Prevention of Bacterial Contamination of a Silica Matrix Containing Entrapped β-Galactosidase through the Action of Covalently Bound Lysozymes
Abstract
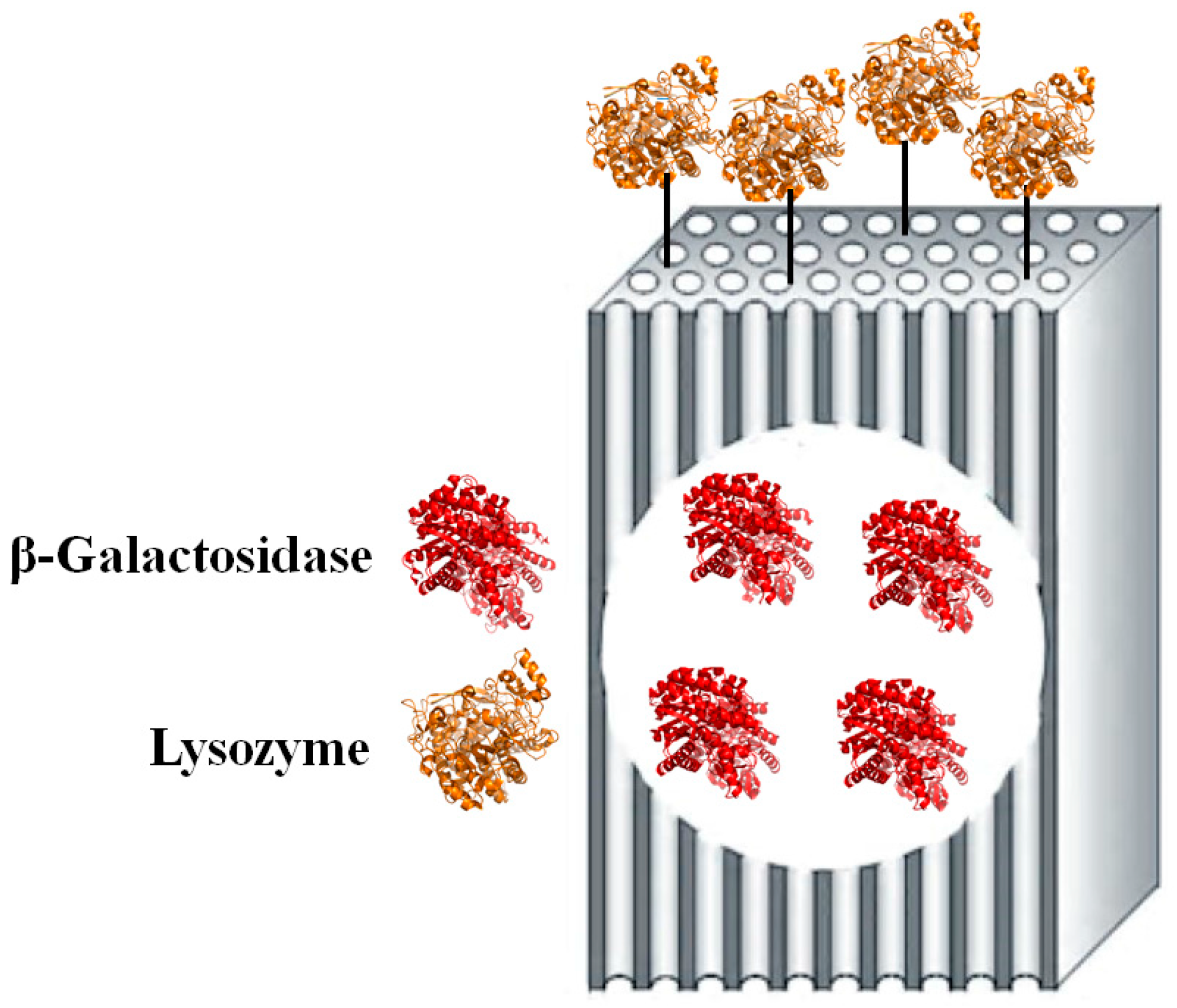
:1. Introduction
2. Results and Discussion
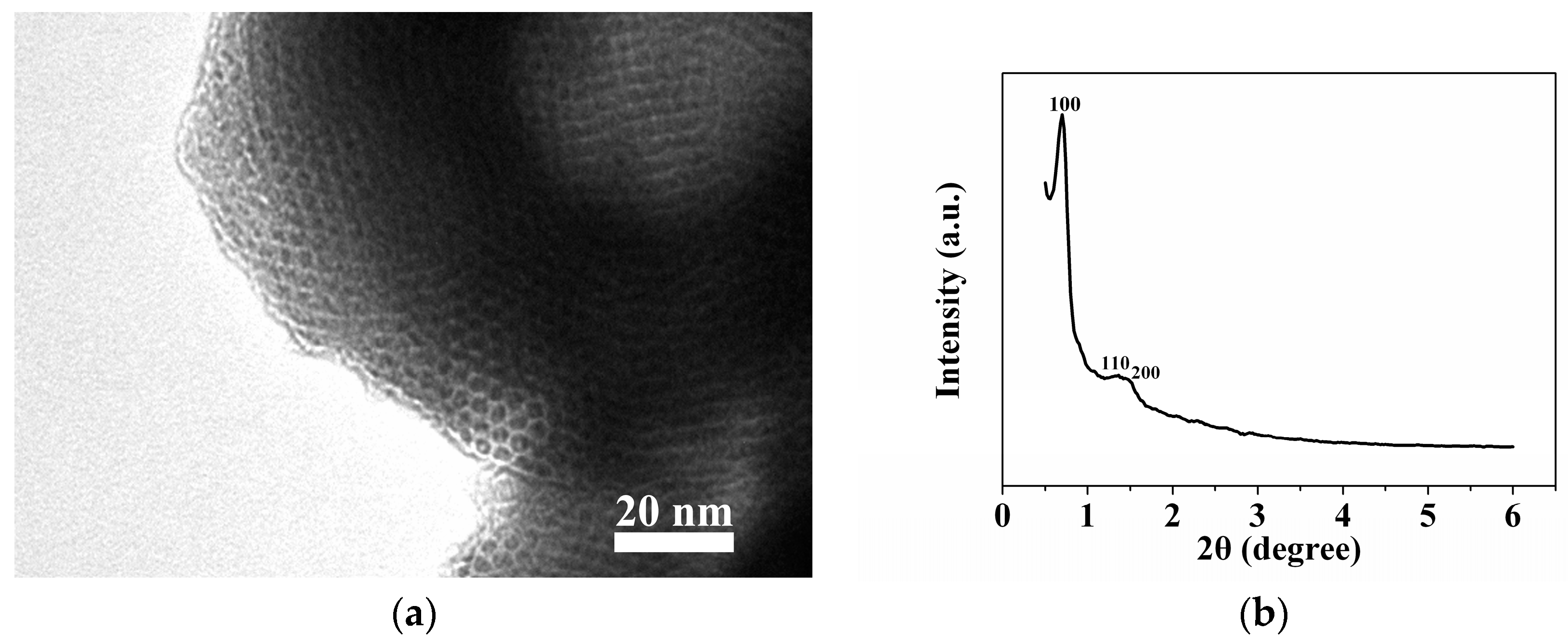
2.1. Sample Characterization by TEM and XRD
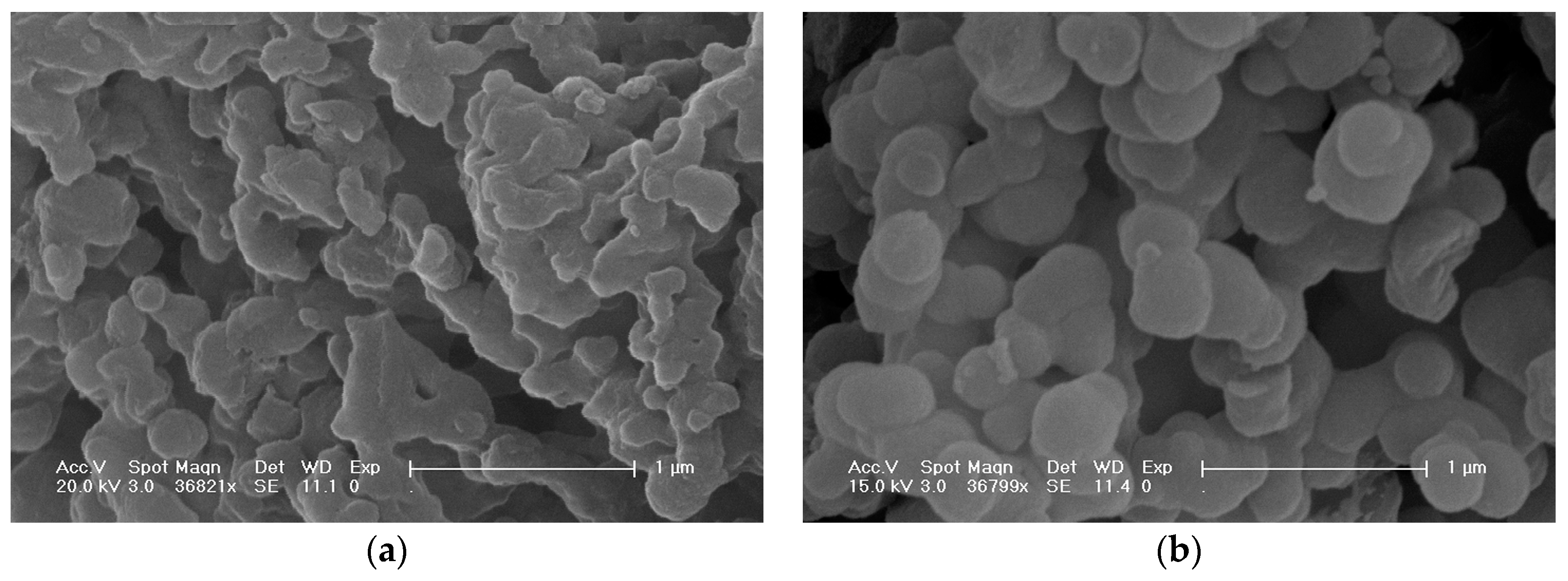
2.2. Sample Characterization by SEM
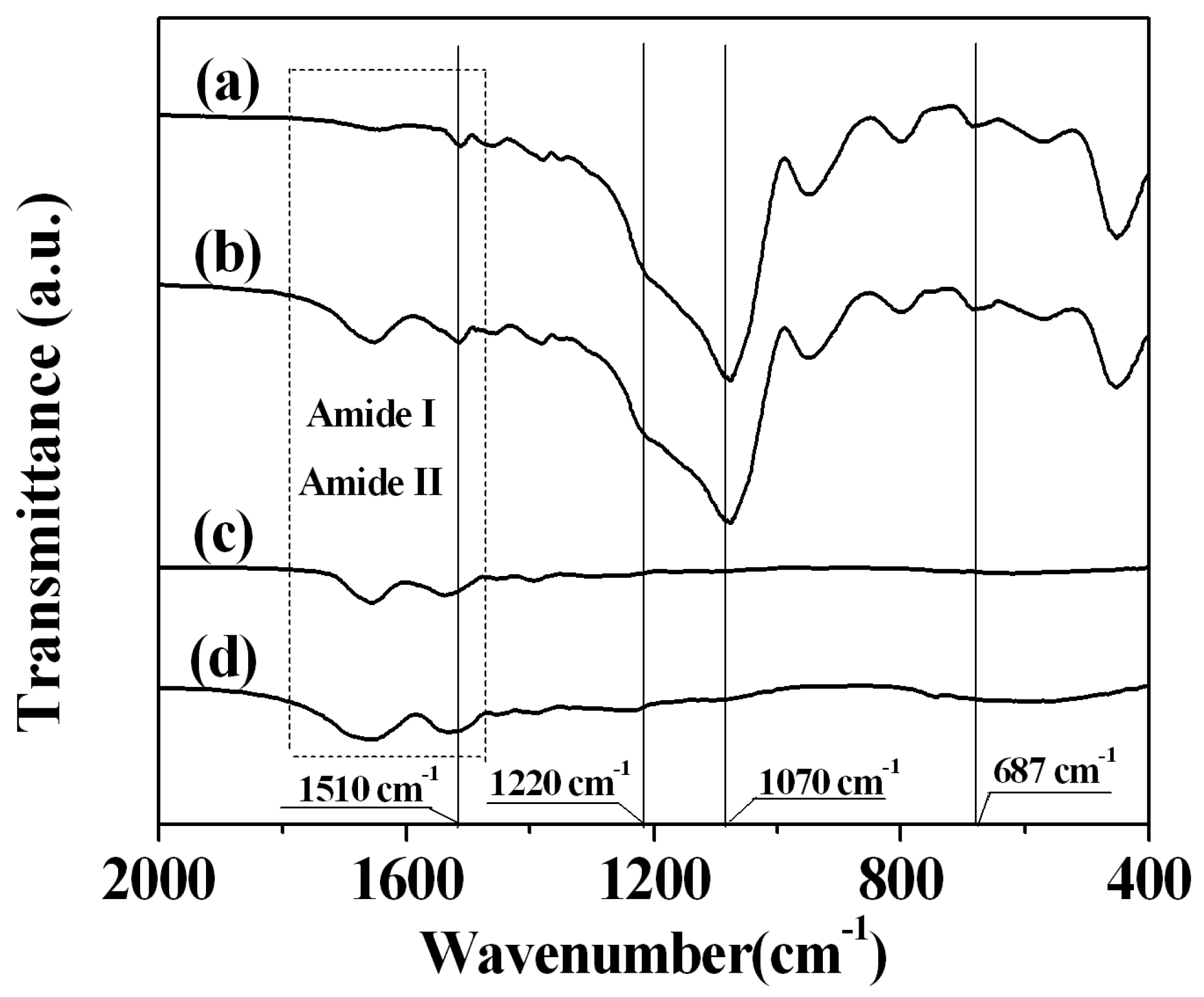
2.3. Sample Characterization by FTIR Spectra
2.4. The Activities of the Co-Immobilised Enzymes
2.5. The Operational Stability of the Co-Immobilised Enzymes
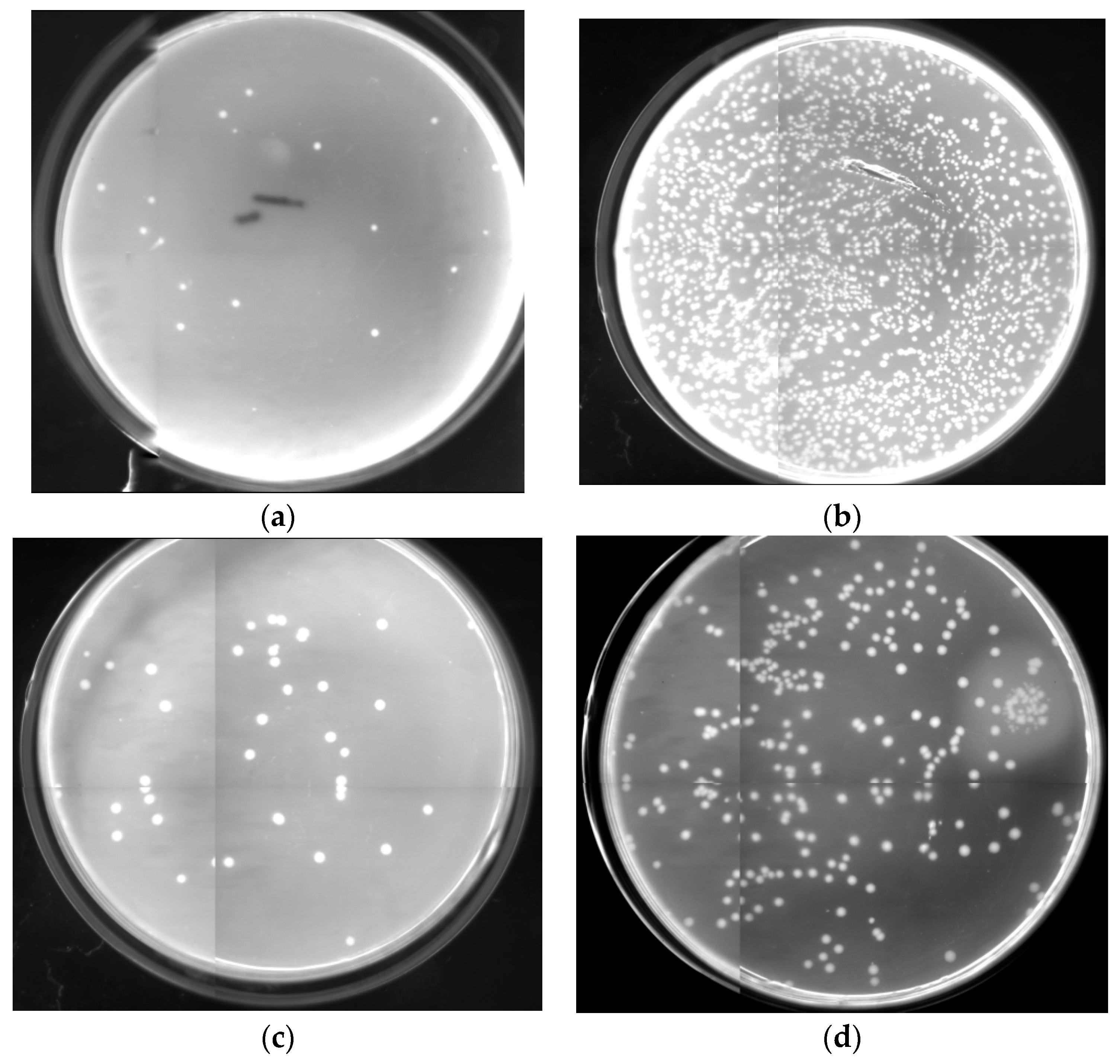
2.6. Antibacterial Activity Assessments
2.7. The Properties of the Co-Immobilised Enzymes in Milk
3. Materials and Methods
3.1. Material
3.2. Modified “Fish-in-Net” Encapsulation
3.3. Glutaraldehyde Coupling Procedure
3.4. Characterisation of the Matrix
3.5. The Catalytic Behaviour of the Co-Immobilised Enzymes
3.5.1. Lactose Assay
3.5.2. Antimicrobial Activity of Bound Lysozyme
3.5.3. Antimicrobial and Lactose Hydrolysis Assays in Milk
3.6. Reusability
3.7. Antimicrobial Tests
3.8. The Storage Stability of the Co-Immobilised Enzymes
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suarez, F.; Savaiano, D.; Levitt, M. Review article the treatment of lactose intolerance. Aliment. Pharmacol. Ther. 1995, 9, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.C.; Jeon, B.J.; Ahn, J.; Kwak, H.S. In vitro study of microencapsulated isoflavone and β-galactosidase. J. Agric. Food Chem. 2006, 54, 2582–2586. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, K.; Martínez-Gil, A.; Moreno-Simunovic, Y.; Illanes, A.; Wilson, L. Aroma release in wine using co-immobilized enzyme aggregates. Molecules 2016, 21, 1485. [Google Scholar] [CrossRef] [PubMed]
