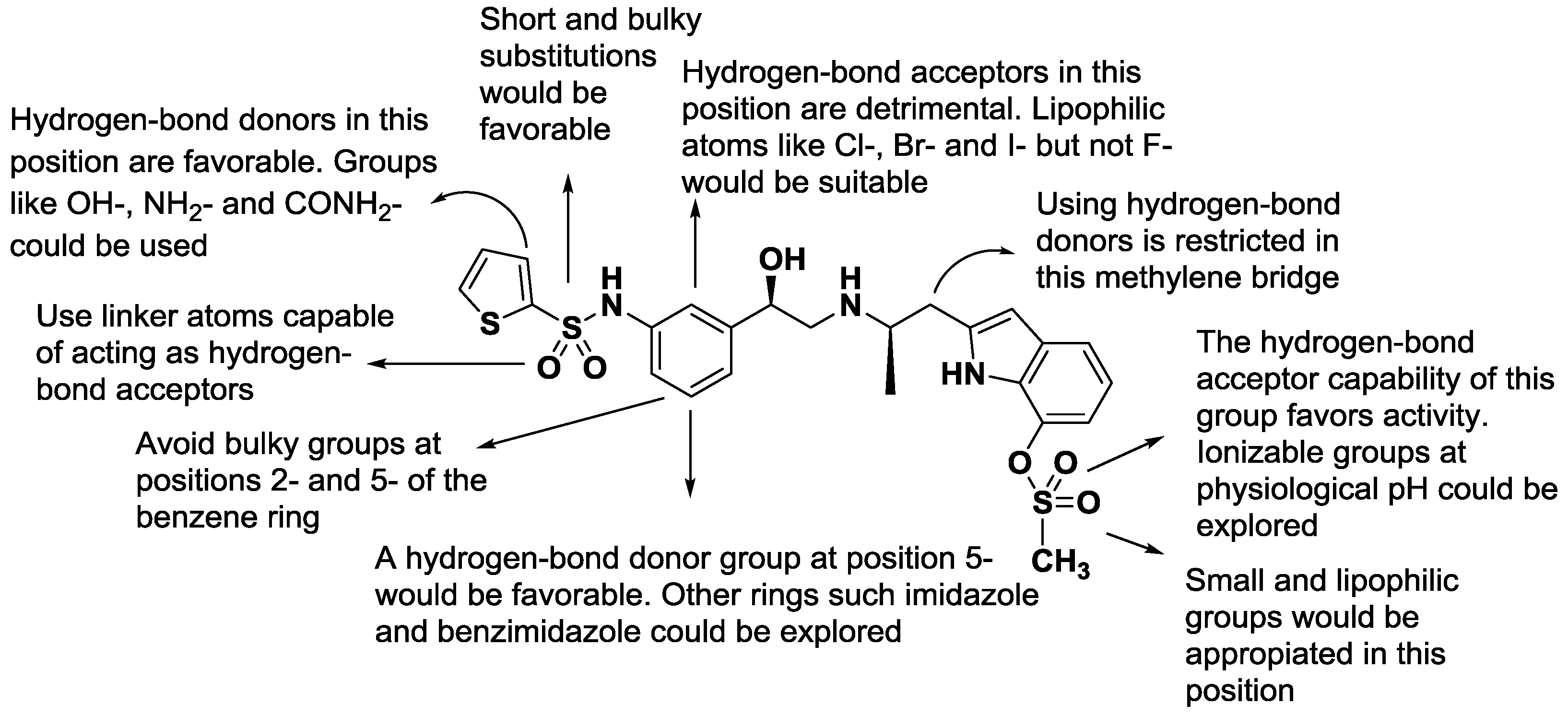
2.3. 2D-QSAR Model
3. Materials and Methods
3.1. Data Set Selection and β3 Adrenergic Activity
3.2. Parameter Calculations and Statistical Analysis
3.3. Selection of Conformers and Molecular Alignment
3.4. CoMSIA Field Calculation
3.5. Internal Validation and Partial Least Squares (PLS) Analysis
3.6. External Validation of the CoMSIA Model
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lands, A.M.; Arnold, A.; McAuliff, J.P.; Luduena, F.P.; Brown, T.G., Jr. Differentiation of receptor systems activated by sympathomimetic amines. Nature 1967, 214, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Frielle, T.; Collins, S.; Daniel, K.W.; Caron, M.G.; Lefkowitz, R.J.; Kobilka, B.K. Cloning of the cDNA for the human β1-adrenergic receptor. Proc. Natl. Acad. Sci. 1987, 84, 7920–7924. [Google Scholar] [CrossRef] [PubMed]
- Kobilka, B.K.; Dixon, R.A.; Frielle, T.; Dohlman, H.G.; Bolanowski, M.A.; Sigal, I.S.; Yang-Feng, T.L.; Francke, U.; Caron, M.G.; Lefkowitz, R.J. cDNA for the human β2-adrenergic receptor: A protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc. Natl. Acad. Sci. 1987, 84, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Arch, J.R. The brown adipocyte beta-adrenoceptor. Proc. Nutr. Soc. 1989, 48, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Emorine, L.J.; Marullo, S.; Briend-Sutren, M.M.; Patey, G.; Tate, K.; Delavier-Klutchko, C.; Strosberg, A.D. Molecular characterization of the human β3-adrenergic receptor. Science 1989, 245, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Arch, J.R.; Ainsworth, A.T.; Cawthorne, M.A.; Piercy, V.; Sennitt, M.V.; Thody, V.E.; Wilson, C.; Wilson, S. Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature 1984, 309, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Bardou, M.; Loustalot, C.; Cortijo, J.; Simon, B.; Naline, E.; Dumas, M.; Esteve, S.; Croci, T.; Chalon, P.; Frydman, R.; et al. Functional, biochemical and molecular biological evidence for a possible β3-adrenoceptor in human near-term myometrium. Br. J. Pharmacol. 2000, 130, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Igawa, Y.; Yamazaki, Y.; Takeda, H.; Hayakawa, K.; Akahane, M.; Ajisawa, Y.; Yoneyama, T.; Nishizawa, O.; Andersson, K.E. Functional and molecular biological evidence for a possible β3-adrenoceptor in the human detrusor muscle. Br. J. Pharmacol. 1999, 126, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Obara, K.; Mizusawa, T.; Tomita, Y.; Arai, K.; Tsutsui, T.; Hatano, A.; Takahashi, K.; Nomura, S. Evidence for β3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: Analysis by molecular biological and pharmacological methods. J. Pharmacol. Exp. Ther. 1999, 288, 1367–1373. [Google Scholar] [PubMed]
- Michel, M.C.; Vrydag, W. α1-, α2- and β-adrenoceptors in the urinary bladder, urethra and prostate. Br. J. Pharmacol. 2006, 147, S88–S119. [Google Scholar] [CrossRef] [PubMed]
