Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives
Abstract
:1. Introduction
2. Biological Properties of Imidazo[4,5-b]pyridines and Imidazo[4,5-c]pyridines
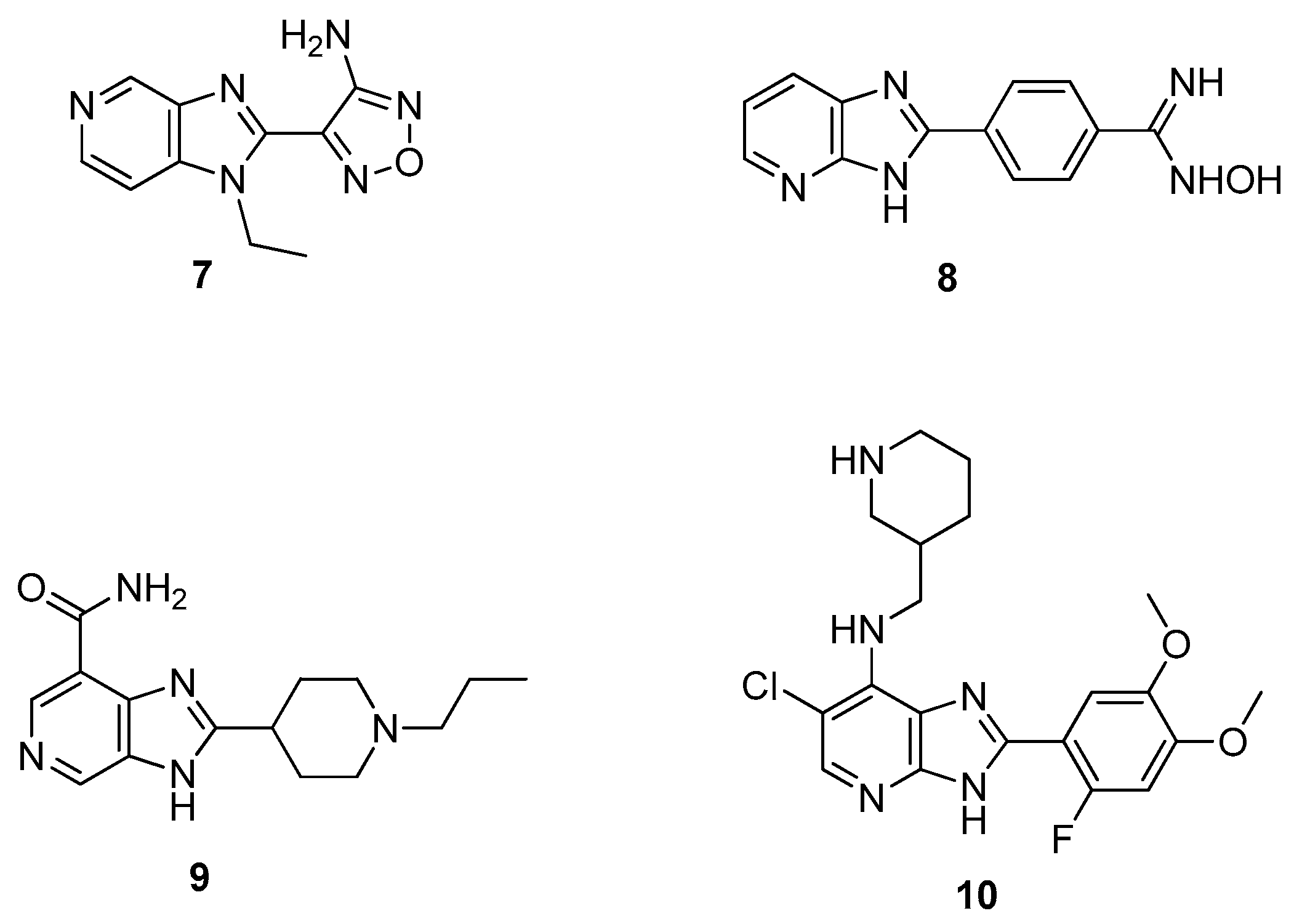
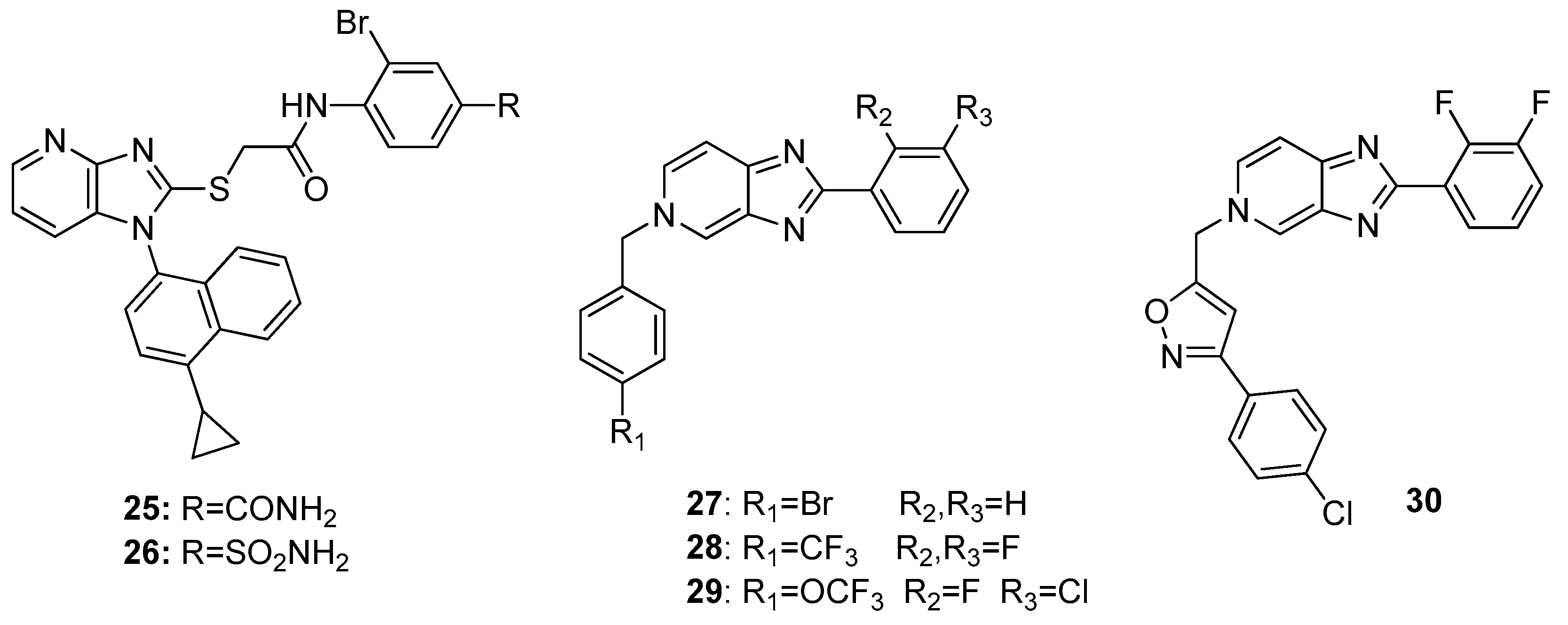
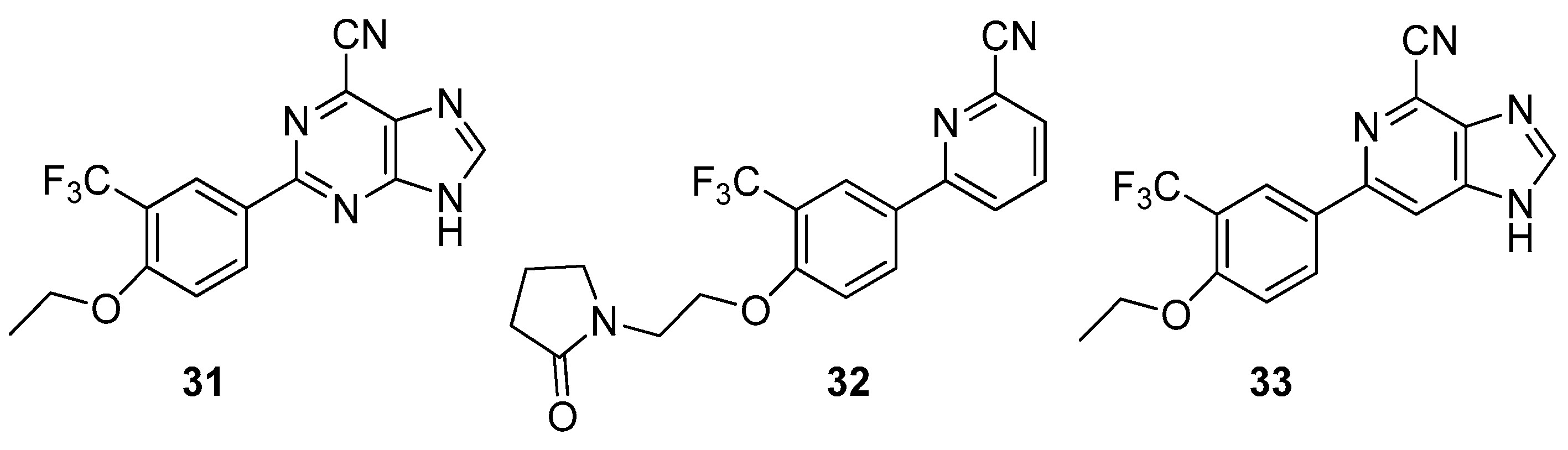
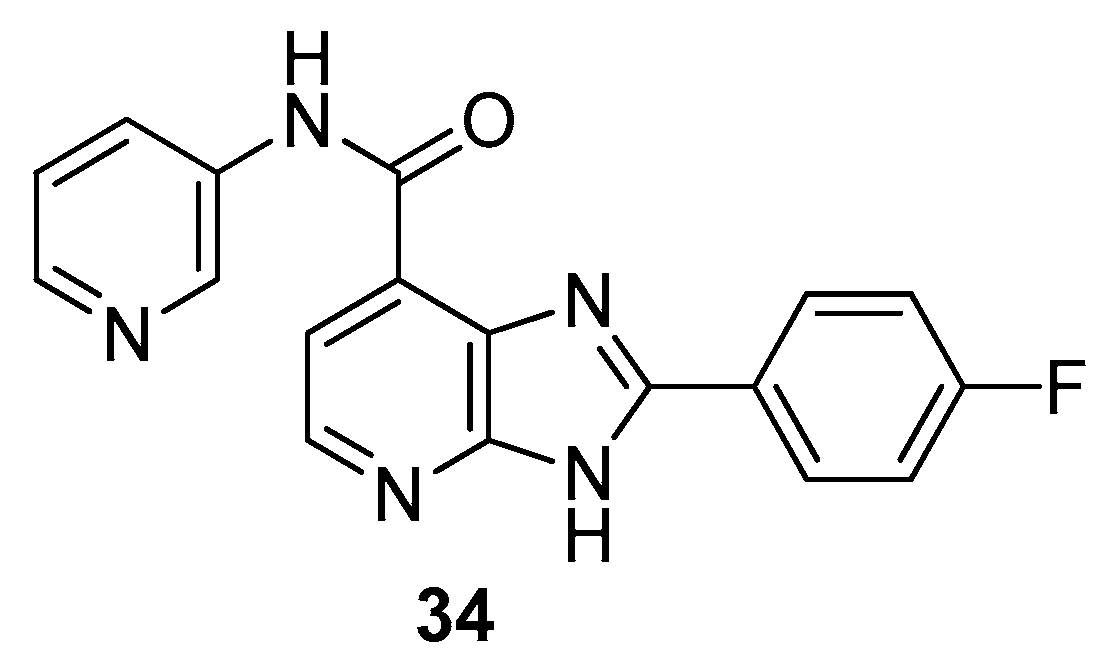
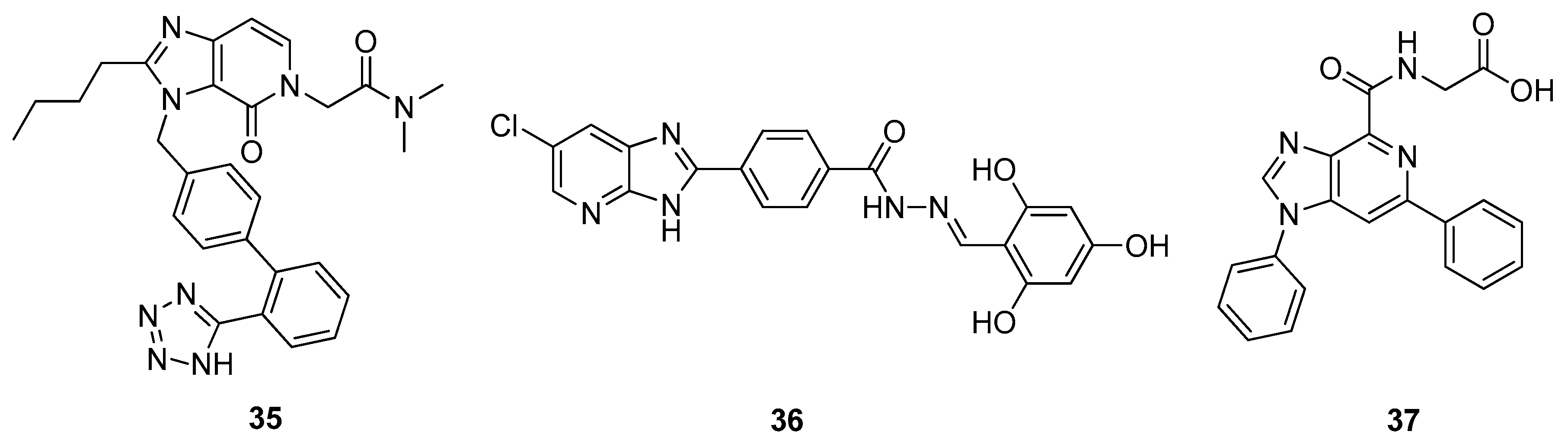
2.1. Antitumor Activity
2.2. Antimicrobial Activity
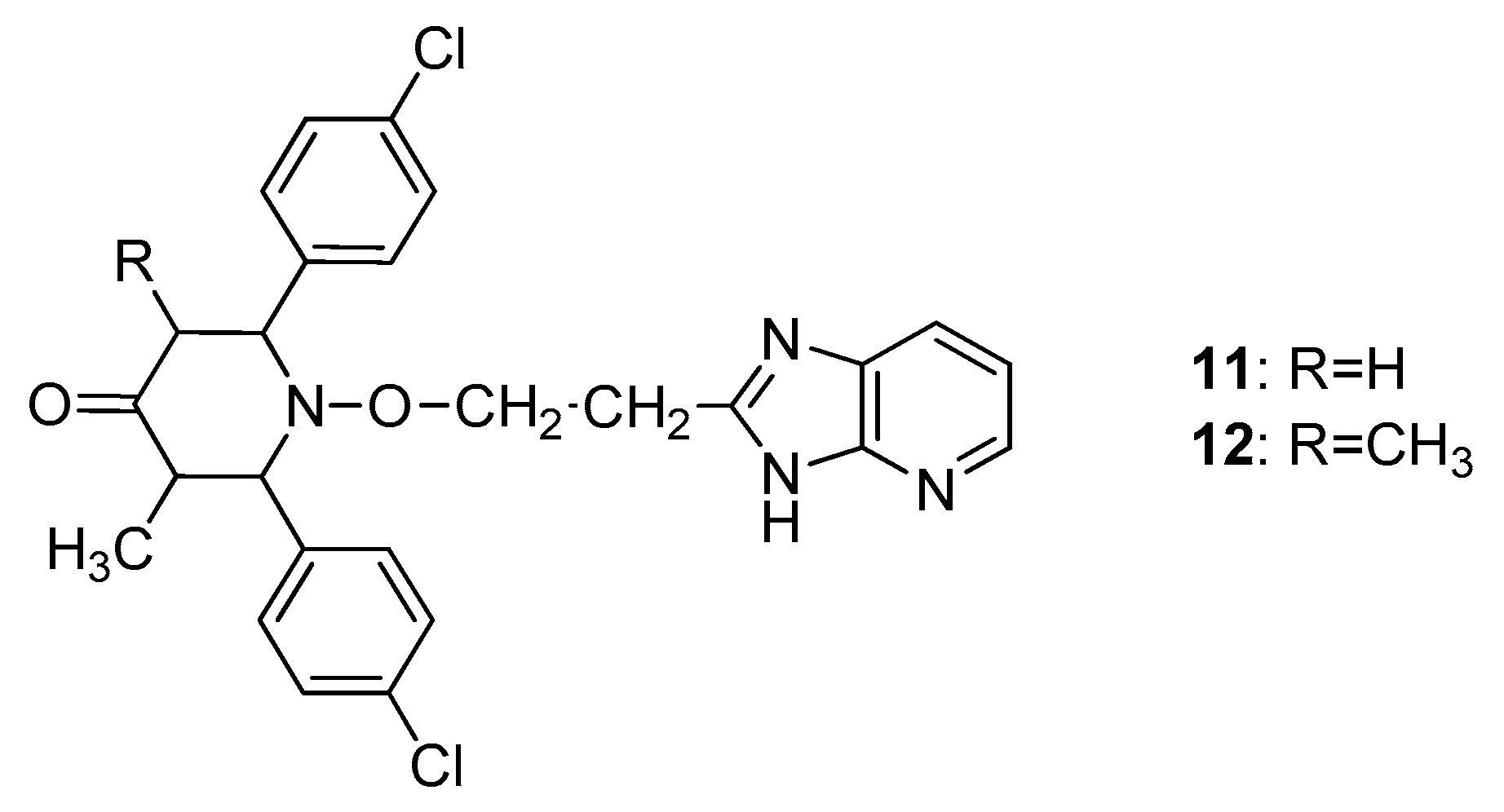
2.3. Anti-Inflammatory
2.4. Antiviral Activity
2.5. Autoimmune Disorders
2.6. Antidiabetic Activity
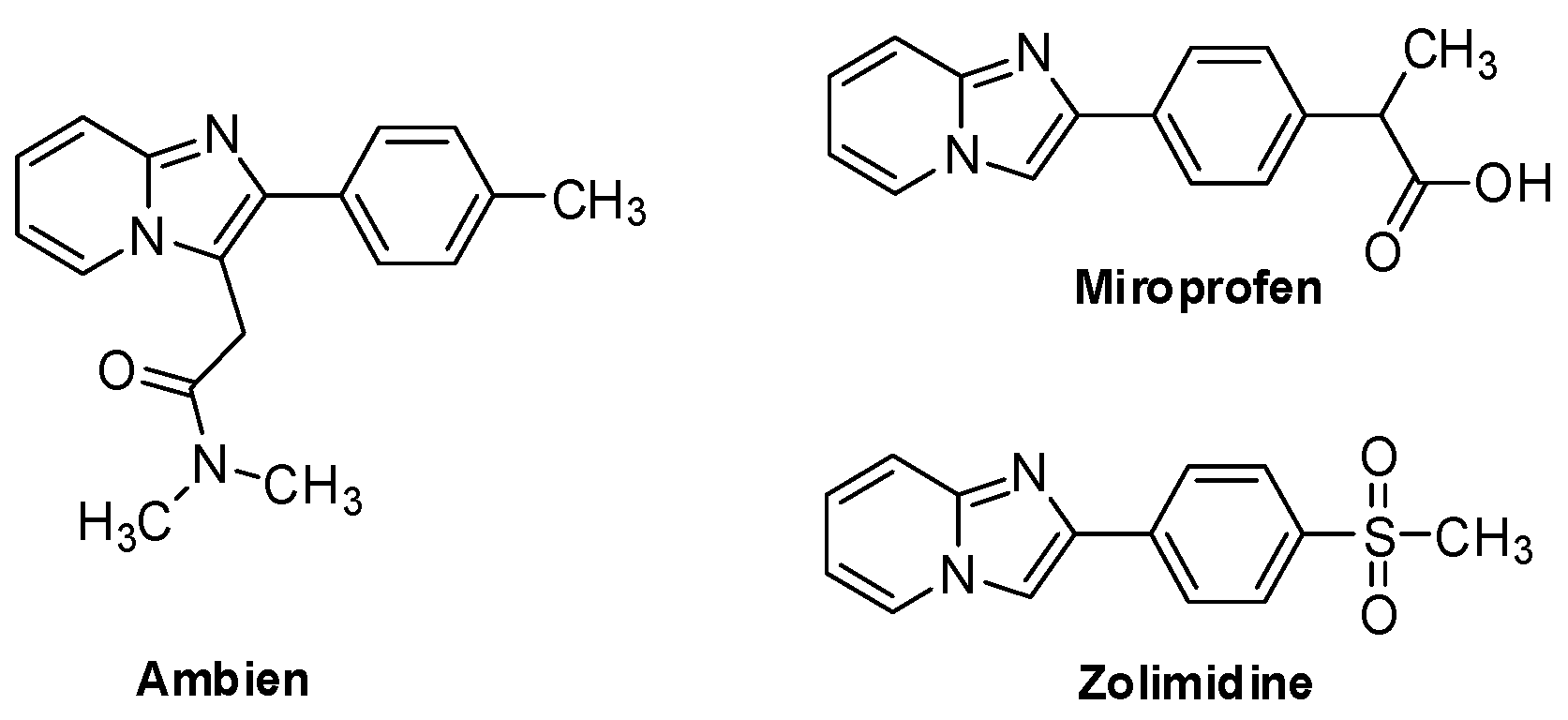
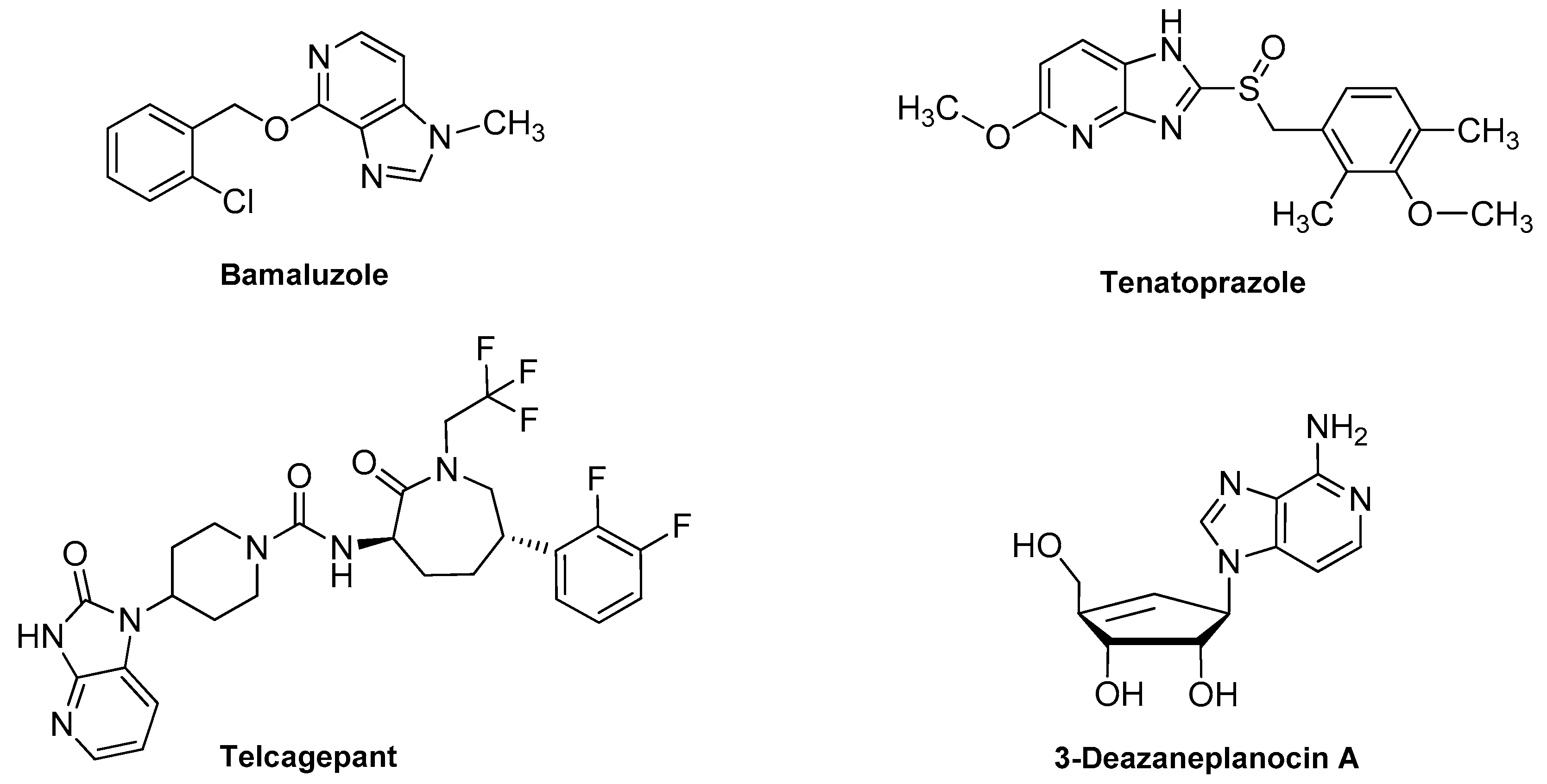
2.7. Miscellaneous Activities
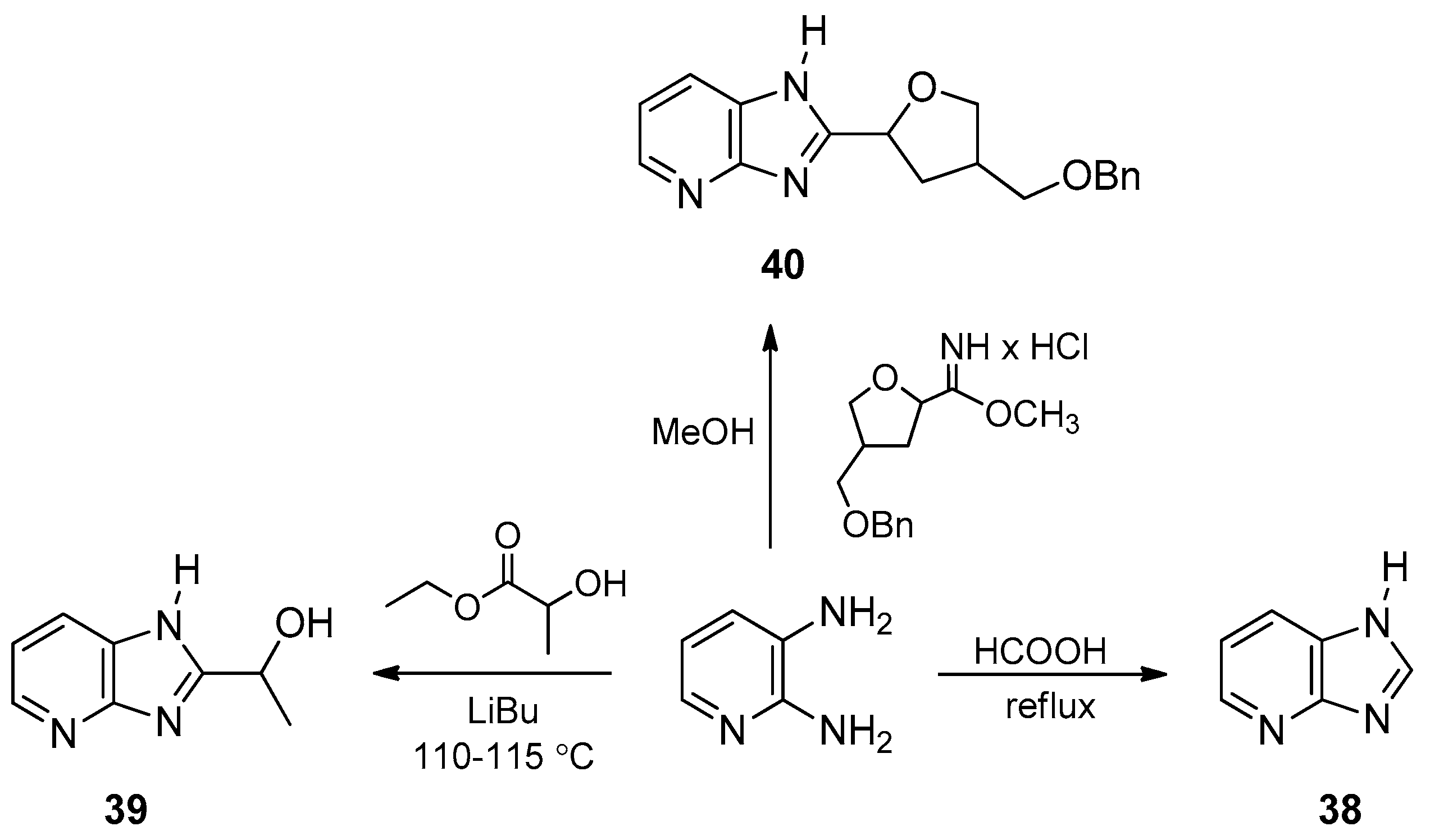
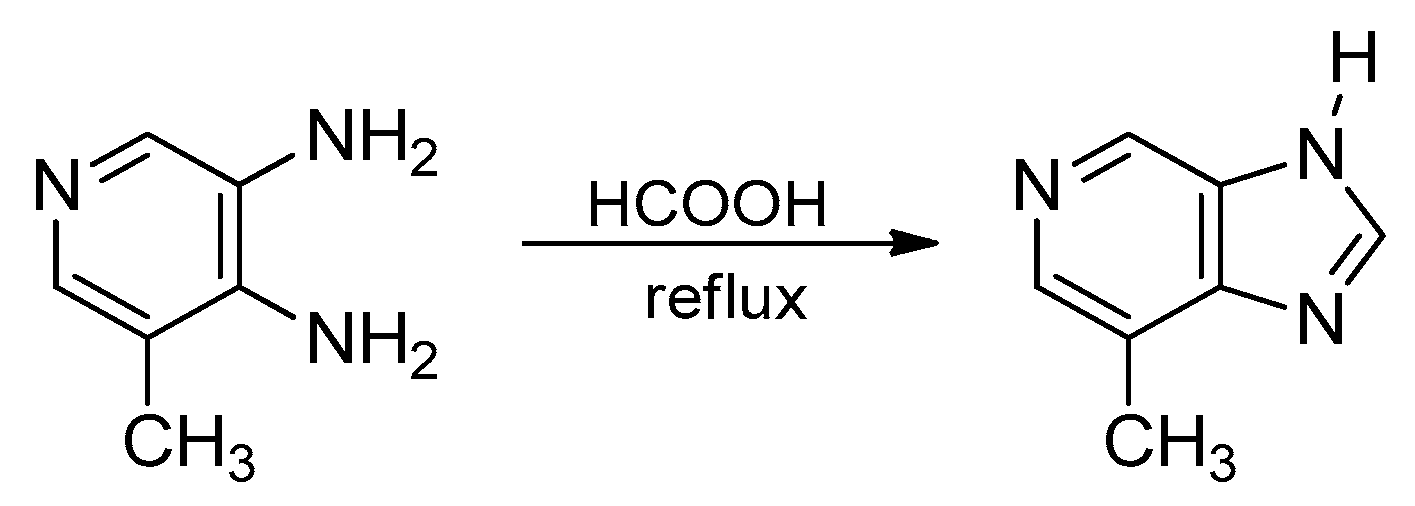
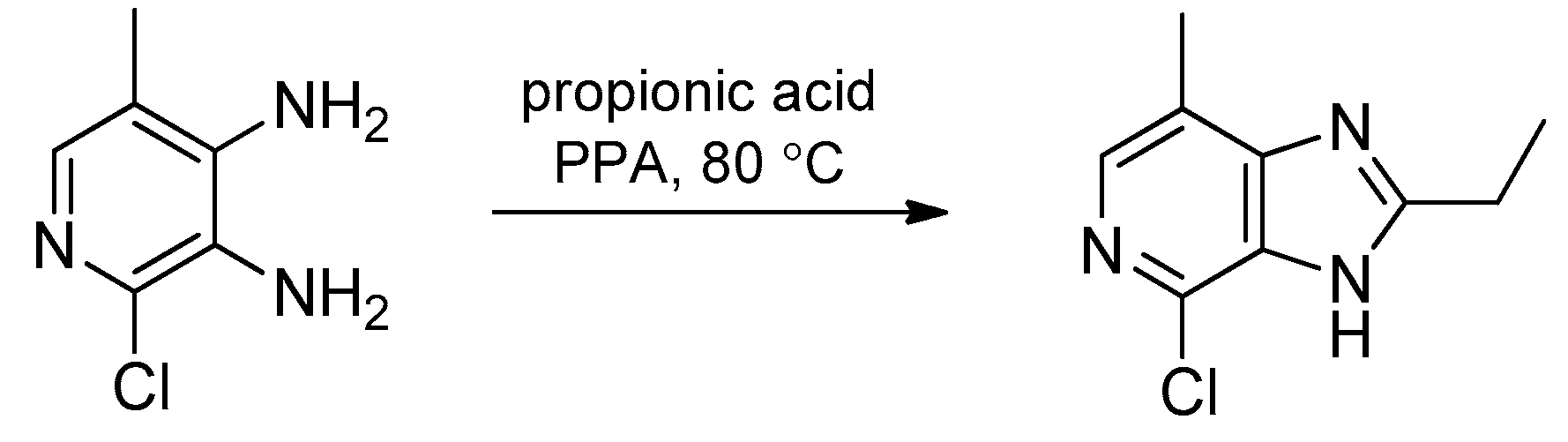
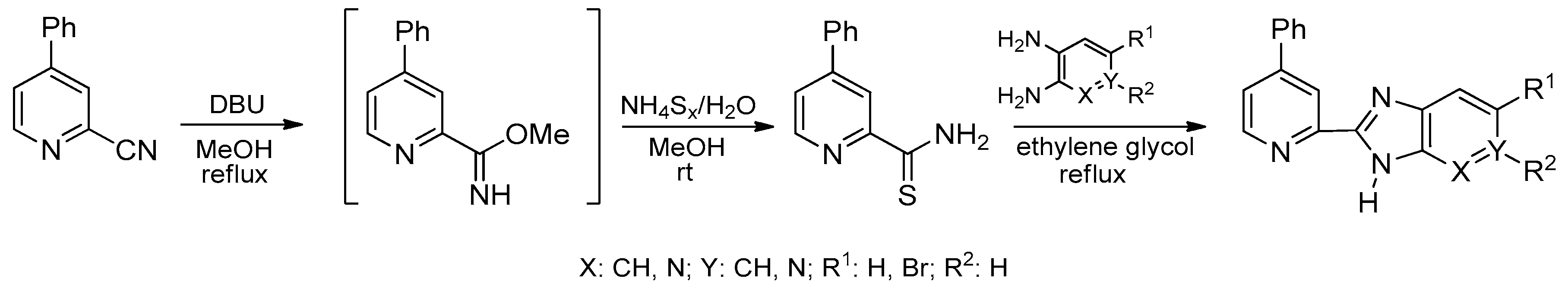
3. Synthetic Routes to Imidazo[4,5-b]pyridines and Imidazo[4,5-c]pyridines
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Foster, A.C.; Kemp, J.A. Glutamate- and GABA-based CNS therapeutics. Curr. Opin. Pharmacol. 2006, 6, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Bamford, M. 3 H+/K+ ATPase inhibitors in the treatment of acid-related disorders. Prog. Med. Chem. 2009, 47, 75–162. [Google Scholar] [PubMed]
- Dowsett, M.; Smithers, D.; Moore, J.; Trunet, P.F.; Coombes, R.C.; Powles, T.J.; Rubens, R.; Smith, I.E. Endocrine changes with the aromatase inhibitor fadrozole hydrochloride in breast cancer. Eur. J. Cancer 1994, 30, 1453–1458. [Google Scholar] [CrossRef]
- Mikashima, H.; Goto, K. Inhibitory effect of 2-(4-(2-imidazo(1,2-a)pyridyl)phenyl) propionic acid (miroprofen) on platelet aggregation and prostaglandin I2 generation. Yakugaku Zasshi 1982, 102, 99–103. [Google Scholar] [PubMed]
- Autorzy, W.A. Pharmaceutical Manufacturing Encyclopedia, 3rd ed.; William Andrew Publishing: Norwich, NY, USA, 2006. [Google Scholar]
- Edvinsson, L.; Linde, M. New drugs in migraine treatment and prophylaxis: Telcagepant and topiramate. Lancet 2010, 376, 645–655. [Google Scholar] [CrossRef]
- Scarpignato, C.; Hunt, R.H. Proton pump inhibitors: The beginning of the end or the end of the beginning? Curr. Opin. Pharmacol. 2008, 8, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K. DZNep, inhibitor of S-adenosylhomocysteine hydrolase, down-regulates expression of SETDB1 H3K9me3 HMTase in human lung cancer cells. Biochem. Biophys. Res. Commun. 2013, 438, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Terashima, S. Synthesis and matrix metalloproteinase (MMP)-12 inhibitory activity of ageladine A and its analogs. Bioorg. Med. Chem. Lett. 2007, 17, 4495–4499. [Google Scholar] [CrossRef] [PubMed]