- Benavente, R.; Pessela, B.; Curiel, J.; de las Rivas, B.; Muñoz, R.; Guisán, J.; Mancheño, J.; Cardelle-Cobas, A.; Ruiz-Matute, A.; Corzo, N. Improving properties of a novel β-galactosidase from Lactobacillus plantarum by covalent immobilization. Molecules 2015, 20, 7874. [Google Scholar] [CrossRef] [PubMed]
- Peirce, S.; Tacias-Pascacio, V.; Russo, M.; Marzocchella, A.; Virgen-Ortíz, J.; Fernandez-Lafuente, R. Stabilization of Candida antarctica lipase b (calb) immobilized on octyl agarose by treatment with polyethyleneimine (pei). Molecules 2016, 21, 751. [Google Scholar] [CrossRef] [PubMed]
- Rueda, N.; Albuquerque, T.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Torres, R.; Ortiz, C.; dos Santos, J.; Barbosa, O.; Fernandez-Lafuente, R. Reversible immobilization of lipases on heterofunctional octyl-amino agarose beads prevents enzyme desorption. Molecules 2016, 21, 646. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.C.S.D.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the support properties for immobilization or purification of enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Wu, Z.; Dong, M.; Lu, M.; Li, Z. Encapsulation of β-galactosidase from Aspergillus oryzae based on “fish-in-net” approach with molecular imprinting technique. J. Mol. Catal. B Enzym. 2010, 63, 75–80. [Google Scholar] [CrossRef]