- De Ponti, F.; Gibelli, G.; Croci, T.; Arcidiaco, M.; Crema, F.; Manara, L. Functional evidence of atypical β3-adrenoceptors in the human colon using the β3-selective adrenoceptor antagonist, SR 59230A. Br. J. Pharmacol. 1996, 117, 1374–1376. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Omu, A.E.; Fatinikun, T.; Chandrasekhar, B.; Kadavil, E.A.; Oriowo, M.A. Evidence for the presence of beta-3-adrenoceptors mediating relaxation in the human oviduct. Pharmacology 2005, 74, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Anthony, A.; Sim, R.; Guillaume, J.L.; Strosberg, A.D.; Dhillon, A.P.; Pounder, R.E.; Wakefield, A.J. Beta(β)3-adrenergic receptors in human pancreatic islet and duodenal somatostatin neuroendocrine cells. Aliment. Pharmacol. Ther. 2000, 14, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Carillon, C.; Coquerel, A.; Le Fur, G.; Ferrara, P.; Caput, D.; Shire, D. Evidence for the presence of β3-adrenergic receptor mRNA in the human brain. Brain. Res. Mol. Brain. Res. 1995, 29, 369–375. [Google Scholar] [CrossRef]
- Grazia Perrone, M.; Scilimati, A. β3-Adrenoceptor agonists and (antagonists as) inverse agonists history, perspective, constitutive activity, and stereospecific binding. Methods Enzymol. 2010, 484, 197–230. [Google Scholar] [PubMed]
- Yoshida, T.; Sakane, N.; Wakabayashi, Y.; Umekawa, T.; Kondo, M. Anti-obesity and anti-diabetic effects of CL 316,243, a highly specific β3-adrenoceptor agonist, in yellow KK mice. Life Sci. 1994, 54, 491–498. [Google Scholar] [CrossRef]
- Ursino, M.G.; Vasina, V.; Raschi, E.; Crema, F.; De Ponti, F. The β3-adrenoceptor as a therapeutic target: Current perspectives. Pharmacol. Res. 2009, 59, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Cawthorne, M.A.; Stock, M.J. Biphasic effects of the beta-adrenoceptor agonist, BRL 37344, on glucose utilization in rat isolated skeletal muscle. Br. J. Pharmacol. 1996, 117, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Simiand, J.; Keane, P.E.; Guitard, J.; Langlois, X.; Gonalons, N.; Martin, P.; Bianchetti, A.; Le Fur, G.; Soubrie, P. Antidepressant profile in rodents of SR 58611A, a new selective agonist for atypical beta-adrenoceptors. Eur. J. Pharmacol. 1992, 219, 193–201. [Google Scholar] [CrossRef]
- Stemmelin, J.; Cohen, C.; Yalcin, I.; Keane, P.; Griebel, G. Implication of β3-adrenoceptors in the antidepressant-like effects of amibegron using Adrb3 knockout mice in the chronic mild stress. Behav. Brain. Res. 2010, 206, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.V.; Lozinsky, I.T. Is the β3-adrenergic receptor a new target for treatment of post-infarct ventricular tachyarrhythmias and prevention of sudden cardiac death? Heart Rhythm 2008, 5, 298–299. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.T.; Li, W.M.; Xiu, C.H.; Shen, J.X.; Wang, X.; Wu, S.; Kong, Y.H. Chronic blocking of β3-adrenoceptor ameliorates cardiac function in rat model of heart failure. Chin. Med. J. 2007, 120, 2250–2255. [Google Scholar] [PubMed]