- Hranjec, M.; Lučić, B.; Ratkaj, I.; Pavelić, S.K.; Piantanida, I.; Pavelić, K.; Karminski-Zamola, G. Novel imidazo[4,5-b]pyridine and triaza-benzo[c]fluorene derivatives: Synthesis, antiproliferative activity and DNA binding studies. Eur. J. Med. Chem. 2011, 46, 2748–2758. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, S.; Mamalis, A. Synthesis and antitumor activity of new thiosemicarbazones of 2-acetylimidazo[4,5-b]pyridine. J. Heterocycl. Chem. 2005, 42, 1273–1281. [Google Scholar] [CrossRef]
- Newhouse, B.J.; Wenglowsky, S.; Grina, J.; Laird, E.R.; Voegtli, W.C.; Ren, L.; Ahrendt, K.; Buckmelter, A.; Gloor, S.L.; Klopfenstein, N.; et al. Imidazo[4,5-b]pyridine inhibitors of B-Raf kinase. Bioorg. Med. Chem. Lett. 2013, 23, 5896–5899. [Google Scholar] [CrossRef] [PubMed]
- Sajith, A.M.; Abdul Khader, K.K.; Joshi, N.; Reddy, M.N.; Padusha, M.S.A.; Nagaswarupa, H.P.; Nibin Joy, M.; Bodke, Y.D.; Karuvalam, R.P.; Banerjee, R.; et al. Design, synthesis and structure–activity relationship (SAR) studies of imidazo[4,5-b]pyridine derived purine isosteres and their potential as cytotoxic agents. Eur. J. Med. Chem. 2015, 89, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Satam, V.; Babu, B.; Patil, P.; Brien, K.A.; Olson, K.; Savagian, M.; Lee, M.; Mepham, A.; Jobe, L.B.; Bingham, J.P.; et al. AzaHx, a novel fluorescent, DNA minor groove and G·C recognition element: Synthesis and DNA binding properties of a p-anisyl-4-aza-benzimidazole-pyrrole-imidazole (azaHx-PI) polyamide. Bioorg. Med. Chem. Lett. 2015, 25, 3681–3685. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lin, X.; Kang, D.; Li, X.; Zhan, P.; Liu, X.; Zhang, Q. Discovery and characterization of novel imidazopyridine derivative CHEQ-2 as a potent CDC25 inhibitor and promising anticancer drug candidate. Eur. J. Med. Chem. 2014, 82, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.; Jin, Y.; Hwan, S.; Sook, N.; Lee, H.; Park, S.Y. The discovery and the structural basis of an imidazo[4,5-b]pyridine based p21-activated kinase 4 inhibitor. Bioorg. Med. Chem. Lett. 2016, 26, 2580–2583. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, L.; Zhang, J.; Xie, X.; Qiu, K. A combined pharmacophore modeling, 3D QSAR and virtual screening studies on imidazopyridines as B-Raf inhibitors. Int. J. Mol. Sci. 2015, 16, 12307–12323. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, Y.; Yu, F.; Xie, X.; Qiu, K.; Fu, J. An investigation of molecular docking and molecular dynamic simulation on imidazopyridines as B-Raf kinase inhibitors. Int. J. Mol. Sci. 2015, 16, 27350–27361. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Chen, W.-N.; Chen, W.-M. Molecular modeling studies on imidazo[4,5-b]pyridine derivatives as Aurora A kinase inhibitors using 3D-QSAR and docking approaches. Eur. J. Med. Chem. 2011, 46, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Bavetsias, V.; Sun, C.; Bouloc, N.; Reynisson, J.; Workman, P.; Linardopoulos, S.; McDonald, E. Hit generation and exploration: Imidazo[4,5-b]pyridine derivatives as inhibitors of Aurora kinases. Bioorg. Med. Chem. Lett. 2007, 17, 6567–6571. [Google Scholar] [CrossRef] [PubMed]
- Bababdani, B.M.; Mousavi, M. Gravitational search algorithm: A new feature selection method for QSAR study of anticancer potency of imidazo[4,5-b]pyridine derivatives. Chemom. Intell. Lab. Syst. 2013, 122, 1–11. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Cai, S.-H.; Cheng, H. Identification of TBK1 and IKKE, the non-canonical IkB kinases, as crucial pro-survival factors in HTLV-1-transformed T lymphocytes. Leuk. Res. 2016, 46, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Thu, Y.M.; Richmond, A. NF-kB inducing kinase: A key regulator in the immune system and in cancer. Cytokine Growth Factor Rev. 2010, 21, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Block, M.A.; Cowen, S.; Davies, A.M.; Devereaux, E.; Gingipalli, L.; Johannes, J.; Larsen, N.A.; Su, Q.; Tucker, J.A.; et al. Discovery of azabenzimidazole derivatives as potent, selective inhibitors of TBK1/IKKε kinases. Bioorg. Med. Chem. Lett. 2012, 22, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Johannes, J.W.; Chuaqui, C.; Cowen, S.; Devereaux, E.; Gingipalli, L.; Molina, A.; Wang, T.; Whitston, D.; Wu, X.; Zhang, H.-J.; et al. Discovery of 6-aryl-azabenzimidaoles that inhibit the TBK1/IKK-ε kinases. Bioorg. Med. Chem. Lett. 2014, 24, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Bamford, M.J.; Alberti, M.J.; Bailey, N.; Davies, S.; Dean, D.K.; Gaiba, A.; Garland, S.; Harling, J.D.; Jung, D.K.; Panchal, T.A.; et al. (1H-Imidazo[4,5-c]pyridin-2-yl)-1,2,5-oxadiazol-3-ylamine derivatives: A novel class of potent MSK-1-inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 3402–3406. [Google Scholar] [CrossRef] [PubMed]