- Oliver, S.P.; Jayarao, B.M.; Almeida, R.A. Foodborne pathogens in milk and the dairy farm environment: Food safety and public health implications. Foodborne Pathog. Dis. 2005, 2, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Chlebicki, J.; Węgrzyńska, J.; Maliszewska, I.; Oświęcimska, M. Preparation, surface-active properties, and antimicrobial activities of bis-quaternary ammonium salts from amines and epichlorohydrin. J. Surfactants Deterg. 2005, 8, 227–232. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, Y.-S.; Shin, D.-H. Antimicrobial synergistic effect of linolenic acid and monoglyceride against Bacillus cereus and Staphylococcus aureus. J. Agric. Food Chem. 2002, 50, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Ortega, P.; Copa-Patiño, J.L.; Muñoz-Fernandez, M.A.; Soliveri, J.; Gomez, R.; de la Mata, F.J. Amine and ammonium functionalization of chloromethylsilane-ended dendrimers. Antimicrobial activity studies. Org. Biomol. Chem. 2008, 6, 3264–3269. [Google Scholar] [CrossRef] [PubMed]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. Coli as a model for gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, T. Antibacterial and bacterium adsorbing macromolecules. Macromol. Mater. Eng. 2001, 286, 63–87. [Google Scholar] [CrossRef]
- Nonaka, T.; Noda, E.; Kurihara, S. Graft copolymerization of vinyl monomers bearing positive charges or episulfide groups onto loofah fibers and their antibacterial activity. J. Appl. Polym. Sci. 2000, 77, 1077–1086. [Google Scholar] [CrossRef]
- Engel, Y.; Schiffman, J.D.; Goddard, J.M.; Rotello, V.M. Nanomanufacturing of biomaterials. Mater. Today 2012, 15, 478–485. [Google Scholar] [CrossRef]