- Bianchetti, A.; Manara, L. In vitro inhibition of intestinal motility by phenylethanolaminotetralines: Evidence of atypical beta-adrenoceptors in rat colon. Br. J. Pharmacol. 1990, 100, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Sacco, E.; Bientinesi, R. Mirabegron: A review of recent data and its prospects in the management of overactive bladder. Ther. Adv. Urol. 2012, 4, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Kar, N.F.; Li, B.; Costa, M.; Dingley, K.H.; Di Salvo, J.; Ha, S.N.; Hurley, A.L.; Li, X.; Miller, R.R.; et al. Discovery of benzamides as potent human β3 adrenergic receptor agonists. Bioorg. Med. Chem. Lett. 2016, 26, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Shirahashi, H.; Iwanami, T.; Ogawa, M.; Nakano, S.; Morimoto, A.; Kasahara, K.; Tanaka, E.; Takada, Y.; Ohashi, S.; et al. Discovery of novel indazole derivatives as highly potent and selective human β3-adrenergic receptor agonists with the possibility of having no cardiovascular side effects. J. Med. Chem. 2015, 58, 6048–6057. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, S.D.; Zhu, C.; Kar, N.F.; Di Salvo, J.; Nagabukuro, H.; Sacre-Salem, B.; Dingley, K.; Berger, R.; Goble, S.D.; Morriello, G.; et al. Discovery of vibegron: A potent and selective β3 adrenergic receptor agonist for the treatment of overactive bladder. J. Med. Chem. 2016, 59, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Bharatam, P.V. CoMFA study on selective human β3-adrenoceptor agonists. ARKIVOC 2005, 13, 67–79. [Google Scholar]
- Kashaw, S.K.; Rathi, L.; Mishra, P.; Saxena, A.K. Development of 3D-QSAR models in cyclic ureidobenzenesulfonamides: Human β3-adrenergic receptor agonist. Bioorg. Med. Chem. Lett. 2003, 13, 2481–2484. [Google Scholar] [CrossRef]
- Telvekar, V.N.; Patel, D.J.; Jadhav, N.C.; Mishra, S.J. Three-dimensional QSAR and pharmacophore mapping of biphenyl benzoic acid derivatives as selective human β3-adrenergic receptor agonists. Med. Chem. Res. 2010, 19, 1174–1190. [Google Scholar] [CrossRef]
- Mella-Raipan, J.; Hernandez-Pino, S.; Morales-Verdejo, C.; Pessoa-Mahana, D. 3D-QSAR/CoMFA-based structure-affinity/selectivity relationships of aminoalkylindoles in the cannabinoid CB1 and CB2 receptors. Molecules 2014, 19, 2842–2861. [Google Scholar] [CrossRef] [PubMed]
- Mella-Raipan, J.A.; Lagos, C.F.; Recabarren-Gajardo, G.; Espinosa-Bustos, C.; Romero-Parra, J.; Pessoa-Mahana, H.; Iturriaga-Vasquez, P.; Pessoa-Mahana, C.D. Design, synthesis, binding and docking-based 3D-QSAR studies of 2-pyridylbenzimidazoles—A new family of high affinity CB1 cannabinoid ligands. Molecules 2013, 18, 3972–4001. [Google Scholar] [CrossRef] [PubMed]