- Cohen, F.; Koehler, M.F.T.; Bergeron, P.; Elliott, L.O.; Flygare, J.A.; Franklin, M.C.; Gazzard, L.; Keteltas, S.F.; Lau, K.; Ly, C.Q.; et al. Antagonists of inhibitor of apoptosis proteins based on thiazole amide isosteres. Bioorg. Med. Chem. Lett. 2010, 20, 2229–2233. [Google Scholar] [CrossRef] [PubMed]
- Lukasik, P.M.; Elabar, S.; Lam, F.; Shao, H.; Liu, X.; Abbas, A.Y.; Wang, S. Synthesis and biological evaluation of imidazo[4,5-b]pyridine and 4-heteroaryl-pyrimidine derivatives as anti-cancer agents. Eur. J. Med. Chem. 2012, 57, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Püsküllü, M.O.; Karaaslan, C.; Bakar, F.; Göker, H. Synthesis and potent cytotoxicity of some novel imidazopyridine derivatives against MCF-7 human breast adenocarcinoma cell line. Chem. Heterocycl. Compd. 2015, 51, 723–733. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, X.; Hu, Y.; He, X.; Gong, G.; Xu, Y. Discovery and SAR study of 2-(1-propylpiperidin-4-yl)-3H-imidazo[4,5-c]pyridine-7-carboxamide: A potent inhibitor of poly(ADP-ribose) polymerase-1 (PARP-1) for the treatment of cancer. Bioorg. Med. Chem. 2015, 23, 6551–6559. [Google Scholar] [CrossRef] [PubMed]
- Vasbinder, M.M.; Alimzhanov, M.; Augustin, M.; Bebernitz, G.; Bell, K.; Chuaqui, C.; Deegan, T.; Ferguson, A.D.; Goodwin, K.; Huszar, D.; et al. Identification of azabenzimidazoles as potent JAK1 selective inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 60–67. [Google Scholar] [CrossRef] [PubMed]
- An, X.-D.; Liu, H.; Xu, Z.-L.; Jin, Y.; Peng, X.; Yao, Y.-M.; Geng, M.; Long, Y.-Q.; et al. Discovery of potent 1H-imidazo[4,5-b]pyridine-based c-Met kinase inhibitors via mechanism-directed structural optimization. Bioorg. Med. Chem. Lett. 2015, 25, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Bürli, R.W.; Jones, P.; McMinn, D.; Le, Q.; Duan, J.-X.; Kaizerman, J.A.; Difuntorum, S.; Moser, H.E. DNA binding ligands targeting drug-resistant Gram-positive bacteria. Part 2: C-terminal benzimidazoles and derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Yildiz-Oren, I.; Yalcin, I.; Aki-Sener, E.; Ucarturk, N. Synthesis and structure–activity relationships of new antimicrobial active multisubstituted benzazole derivatives. Eur. J. Med. Chem. 2004, 39, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Global Tuberculosis Report 2015. Available online: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1n.d (accessed on 3 March 2017).