- Smid, E.J.; Gorris, L.G. Natural antimicrobials for food preservation. In Food Science and Technology-New York-Marcel Dekker; Springer: New York, NY, USA, 1999; pp. 285–308. [Google Scholar]
- Ibrahim, H.R.; Matsuzaki, T.; Aoki, T. Genetic evidence that antibacterial activity of lysozyme is independent of its catalytic function. FEBS Lett. 2001, 506, 27–32. [Google Scholar] [CrossRef]
- Proctor, V.A.; Cunningham, F.; Fung, D.Y. The chemistry of lysozyme and its use as a food preservative and a pharmaceutical. Crit. Rev. Food Sci. Nutr. 1988, 26, 359–395. [Google Scholar] [CrossRef] [PubMed]
- Germaine, G.R.; Tellefson, L. Potential role of lysozyme in bactericidal activity of in vitro-acquired salivary pellicle against Streptococcus faecium 9790. Infect. Immun. 1986, 54, 846–854. [Google Scholar] [PubMed]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-enzymatic cascade reactions: Overview and perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Van Oers, M.; Rutjes, F.; Van Hest, J. Cascade reactions in nanoreactors. Curr. Opin. Biotechnol. 2014, 28, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-L.; Palomec, L.; Wheeldon, I. Design and analysis of enhanced catalysis in scaffolded multienzyme cascade reactions. ACS Catal. 2014, 4, 505–511. [Google Scholar] [CrossRef]
- Lopez-Gallego, F.; Schmidt-Dannert, C. Multi-enzymatic synthesis. Curr. Opin. Chem. Biol. 2010, 14, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lafuente, R.; Rodriguez, V.; Guisán, J.M. The coimmobilization of d-amino acid oxidase and catalase enables the quantitative transformation of d-amino acids (d-phenylalanine) into α-keto acids (phenylpyruvic acid). Enzyme Microb. Technol. 1998, 23, 28–33. [Google Scholar] [CrossRef]