- Romero-Parra, J.; Mella-Raipán, J.; Palmieri, V.; Allarà, M.; Torres, M.J.; Pessoa-Mahana, H.; Iturriaga-Vásquez, P.; Escobar, R.; Faúndez, M.; Marzo, V.D.; et al. Synthesis, binding assays, cytotoxic activity and docking studies of benzimidazole and benzothiophene derivatives with selective affinity for the CB2 cannabinoid receptor. Eur. J. Med. Chem. 2016, 124, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Klebe, G.; Abraham, U.; Mietzner, T. Molecular similarity indices in a comparative analysis (CoMSIA) of drug molecules to correlate and predict their biological activity. J. Med. Chem. 1994, 37, 4130–4146. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Hirokawa, Y.; Suzuki, K.; Hiyama, Y.; Oue, M.; Kawashima, H.; Yoshida, N.; Furutani, Y.; Kato, S. Novel and potent human and rat β3-adrenergic receptor agonists containing substituted3-indolylalkylamines. Bioorg. Med. Chem. Lett. 2003, 13, 1301–1305. [Google Scholar] [CrossRef]
- Mizuno, K.; Sawa, M.; Harada, H.; Tateishi, H.; Oue, M.; Tsujiuchi, H.; Furutani, Y.; Kato, S. Tryptamine-based human β3-adrenergic receptor agonists. Part 1: SAR studies of the 7-position of the indole ring. Bioorg. Med. Chem. Lett. 2004, 14, 5959–5962. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Sawa, M.; Harada, H.; Taoka, I.; Yamashita, H.; Oue, M.; Tsujiuchi, H.; Arai, Y.; Suzuki, S.; Furutani, Y.; Kato, S. Discovery of 1,7-cyclized indoles as a new class of potent and highly selective human β3-adrenergic receptor agonists with high cell permeability. Bioorg. Med. Chem. 2005, 13, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Sawa, M.; Tateishi, H.; Mizuno, K.; Harada, H.; Oue, M.; Tsujiuchi, H.; Furutani, Y.; Kato, S. Tryptamine-based human β3-adrenergic receptor agonists. Part 2: SAR of the methylene derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 5963–5966. [Google Scholar] [CrossRef] [PubMed]
- Sawa, M.; Mizuno, K.; Harada, H.; Tateishi, H.; Arai, Y.; Suzuki, S.; Oue, M.; Tsujiuchi, H.; Furutani, Y.; Kato, S. Tryptamine-based human β3-adrenergic receptor agonists. Part 3: Improved oral bioavailability via modification of the sulfonamide moiety. Bioorg. Med. Chem. Lett. 2005, 15, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D.M. The problem of overfitting. J. Chem. Inf. Comput. Sci. 2004, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Topliss, J.G.; Edwards, R.P. Chance factors in studies of quantitative structure-activity relationships. J. Med. Chem. 1979, 22, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.I.; Waller, C.L.; Marshall, G.R. Three-dimensional quantitative structure-activity relationship of human immunodeficiency virus (I) protease inhibitors. 2. Predictive power using limited exploration of alternate binding modes. J. Med. Chem. 1994, 37, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- SYBYL-X 1.2. Tripos International: St. Louis, MI, USA, 2011.