- Aridoss, G.; Balasubramanian, S.; Parthiban, P.; Kabilan, S. Synthesis and in vitro microbiological evaluation of imidazo[4,5-b]pyridinylethoxypiperidones. Eur. J. Med. Chem. 2006, 41, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Milewski, S. Glucosamine-6-phosphate synthase—The multi-facets enzyme. Biochem. Biophys. Acta 2002, 1597, 173–192. [Google Scholar] [CrossRef]
- Mouilleron, S.; Badet-Denisot, M.; Badet, B.; Golinelli-Pimpaneau, B. Dynamics of glucosamine-6-phosphate synthase catalysis. Arch. Biochem. Biophys. 2011, 505, 1–12. [Google Scholar] [CrossRef] [PubMed]
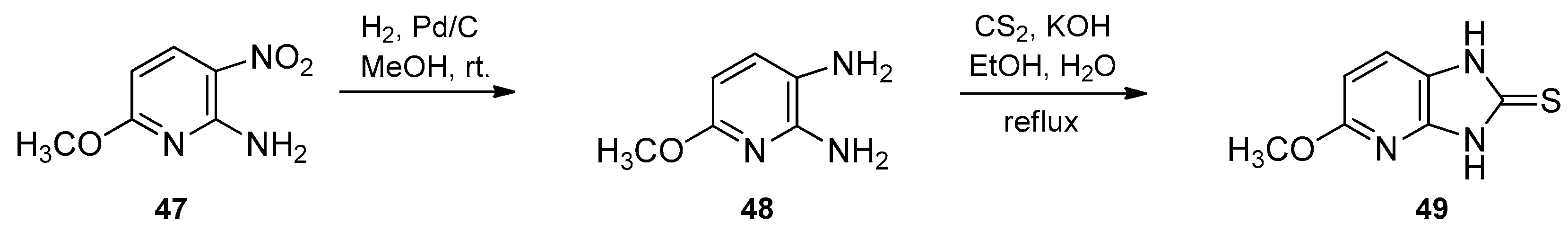
- Jose, G.; Suresha Kumara, T.H.; Nagendrappa, G.; Sowmya, H.B.V.; Jasinski, J.P.; Millikan, S.P.; Chandrika, N.; More, S.S.; Harish, B.G. New polyfunctional imidazo[4,5-c]pyridine motifs: Synthesis, crystal studies, docking studies and antimicrobial evaluation. Eur. J. Med. Chem. 2014, 77, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Khoje, A.D.; Charnock, C.; Wan, B.; Franzblau, S.; Gundersen, L. Synthesis and antimycobacterial activities of non-purine analogs of 6-aryl-9-benzylpurines: Imidazopyridines, pyrrolopyridines, benzimidazoles, and indoles. Bioorg. Med. Chem. 2011, 19, 3483–3491. [Google Scholar] [CrossRef] [PubMed]
- Masand, V.H.; El-sayed, N.N.E.; Mahajan, D.T.; Mercader, A.G.; Alafeefy, A.M.; Shibi, I.G. QSAR modeling for anti-human African trypanosomiasis activity of substituted 2-phenylimidazopyridines. J. Mol. Struct. 2017, 1130, 711–718. [Google Scholar] [CrossRef]
- Zhang, Z.; Koh, C.Y.; Ranade, R.M.; Shibata, S.; Gillespie, J.R.; Hulverson, M.A.; Huang, W.; Nguyen, J.; Pendem, N.; Gelb, M.H.; et al. 5-Fluoroimidazo[4,5-b]pyridine is a privileged fragment that conveys bioavailability to potent trypanosomal methionyl-tRNA synthetase inhibitors. ACS Infect. Dis. 2016, 2, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.Y.; Kim, J.E.; Shibata, S.; Ranade, R.M.; Yu, M.; Liu, J.; Gillespie, J.R.; Buckner, F.S.; Verlinde, C.L.; Fan, E.; et al. Distinct states of methionyl-tRNA synthetase indicate inhibitor binding by conformational selection. Structure 2012, 20, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, Z.; Alvarez, X.B.; Yeow, C.; Ranade, R.M.; Gillespie, J.R.; Creason, S.A.; Shibata, S.; Verlinde, C.L.M.J.; Hol, W.G.J. Structure-guided design of novel Trypanosoma brucei Methionyl-tRNA synthetase inhibitors. Eur. J. Med. Chem. 2016, 124, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Tao, J.; Chen, H.; Bian, Y.; Yang, X.; Chen, G.; Zhang, X.; Liang, G.; Wu, W.; Song, Z.; et al. Myeloid differentiation protein 2-dependent mechanisms in retinal ischemia-reperfusion injury. Toxicol. Appl. Pharmacol. 2017, 317, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fang, I.; Yang, C.; Yang, C. Chitosan oligosaccharides prevented retinal ischemia and reperfusion injury via reduced oxidative stress and in fl ammation in rats. Exp. Eye Res. 2015, 130, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Ren, L.; Wang, L.; Xu, S.; Tao, J. A novel imidazopyridine derivative, X22, prevents the retinal ischemia-reperfusion injury via inhibition of MAPKs. Exp. Eye Res. 2015, 135, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, L.; Huang, W.; Skibba, M.; Fang, Q.; Xie, L.; Wei, T.; Feng, Z.; Liang, G. Inhibition of ROS and inflammation by an imidazopyridine derivative X22 attenuate high fat diet-induced arterial injuries. Vascul. Pharmacol. 2015, 72, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhang, Y.; Zhong, P.; Peng, K.; Xu, Z.; Chen, X. Inhibition of inflammation and oxidative stress by an imidazopyridine derivative X22 prevents heart injury from obesity. J. Cell. Mol. Med. 2016, 20, 1427–1442. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Shimokawa, H. Divergent roles of endothelial nitric oxide synthases system in maintaining cardiovascular homeostasis. Free Radic. Biol. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Paula, G.H.; Lacchini, R.; Tanus-Santos, J.E. Clinical and pharmacogenetic impact of endothelial nitric oxide synthase polymorphisms on cardiovascular diseases. Nitric Oxide 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Grädler, U.; Fuchß, T.; Ulrich, W.; Boer, R.; Strub, A.; Hesslinger, C.; Anézo, C.; Diederichs, K.; Zaliani, A. Novel nanomolar imidazo[4,5-b]pyridines as selective nitric oxide synthase (iNOS) inhibitors: SAR and structural insights. Bioorg. Med. Chem. Lett. 2011, 21, 4228–4232. [Google Scholar] [CrossRef] [PubMed]
- Gierse, J.; Nickols, M.; Leahy, K.; Warner, J.; Zhang, Y.; Cortes-Burgos, L.; Carter, J.; Seibert, K.; Masferrer, J. Evaluation of COX-1/COX-2 selectivity and potency of a new class of COX-2 inhibitors. Eur. J. Pharmocol. 2008, 588, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, S.S.R.; Elshemy, H.A.H.; Abdelgawad, M.A.; Abdel-latif, M.S.; Abdellatif, K.R.A. Design, synthesis and biological screening of some novel celecoxib and etoricoxib analogs with promising COX-2 selectivity, anti-inflammatory activity and gastric safety profile. Bioorg. Chem. 2016, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kirwen, E.; Batra, T.; Karthikeyan, C.; Singh, G.; Rathore, V.; Mulakayala, C.; Mulakayala, N.; Nusbaum, A.C.; Chen, J.; Amawi, H.; et al. 2,3-Diaryl-3H-imidazo[4,5-b]pyridine derivatives as potential anticancer and anti-inflammatory agents. Acta Pharm. Sin. B 2017, 7, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, B.; Zhou, Z.; Gao, P.; Pannecouque, C.; Daelemans, D.; De Clercq, E.; Zhan, P.; Liu, X. Arylazolyl(azinyl)thioacetanilides: Part 19: Discovery of novel substituted imidazo[4,5-b]pyridin-2-ylthioacetanilides as potent HIV NNRTIs via a structure-based bioisosterism approach. Chem. Biol. Drug Des. 2016, 88, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Puerstinger, G.; Paeshuyse, J.; Herdewijn, P.; Rozenski, J.; De Clercq, E.; Neyts, J. Substituted 5-benzyl-2-phenyl-5-H-imidazo[4,5-c]pyridines: A new class of pestivirus inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 5345–5349. [Google Scholar] [CrossRef] [PubMed]
- Puerstinger, G.; Paeshuyse, J.; Heinrich, S.; Mohr, J.; Schraffl, N.; De Clercq, E.; Neyts, J. Antiviral 2,5-disubstituted imidazo[4,5-c]pyridines: Further optimization of anti-hepatitis C virus activity. Bioorg. Med. Chem. Lett. 2007, 17, 5111–5114. [Google Scholar] [CrossRef] [PubMed]
- Vliegen, I.; Paeshuyse, J.; Burghgraeve T, D.e.; Lehman, L.S.; Paulson, M.; Shih, I.; Mabery, E.; Boddeker, N.; De Clercq, E.; Reiser, H.; et al. Substituted imidazopyridines as potent inhibitors of HCV replication. J. Hepatol. 2009, 50, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Steimle, A.; Kalbacher, H.; Maurer, A.; Beifuss, B.; Bender, A.; Schäfer, A.; Müller, R.; Autenrieth, I.B.; Frick, J.S. A novel approach for reliable detection of cathepsin S activities in mouse antigen presenting cells. J. Immunol. Methods 2016, 432, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Repnik, U.; Stoka, V.; Turk, V.; Turk, B. Lysosomes and lysosomal cathepsins in cell death. Biochim. Biophys. Acta 2012, 1824, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Baugh, M.; Black, D.; Long, C.; Bennett, D.J.; Dempster, M.; Fradera, X.; Gillespie, J.; Andrews, F.; Boucharens, S.; et al. 6-Phenyl-1-H-imidazo[4,5-c] pyridine-4-carbonitrile as cathepsin S inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Global Report for Diabetes. Available online: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf (accessed on 3 March 2017).