- Hernandez, K.; Berenguer-Murcia, A.; Rodrigues, C.R.; Fernandez-Lafuente, R. Hydrogen peroxide in biocatalysis. A dangerous liaison. Curr. Org. Chem. 2012, 16, 2652–2672. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Rivas, B.D.L.; Muñoz, R.; Guisán, J.M.; López-Gallego, F. Rational co-immobilization of bi-enzyme cascades on porous supports and their applications in bio-redox reactions with in situ recycling of soluble cofactors. ChemCatChem 2012, 4, 1279–1288. [Google Scholar] [CrossRef]
- Peirce, S.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Rueda, N.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Russo, M.E.; Marzocchella, A.; Fernandez-Lafuente, R. Development of simple protocols to solve the problems of enzyme coimmobilization. Application to coimmobilize a lipase and a β-galactosidase. RSC Adv. 2016, 6, 61707–61715. [Google Scholar] [CrossRef]
- Bavaro, T.; Cattaneo, G.; Serra, I.; Benucci, I.; Pregnolato, M.; Terreni, M. Immobilization of neutral protease from Bacillus subtilis for regioselective hydrolysis of acetylated nucleosides: Application to capecitabine synthesis. Molecules 2016, 21, 1621. [Google Scholar] [CrossRef] [PubMed]
- Rivero, C.; Palomo, J. Covalent immobilization of Candida rugosa lipase at alkaline pH and their application in the regioselective deprotection of per-o-acetylated thymidine. Catalysts 2016, 6, 115. [Google Scholar] [CrossRef]