- Vinter, J.G.; Davis, A.; Saunders, M.R. Strategic approaches to drug design. I. An integrated software framework for molecular modelling. J. Comput. Aided Mol. Des. 1987, 1, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity—A rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Clark, M.; Cramer, R.D.; Van Opdenbosch, N. Validation of the general purpose Tripos 5.2 force field. J. Comput. Chem. 1989, 10, 982–1012. [Google Scholar] [CrossRef]
- Waller, C.L.; Oprea, T.I.; Giolitti, A.; Marshall, G.R. Three-dimensional QSAR of human immunodeficiency virus (I) protease inhibitors. 1. A CoMFA study employing experimentally-determined alignment rules. J. Med. Chem. 1993, 36, 4152–4160. [Google Scholar] [CrossRef] [PubMed]
- Golbraikh, A.; Tropsha, A. Predictive QSAR modeling based on diversity sampling of experimental datasets for the training and test set selection. J. Comput. Aided Mol. Des. 2002, 16, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
| Model No. | Model | q2 | N | SEP | SEE | r2 | F | Field Contributions | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | E | H | D | A | ||||||||
| 1 | CoMSIA-S | 0.393 | 1 | 0.668 | 0.432 | 0.746 | 47.005 | 1 | ||||
| 2 | CoMSIA-E | 0.414 | 9 | 0.928 | 0.004 | 1.000 | 75,589.127 | 1 | ||||
| 3 | CoMSIA-H | 0.493 | 9 | 0.863 | 0.007 | 1.000 | 26,900.499 | 1 | ||||
| 4 | CoMSIA-D | 0.251 | 8 | 0.989 | 0.02 | 1.000 | 3582.73 | 1 | ||||
| 5 | CoMSIA-A | 0.493 | 3 | 0.652 | 0.288 | 0.901 | 42.56 | 1 | ||||
| 6 | CoMSIA-SE | 0.443 | 7 | 0.809 | 0.004 | 1.000 | 83,581.585 | 0.335 | 0.665 | |||
| 7 | CoMSIA-SEH | 0.439 | 2 | 0..663 | 0.203 | 0.948 | 135.576 | 0.193 | 0.432 | 0.375 | ||
| 8 | CoMSIA-SEHD | 0.437 | 10 | 0.973 | 0.000 | 1.000 | 5.92 × 106 | 0.143 | 0.308 | 0.251 | 0.298 | |
| 9 | CoMSIA-SEHA | 0.530 | 2 | 0.607 | 0.190 | 0.954 | 155.022 | 0.135 | 0.285 | 0.258 | 0.322 | |
| 10 | CoMSIA-SED | 0.448 | 4 | 0.707 | 0.037 | 0.998 | 2115.698 | 0.193 | 0.412 | 0.394 | ||
| 11 | CoMSIA-SEA | 0.552 | 2 | 0.593 | 0.209 | 0.944 | 126.755 | 0.185 | 0.38 | 0.434 | ||
| 12 | CoMSIA-SEDA | 0.581 | 7 | 0.702 | 0.007 | 1.000 | 33,768.991 | 0.129 | 0.28 | 0.296 | 0.294 | |