- Yokoyama, H.; Araki, S.; Kawai, K.; Hirao, K.; Oishi, M.; Sugimoto, K.; Sone, H.; Maegawa, H.; Kashiwagi, A.; on behalf of Japan Diabetes Clinical Data Management Study Group. Pioglitazone treatment and cardiovascular event and death in subjects with type 2 diabetes without established cardiovascular disease (JDDM 36). Diabetes Res. Clin. Pract. 2015, 109, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.C.M.; Woodward, M.; Colagiuri, S. Triple therapy combinations for the treatment of type 2 diabetes–A network meta-analysis. Diabetes Res. Clin. Pract. 2016, 116, 149–158. [Google Scholar]
- Fedejko, B.; Mazerska, Z. UDP-glukuronylotranferazy w metabolizmie detoksykacyjnym i aktywacyjnym związków endogennych oraz ksenobiotyków. Postepy Biochem. 2011, 57, 49–62. [Google Scholar] [PubMed]
- Lee, S.-C.; Kim, H.T.; Park, C.-H.; Lee, D.Y.; Chang, H.-J.; Park, S.; Cho, J.M.; Ro, S.; Suh, Y.-G. Design, synthesis and biological evaluation of novel imidazopyridines as potential antidiabetic GSK3β inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 4221–4224. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.C.; Kohli, D.V. A comprehensive structure -activity analysis 2,3,5-trisubstituted 4,5-dihydro-4-oxo-3H-imidazo[4,5-c]pyridine derivatives as angiotensin II receptor antagonists: Using 2D- and 3D-QSAR approach. Med. Chem. Res. 2013, 22, 588–605. [Google Scholar] [CrossRef]
- Warwas, M.; Piwowar, A.; Kopiec, G. Zaawansowane produkty glikacji (AGE) w organizmie-powstawanie, losy, interakcja z receptorami i jej następstwa. Farm. Pol. 2010, 8, 585–590. [Google Scholar]
- Klenina, O.; Drapak, I.; Chaban, T.; Ogurtsov, V.; Chaban, I.; Golos, I. QSAR Studies of some thiazolo[4,5-b]pyridines as novel antioxidant agents: Enhancement of activity. Chem. Chem. Technol. 2013, 7, 5–12. [Google Scholar]
- Taha, M.; Alkadi, K.A.A.; Ismail, N.H.; Imran, S.; Adam, A.; Kashif, S.M.; Shah, S.A.A.; Jamil, W.; Sidiqqui, S.; Khan, K.M. Antiglycation and antioxidant potential of novel imidazo[4,5-b]pyridine benzohydrazones. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Taha, M.; Hadiani, N.; Imran, S.; Rashwan, H.; Jamil, W.; Ali, S.; Kashif, S.M.; Rahim, F.; Salar, U.; Khan, K.M. Synthesis of 6-chloro-2-Aryl-1H-imidazo[4,5-b]pyridine derivatives: Antidiabetic, antioxidant, b-glucuronidase inhibiton and their molecular docking studies. Bioorg. Chem. 2016, 65, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Frohn, M.; Viswanadhan, V.; Pickrell, A.J.; Golden, J.E.; Muller, K.M.; Bürli, R.W.; Biddlecome, G.; Yoder, S.C.; Rogers, N.; Dao, J.H. Structure-guided design of substituted aza-benzimidazoles as potent hypoxia inducible factor-1 a prolyl hydroxylase-2 inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 5023–5026. [Google Scholar] [CrossRef] [PubMed]
- Baugh, J.A.; Gantier, M.; Li, L.; Byrne, A.; Buckley, A.; Donnelly, S.C. Dual regulation of macrophage migration inhibitory factor (MIF) expression in hypoxia by CREB and HIF-1. Biochem. Biophys. Res. Commun. 2006, 347, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Dymińska, L.; Gągor, A.; Talik, Z.; Lorenc, J.; Hanuza, J. Vibrational spectra and structure of methyl-derivatives of imidazo[4,5-c]pyridine based on DFT quantum chemical calculations and XRD studies. Vib. Spectrosc. 2011, 57, 229–241. [Google Scholar] [CrossRef]
- Dekhane, D.V.; Pawar, S.S.; Gupta, S.V.; Shingare, M.S.; Thore, S.N. Lithium bromide catalyzed solvent free method for synthesis of 2-substituted benzimidazoles and imidazopyridines. Chin. Chem. Lett. 2010, 21, 519–523. [Google Scholar] [CrossRef]
- Mladenova, G.; Lee-Ruff, E. Cyclobutanone photoadducts of HCN and malononitrile: Useful intermediates for the synthesis of C-nucleosides. Tetrahedron Lett. 2007, 48, 2787–2789. [Google Scholar] [CrossRef]
- Dymińska, L.; Gągor, A.; Mączka, M.; Węgliński, Z.; Hanuza, J. XRD studies, vibrational spectra, and molecular structure of 1H-imidazo[4,5-b]pyridine based on DFT quantum chemical calculations. J. Raman Spectrosc. 2010, 41, 1021–1029. [Google Scholar] [CrossRef]