- Jian, H.; Wang, Y.; Bai, Y.; Li, R.; Gao, R. Site-specific, covalent immobilization of dehalogenase st2570 catalyzed by formylglycine-generating enzymes and its application in batch and semi-continuous flow reactors. Molecules 2016, 21, 895. [Google Scholar] [CrossRef] [PubMed]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.-T.; Meyer, A. Enzymatic cellulose hydrolysis: Enzyme reusability and visualization of β-glucosidase immobilized in calcium alginate. Molecules 2014, 19, 19390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weetall, H.H. Immobilized enzymes. Analytical applications. Anal. Chem. 1974, 46, 602a–615a. [Google Scholar] [PubMed]
- Wang, M.; Shi, H.; Wu, D.; Han, H.; Zhang, J.; Xing, Z.; Wang, S.; Li, Q. Glutaraldehyde cross-linking of immobilized thermophilic esterase on hydrophobic macroporous resin for application in poly(ε-caprolactone) synthesis. Molecules 2014, 19, 9838. [Google Scholar] [CrossRef] [PubMed]
- Betancor, L.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Mateo, G.D.-O.C.; Fernández-Lafuente, R.; Guisán, J.M. Different mechanisms of protein immobilization on glutaraldehyde activated supports: Effect of support activation and immobilization conditions. Enzyme Microb. Technol. 2006, 39, 877–882. [Google Scholar] [CrossRef]
- Wang, X.; Lin, K.S.; Chan, J.C.; Cheng, S. Direct synthesis and catalytic applications of ordered large pore aminopropyl-functionalized sba-15 mesoporous materials. J. Phys. Chem. B 2005, 109, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.N.; Shimizu, K.; Zhou, Y.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D.; Morse, D.E. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, M.B.; Sandhage, K.H.; Naik, R.R. Protein- and peptide-directed syntheses of inorganic materials. Chem. Rev. 2008, 108, 4935–4978. [Google Scholar] [CrossRef] [PubMed]