| 13 | CoMSIA-SH | 0.504 | 5 | 0.697 | 0.067 | 0.995 | 523.658 | 0.349 | 0.651 | |||
| 14 | CoMSIA-SD | 0.356 | 10 | 1.04 | 0.005 | 1.000 | 56,928.168 | 0.342 | 0.658 | |||
| 15 | CoMSIA-SA | 0.639 | 7 | 0.732 | 0.014 | 0.989 | 6334.553 | 0.387 | 0.613 | |||
| 16 | CoMSIA-SHD | 0.434 | 5 | 0.745 | 0.056 | 0.997 | 755.261 | 0.211 | 0.378 | 0.411 | ||
| 17 | CoMSIA-SHA | 0.579 | 7 | 0.703 | 0.021 | 1.000 | 3754.265 | 0.217 | 0.385 | 0.398 | ||
| 18 | CoMSIA-SDA | 0.599 | 10 | 0.821 | 0.001 | 1.000 | 823,177.397 | 0.185 | 0.397 | 0.418 | ||
| 19 | CoMSIA-SHDA | 0.594 | 6 | 0.659 | 0.021 | 1.000 | 4613.236 | 0.137 | 0.251 | 0.3 | 0.312 | |
| 20 | CoMSIA-EH | 0.421 | 2 | 0.674 | 0.216 | 0.941 | 118.677 | 0.533 | 0.467 | |||
| 21 | CoMSIA-ED | 0.404 | 4 | 0.734 | 0.072 | 0.994 | 556.137 | 0.516 | 0.484 | |||
| 22 | CoMSIA-EA | 0.517 | 2 | 0.615 | 0.24 | 0.926 | 94.322 | 0.46 | 0.54 | |||
| 23 | CoMSIA-EHD | 0.424 | 2 | 0.672 | 0.198 | 0.950 | 143.245 | 0.352 | 0.307 | 0.341 | ||
| 24 | CoMSIA-EHA | 0.512 | 2 | 0.619 | 0.205 | 0.947 | 132.798 | 0.328 | 0.299 | 0.373 | ||
| 25 | CoMSIA-EDA | 0.586 | 7 | 0.698 | 0.011 | 1.000 | 13,051.808 | 0.321 | 0.339 | 0.34 | ||
| 26 | CoMSIA-EHDA | 0.557 | 9 | 0.807 | 0.001 | 1.000 | 1.71 × 106 | 0.248 | 0.214 | 0.272 | 0.266 | |
| 27 | CoMSIA-HD | 0.408 | 2 | 0.681 | 0.248 | 0.921 | 87.997 | 0.487 | 0.513 | |||
| 28 | CoMSIA-HA | 0.514 | 2 | 0.618 | 0.239 | 0.927 | 95.784 | 0.45 | 0.55 | |||
| 29 | CoMSIA-HDA | 0.596 | 5 | 0.63 | 0.051 | 0.997 | 907.891 | 0.289 | 0.35 | 0.361 | ||
| 30 | CoMSIA-DA | 0.626 | 8 | 0.813 | 0.003 | 1.000 | 154,958.201 | 0.492 | 0.508 | |||
| 31 | CoMSIA-ALL | 0.557 | 8 | 0.761 | 0.001 | 1.000 | 1.44 × 106 | 0.103 | 0.225 | 0.192 | 0.242 | 0.239 |
| Mol. | Actual pEC50 (M) | CoMSIA-SA | CoMSIA-DA | 2D-QSAR | |||
|---|---|---|---|---|---|---|---|
| Predicted pE50 (M) | Residual | Predicted pE50 (M) | Residual | Predicted pE50 (M) | Residual | ||
| 1 t | 8.260 | 7.890 | 0.37 | 7.607 | 0.65 | 8.229 | 0.03 |
| 2 | 6.650 | 6.637 | 0.01 | 6.664 | 0.01 | 6.453 | 0.20 |
| 3 t | 7.670 | 7.229 | 0.44 | 7.686 | −0.02 | 7.798 | −0.13 |