- Casimiro-Garcia, A.; Heemstra, R.J.; Bigge, C.F.; Chen, J.; Ciske, F.A.; Davis, J.A.; Ellis, T.; Esmaeil, N.; Flynn, D.; Han, S. Design, synthesis, and evaluation of imidazo[4,5-c]pyridin-4-one derivatives with dual activity at angiotensin II type 1 receptor and peroxisome proliferator-activated receptor-gamma. Bioorg. Med. Chem. Lett. 2013, 23, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Palou, R.; Zepeda, L.G.; Hopfl, H.; Montoya, A.; Guzman-Lucero, D.J.; Guzm, J. Parallel and automated library synthesis of 2-long alkyl chain benzoazoles and azole[4,5-b]pyridines under microwave irradiation. Mol. Divers. 2005, 9, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Myllymaki, M.J.; Koskinen, A.M.P. A rapid method for the preparation of 2-substituted oxazolo[4,5-b]pyridines using microwave-assisted direct condensation reactions. Tetrahedron Lett. 2007, 48, 2295–2298. [Google Scholar] [CrossRef]
- René, O.; Souverneva, A.; Magnuson, S.R.; Fauber, B.P. Efficient syntheses of 2-fluoroalkylbenzimidazoles and -benzothiazoles. Tetrahedron Lett. 2013, 54, 201–204. [Google Scholar] [CrossRef]
- Gobis, K.; Foks, H.; Serocki, M.; Augustynowicz-Kopeć, E. Synthesis and evaluation of in vitro antimycobacterial activity of novel 1H-benzo[d]imidazole derivatives and analogues. Eur. J. Med. Chem. 2015, 89, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Zeinyeh, W.; Pilmé, J.; Radix, S.; Walchshofer, N. Regioselective N-alkylation of imidazo[4,5-b]pyridine-4-oxide derivatives: An experimental and DFT study. Tetrahedron Lett. 2009, 50, 1828–1833. [Google Scholar] [CrossRef]
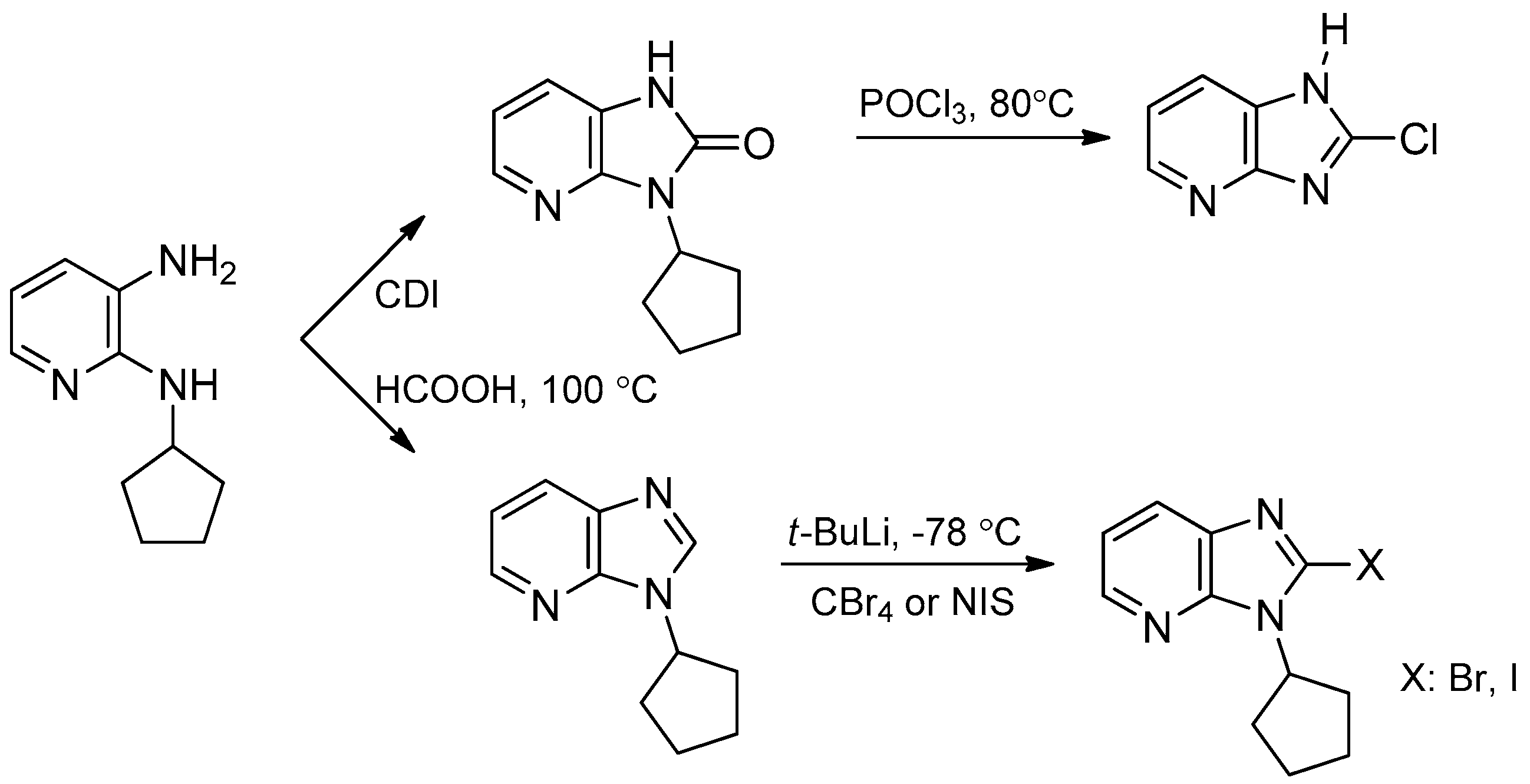
- Rosenberg, A.J.; Zhao, J.; Clark, D.A. Synthesis of imidazo[4,5-b]pyridines and imidazo[4,5-b]pyrazines by palladium catalyzed amidation of 2-chloro-3-amino-heterocycles. Org. Lett. 2012, 14, 1764–1767. [Google Scholar] [CrossRef] [PubMed]
- Al-duaij, O.K.; Zaki, E.A.; El Gazzar, A.B. A simple precursor for highly functionalized fused imidazo[4,5-b]pyridines and imidazo[4,5-b]-1,8-naphthyridine. Molecules 2016, 21, 1646. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.E.A.; Proença, M.F.; Booth, B.L. Efficient synthesis of 3H-imidazo[4,5-b]pyridines from malononitrile and 5-amino-4-(cyanoformimidoyl)imidazoles. J. Org. Chem. 2003, 68, 276–282. [Google Scholar] [CrossRef] [PubMed]
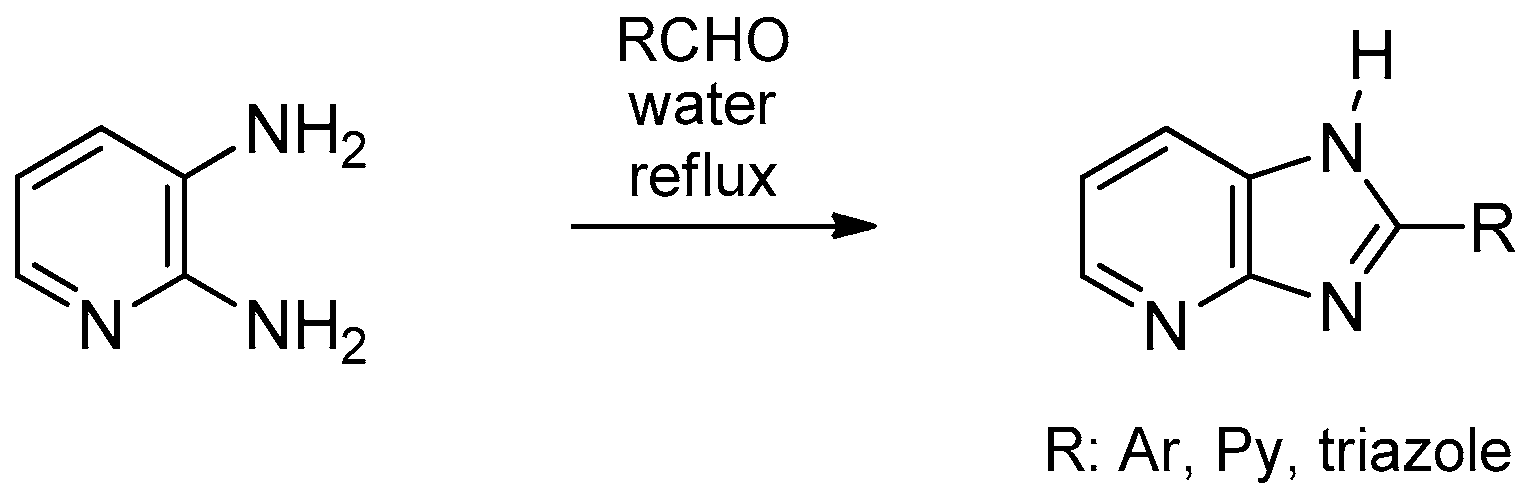
- Kale, R.P.; Shaikh, M.U.; Jadhav, G.R.; Gill, C.H. Eco-friendly and facile synthesis of 2-substituted-1H-imidazo[4,5-b]pyridine in aqueous medium by air oxidation. Tetrahedron Lett. 2009, 50, 1780–1782. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Sviridov, S.I.; Stepanov, A.E. Parallel solution-phase synthesis of substituted 2-(1,2,4-triazol-3-yl)benzimidazoles. Tetrahedron Lett. 2006, 47, 8025–8027. [Google Scholar] [CrossRef]
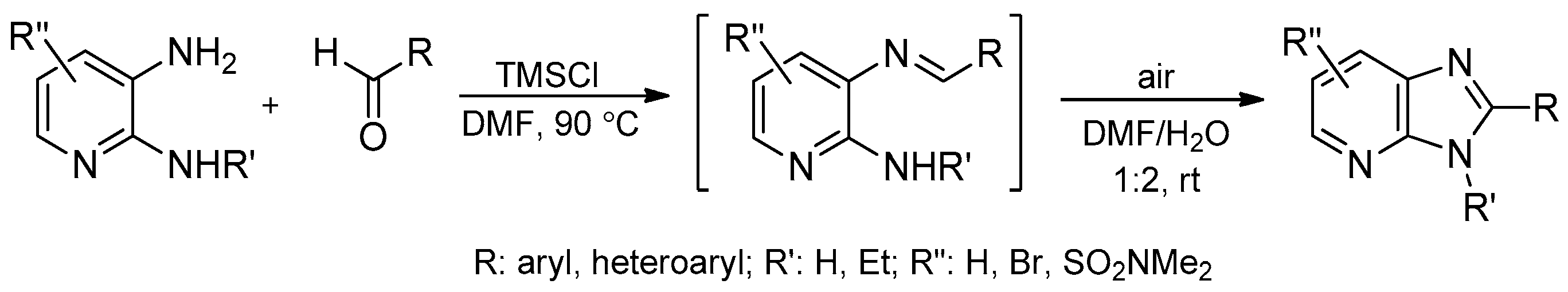
- Ryabukhina, S.V.; Plaskona, A.S.; Volochnyuk, D.M.; Tolmachev, A.A. Synthesis of fused imidazoles and benzothiazoles from (hetero)aromatic ortho-diamines or ortho-aminothiophenol and aldehydes promoted by chlorotrimethylsilane. Synthesis 2006, 21, 3715–3726. [Google Scholar] [CrossRef]
- Gao, M.; Wang, M.; Zheng, Q. Synthesis of carbon-11-labeled imidazopyridine- and purine- thioacetamide derivatives as new potential PET tracers for imaging of nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1). Bioorg. Med. Chem. Lett. 2016, 26, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Sajith, A.M.; Muralidharan, A. Microwave enhanced Suzuki coupling: A diversity-oriented approach to the synthesis of highly functionalised 3-substituted-2-aryl/heteroaryl imidazo[4,5-b]pyridines. Tetrahedron Lett. 2012, 53, 1036–1041. [Google Scholar] [CrossRef]
- Sajith, A.M.; Muralidharan, A. Exploration of copper and amine-free Sonogashira cross coupling reactions of 2-halo-3-alkyl imidazo[4,5-b]pyridines using tetrabutyl ammonium acetate as an activator under microwave enhanced conditions. Tetrahedron Lett. 2012, 53, 5206–5210. [Google Scholar] [CrossRef]
- Avinesh, P.; Jaison, T.J.; Sajith, A.M.; Nagaswarupa, H.P.; Muralidharan, A. Facile synthesis of fully decorated imidazo[4,5-b] and imidazo[4,5-c] pyridines in aqueous DMF via C-H activation under microwave irradiation. Chem. Sel. 2016, 1, 2265–2270. [Google Scholar] [CrossRef]
- Baladi, T.; Granzhan, A.; Piguel, S. C-H Activation microwave-sssisted C-2 direct alkenylation of imidazo [4,5-b]pyridines: Access to fluorescent purine isosteres with remarkably large stokes shifts. Eur. J. Org. Chem. 2016, 2421–2434. [Google Scholar] [CrossRef]
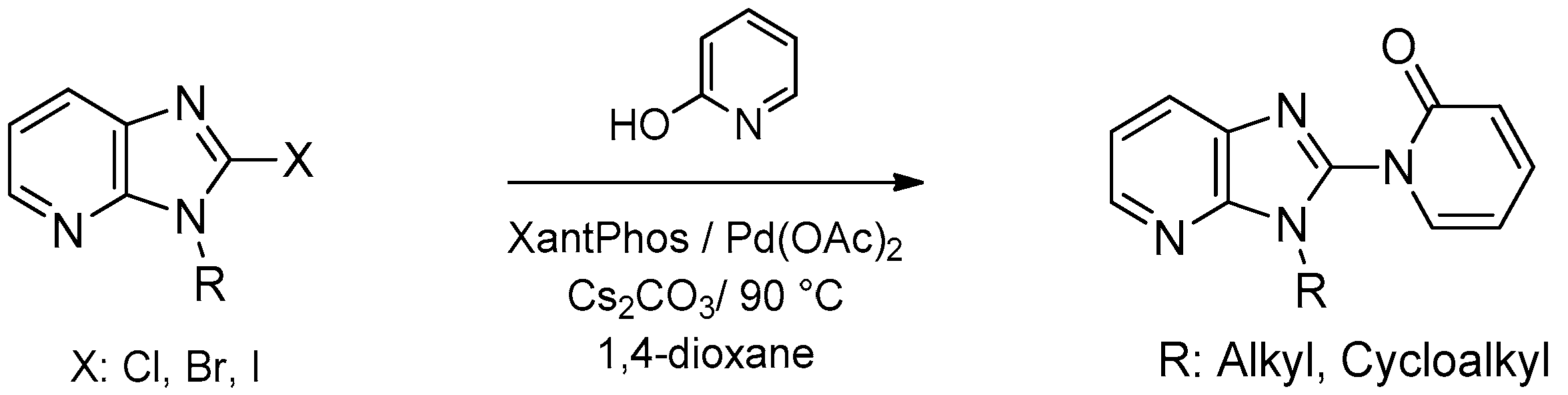
- Khader, K.K.A.; Sajith, A.M.; Padusha, M.S.A.; Nagaswarupa, H.P.; Muralidharan, A. Regioselective synthesis of C-2 substituted imidazo[4,5-c]pyridines utilizing palladium catalysed C-N bond forming reactions with enolizable heterocycles. Tetrahedron Lett. 2014, 55, 1778–1783. [Google Scholar] [CrossRef]
- Mujumdar, P.; Grkovic, T.; Krasavin, M. A simple two-step access to diversely substituted imidazo[4,5-b]pyridines and benzimidazoles from readily available 2-imidazolines. Tetrahedron Lett. 2013, 54, 3336–3340. [Google Scholar] [CrossRef]
- Li, C.; Chen, L.; Steinhuebel, D.; Goodman, A. Rapid construction of imidazopyridines from ortho -haloaminopyridines. Tetrahedron Lett. 2016, 57, 2708–2712. [Google Scholar] [CrossRef]
- Salomé, C.; Schmitt, M.; Bourguignon, J.-J. Rapid synthesis of imidazo[4,5-b]pyridine containing polycyclics by means of palladium-catalyzed amidation of 2-chloro-3-nitropyridine. Tetrahedron Lett. 2009, 50, 3798–3800. [Google Scholar] [CrossRef]
- Wang, F.; Tran-Dubé, M.; Scales, S.; Johnson, S.; McAlpine, I.; Ninkovic, S. A simple and convenient two-step, one-pot synthesis of hetero- imidazoles from nitroaminoaryls catalyzed by Ytterbium triflate. Tetrahedron Lett. 2013, 54, 4054–4057. [Google Scholar] [CrossRef]
- Harer, S.L.; Bhatia, M.S. In-silico docking based design and synthesis of [1H,3H]imidazo[4,5-b]pyridines as lumazine synthase inhibitors for their effective antimicrobial activity. J. Pharm. Bioallied Sci. 2014, 6, 285–296. [Google Scholar] [CrossRef] [PubMed]
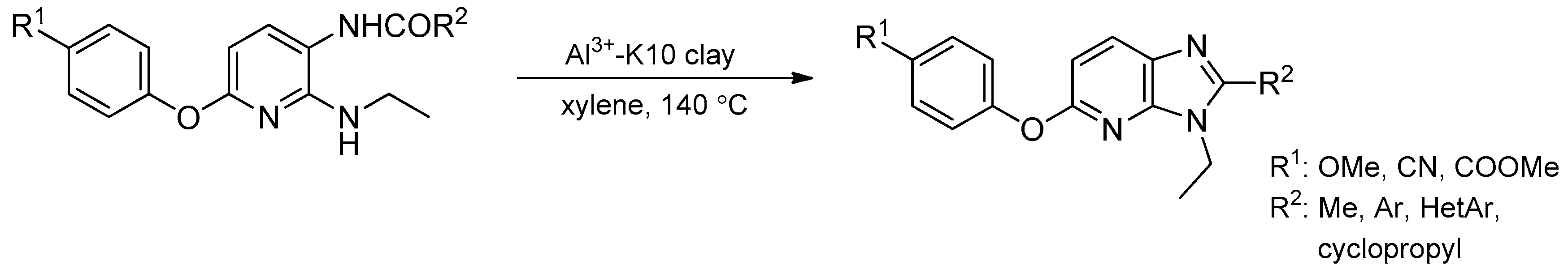
- Suresh, D.; Dhakshinamoorthy, A.; Kanagaraj, K.; Pitchumani, K. Synthesis of 2-substituted 3-ethyl-3H-imidazo[4,5-b] pyridines catalyzed by Al3+-exchanged K10 clay as solid acids. Tetrahedron Lett. 2013, 54, 6479–6484. [Google Scholar] [CrossRef]
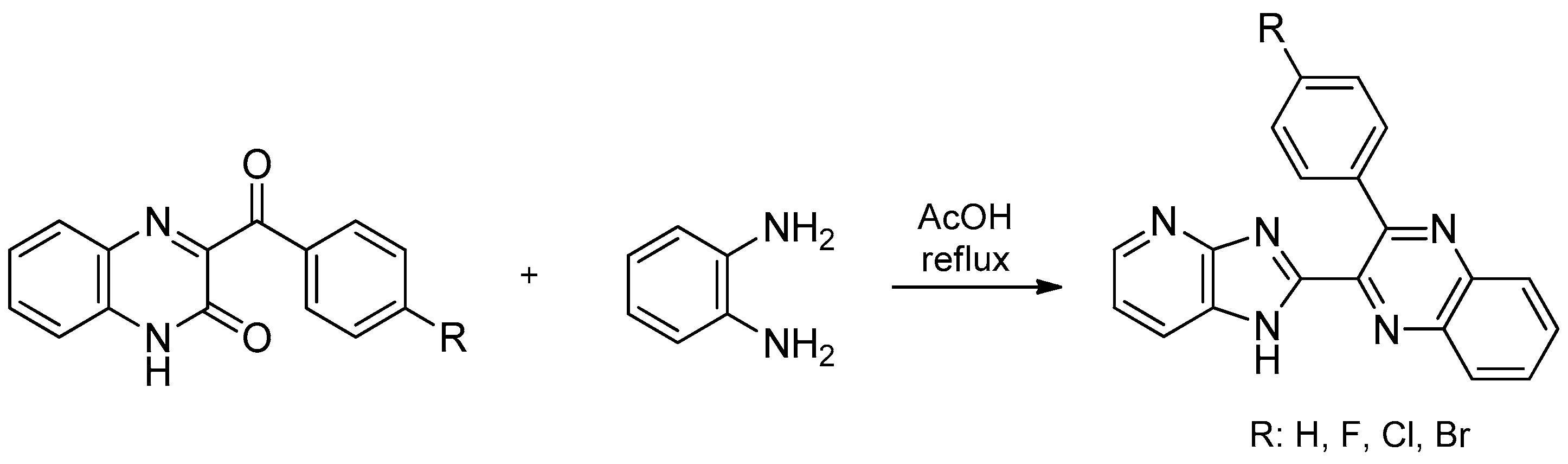
- Mamedov, V.A.; Zhukova, N.A.; Beschastnova, T.N.; Gubaidullin, A.T.; Balandina, A.A.; Latypov, S.K. A reaction for the synthesis of benzimidazoles and 1H-imidazo[4,5-b]pyridines via a novel rearrangement of quinoxalinones and their aza-analogues when exposed to 1,2-arylenediamines. Tetrahedron 2010, 66, 9745–9753. [Google Scholar] [CrossRef]
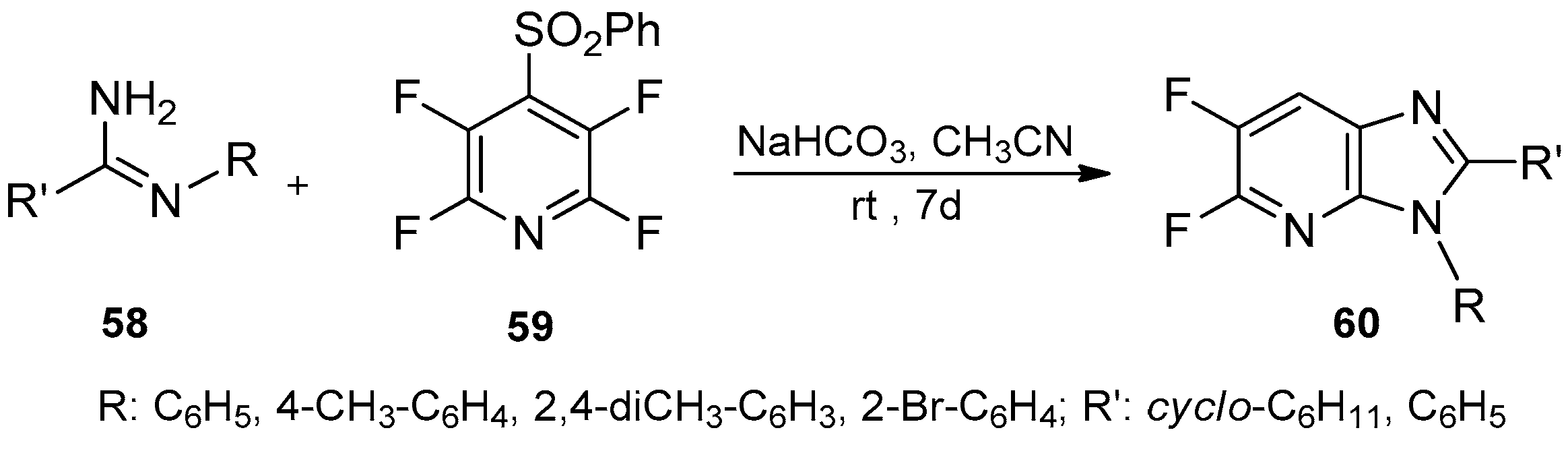
- Poorfreidoni, A.; Ranjbar-Karimi, R. Synthesis of substituted imidazopyridines from perfluorinated pyridine derivatives. Tetrahedron Lett. 2016, 57, 5781–5783. [Google Scholar] [CrossRef]
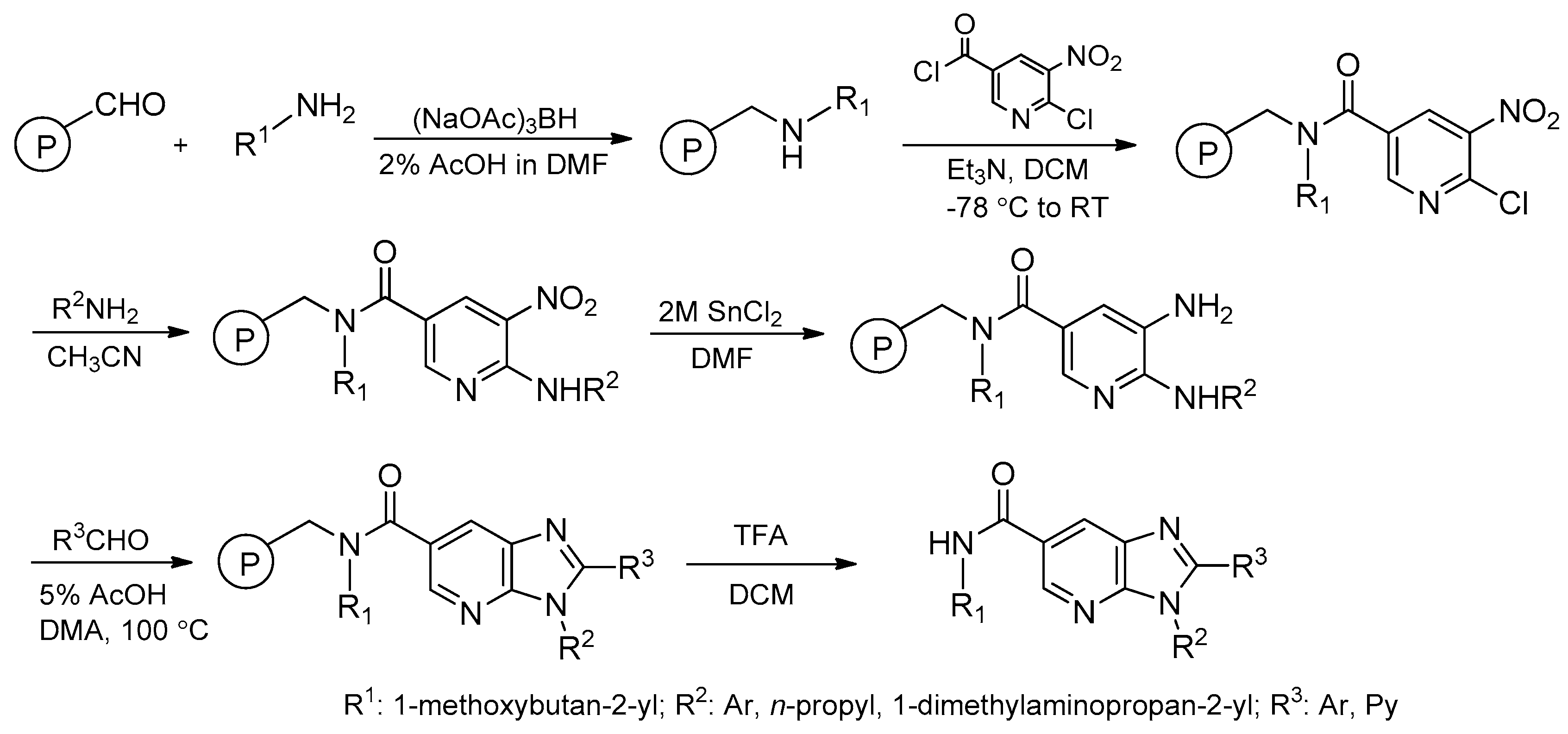
- Farrant, E.; Rahman, S.S. A solid-phase synthetic route to substituted 7-azabenzimidazoles suitable for combinatorial library synthesis. Tetrahedron Lett. 2000, 41, 5383–5386. [Google Scholar] [CrossRef]












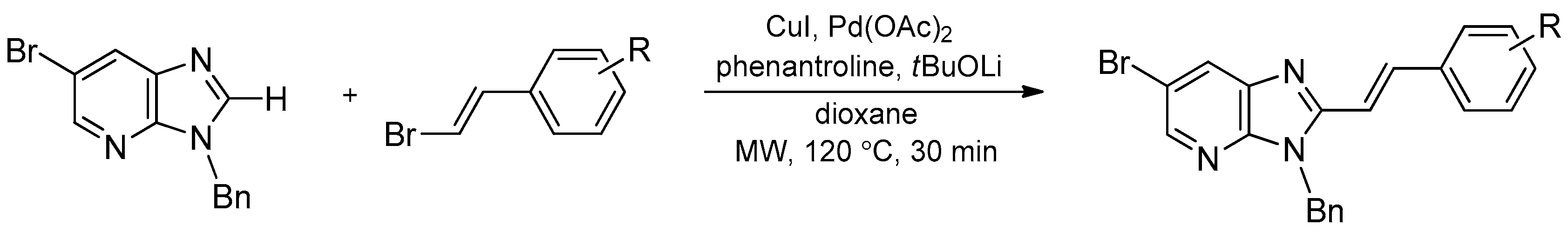






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krause, M.; Foks, H.; Gobis, K. Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives. Molecules 2017, 22, 399. https://doi.org/10.3390/molecules22030399
Krause M, Foks H, Gobis K. Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives. Molecules. 2017; 22(3):399. https://doi.org/10.3390/molecules22030399
Chicago/Turabian StyleKrause, Malwina, Henryk Foks, and Katarzyna Gobis. 2017. "Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives" Molecules 22, no. 3: 399. https://doi.org/10.3390/molecules22030399