| 4 | 9.051 | 9.061 | −0.01 | 9.041 | −0.01 | 9.232 | −0.18 |
| 5 | 9.252 | 9.241 | 0.01 | 9.254 | 0.00 | 9.141 | 0.11 |
| 6 | 9.108 | 9.107 | 0.00 | 9.117 | 0.01 | 9.068 | 0.04 |
| 7 | 8.879 | 8.887 | −0.01 | 8.885 | 0.01 | 9.114 | −0.23 |
| 8 | 8.759 | 8.757 | 0.00 | 8.773 | 0.01 | 8.567 | 0.19 |
| 9 | 9.678 | 9.668 | 0.00 | 9.673 | 0.00 | 9.385 | 0.29 |
| 10 | 9.222 | 9.225 | −0.01 | 9.207 | −0.01 | 8.951 | 0.27 |
| 11 t | 9.553 | 9.519 | 0.03 | 9.286 | 0.26 | 9.535 | 0.01 |
| 12 | 9.292 | 9.296 | −0.01 | 9.296 | 0.01 | 9.064 | 0.23 |
| 13 | 9.060 | 9.058 | 0.00 | 9.065 | 0.01 | 8.973 | 0.09 |
| 14 | 9.585 | 9.568 | 0.01 | 9.593 | 0.01 | 9.499 | 0.08 |
| 15 t | 9.187 | 9.198 | −0.01 | 9.101 | 0.09 | 9.023 | 0.17 |
| 16 | 8.921 | 8.917 | 0.00 | 8.913 | −0.01 | 9.077 | −0.16 |
| 17 t | 9.319 | 9.080 | 0.24 | 9.013 | 0.31 | 9.613 | −0.29 |
| 18 t | 9.260 | 8.830 | 0.43 | 9.101 | 0.16 | 9.527 | −0.27 |
| 19 | 6.790 | 6.790 | 0.00 | 6.790 | 0.00 | 7.173 | −0.38 |
| 20 | 8.921 | 8.899 | 0.02 | 8.931 | 0.01 | 8.842 | 0.08 |
| 21 | 7.440 | 7.900 | −0.46 | 7.603 | −0.16 | 7.740 | −0.30 |
| 22 | 8.000 | 8.019 | −0.02 | 7.975 | −0.03 | 7.455 | 0.54 |
| 23 | 8.530 | 8.532 | 0.00 | 8.532 | 0.00 | 8.295 | 0.23 |
| 24 | 8.350 | 8.356 | −0.01 | 8.341 | −0.01 | 8.666 | −0.32 |
| 25 | 9.000 | 9.010 | −0.01 | 8.989 | −0.01 | 9.026 | −0.03 |
| Entry | Structure | EC50 (nM) β1 (β1/β3) β2 (β2/β3) β3 | pEC50 β3 (M) |
|---|---|---|---|
| 1 |  | 1.9 (0.4) 25 (4.6) 5.50 | 8.260 |
| 2 |  | 47 (0.2) 330 (1.5) 223 | 6.650 |
| 3 |  | 1700 (79.5) 290 (13.6) 21.38 | 7.670 |
| 4 |  | 21 (23.6) 66 (74.2) 0.89 | 9.051 |
| 5 |  | 6.6 (11.8) 29 (51.8) 0.56 | 9.252 |
| 6 |  | 6.6 (8.5) 54 (69.2) 0.78 | 9.108 |
| 7 |  | 6.8 (5.2) 19 (14.4) 1.32 | 8.879 |
| 8 |  | 19 (10.9) 180 (103.4) 1.74 | 8.759 |
| 9 |  | 18 (85.7) 44 (19.1) 0.21 | 9.678 |
| 10 |  | 7.3 (12.2) 26 (43.3) 0.60 | 9.222 |
| 11 |  | 5.6 (20) 20 (71.4) 0.28 | 9.553 |
| 12 |  | 6.2 (12.2) 40 (78.4) 0.51 | 9.292 |
| 13 |  | 3.1 (3.6) 72 (82.8) 0.87 | 9.060 |
| 14 |  | 1.3 (5.0) 22 (84.6) 0.26 | 9.585 |
| 15 |  | 1.2 (1.8) 49 (75.4) 0.65 | 9.187 |
| 16 |  | 7.2 (6.0) 58 (48.3) 1.20 | 8.921 |
| 17 |  | 13 (27.1) 26 (54.2) 0.48 | 9.319 |
| 18 |  | 19 (34.5) 13 (23.6) 0.55 | 9.260 |
| 19 |  | 69 (0.43) 120 (0.74) 162 | 6.790 |
| 20 |  | 10 (8.3) 170 (141.7) 1.20 | 8.921 |
| 21 |  | 36 (1.0) 160 (4.4) 36.31 | 7.440 |
| 22 |  | 9.6 (1.0) 45 (4.5) 10.00 | 8.000 |
| 23 |  | 7.6 (25.8) 44 (14.9) 2.95 | 8.530 |
| 24 |  | 22 (4.9) 32 (7.2) 4.47 | 8.350 |
| 25 |  | 44 (44.0) 53 (53.0) 1.00 | 9.000 |
| Entry | Structure | Predicted pEC50 |
|---|---|---|
| QSAR_1 |  | 9.72 |
| QSAR_2 |  | 9.60 |
| QSAR_3 |  | 9.68 |
| QSAR_4 |  | 9.30 |
| QSAR_5 |  | 10.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apablaza, G.; Montoya, L.; Morales-Verdejo, C.; Mellado, M.; Cuellar, M.; Lagos, C.F.; Soto-Delgado, J.; Chung, H.; Pessoa-Mahana, C.D.; Mella, J. 2D-QSAR and 3D-QSAR/CoMSIA Studies on a Series of (R)-2-((2-(1H-Indol-2-yl)ethyl)amino)-1-Phenylethan-1-ol with Human β3-Adrenergic Activity. Molecules 2017, 22, 404. https://doi.org/10.3390/molecules22030404
Apablaza G, Montoya L, Morales-Verdejo C, Mellado M, Cuellar M, Lagos CF, Soto-Delgado J, Chung H, Pessoa-Mahana CD, Mella J. 2D-QSAR and 3D-QSAR/CoMSIA Studies on a Series of (R)-2-((2-(1H-Indol-2-yl)ethyl)amino)-1-Phenylethan-1-ol with Human β3-Adrenergic Activity. Molecules. 2017; 22(3):404. https://doi.org/10.3390/molecules22030404
Chicago/Turabian StyleApablaza, Gastón, Luisa Montoya, Cesar Morales-Verdejo, Marco Mellado, Mauricio Cuellar, Carlos F. Lagos, Jorge Soto-Delgado, Hery Chung, Carlos David Pessoa-Mahana, and Jaime Mella. 2017. "2D-QSAR and 3D-QSAR/CoMSIA Studies on a Series of (R)-2-((2-(1H-Indol-2-yl)ethyl)amino)-1-Phenylethan-1-ol with Human β3-Adrenergic Activity" Molecules 22, no. 3: 404. https://doi.org/10.3390/molecules22030404
APA StyleApablaza, G., Montoya, L., Morales-Verdejo, C., Mellado, M., Cuellar, M., Lagos, C. F., Soto-Delgado, J., Chung, H., Pessoa-Mahana, C. D., & Mella, J. (2017). 2D-QSAR and 3D-QSAR/CoMSIA Studies on a Series of (R)-2-((2-(1H-Indol-2-yl)ethyl)amino)-1-Phenylethan-1-ol with Human β3-Adrenergic Activity. Molecules, 22(3), 404. https://doi.org/10.3390/molecules22030404