- Nassif, N.; Livage, J. From diatoms to silica-based biohybrids. Chem. Soc. Rev. 2011, 40, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, N.; Sumper, M.; Kroger, N. Biosilica formation in diatoms: Characterization of native silaffin-2 and its role in silica morphogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 12075–12080. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, A.; Samanta, S.; Mal, N.K. Highly active disordered extra large pore titanium silicate. Microporous Mesoporous Mater. 2004, 68, 29–35. [Google Scholar] [CrossRef]
- Chen, Y.; Han, J.; Zhang, H. Structure and acid–base properties of surface-modified mesoporous silica. Appl. Surf. Sci. 2007, 253, 9400–9406. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lin, T.-S.; Mou, C.-Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Virgen-Ortíz, J.; Pedrero, S.; Fernandez-Lopez, L.; Lopez-Carrobles, N.; Gorines, B.; Otero, C.; Fernandez-Lafuente, R. Desorption of lipases immobilized on octyl-agarose beads and coated with ionic polymers after thermal inactivation. Stronger adsorption of polymers/unfolded protein composites. Molecules 2017, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Abaházi, E.; Lestál, D.; Boros, Z.; Poppe, L. Tailoring the spacer arm for covalent immobilization of Candida antarctica lipase b—Thermal stabilization by bisepoxide-activated aminoalkyl resins in continuous-flow reactors. Molecules 2016, 21, 767. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Z.; Guan, B.; Wang, X.; Zhang, Y.; Xiao, Y.; Zhi, B.; Liu, Y.; Li, Z.; Huo, Q. Improving the properties of β-galactosidase from Aspergillus oryzae via encapsulation in aggregated silica nanoparticles. New J. Chem. 2013, 37, 3793–3797. [Google Scholar] [CrossRef]
- Salazar, O.; Asenjo, J.A. Enzymatic lysis of microbial cells. Biotechnol. Lett. 2007, 29, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Alm, L. Effect of fermentation on lactose, glucose, and galactose content in milk and suitability of fermented milk products for lactose intolerant individuals. J. Dairy Sci. 1982, 65, 346–352. [Google Scholar] [CrossRef]
- Paige, D.M.; Bayless, T.M.; Huang, S.S.; Wexler, R. Lactose hydrolyzed milk. Am. J. Clin. Nutr. 1975, 28, 818–822. [Google Scholar] [PubMed]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the porous structure of sba-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shugar, D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim. Biophys. Acta 1952, 8, 302–309. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
| Sample | Specific Activity (U/mg) |
|---|---|
| Lysozyme | |
| Free lysozyme | 10784 |
| Immobilised lysozyme without β-galactosidase | 10380 |
| Immobilised lysozyme entrapped by β-galactosidase | 10385 |
| β-galactosidase | |
| Free β-galactosidase | 390 |
| Encapsulated β-galactosidase before covalent binding of lysozyme | 566 |
| Encapsulated β-galactosidase after covalent binding of lysozyme | 563 |
| Batch | Immobilised Lysozyme | Encapsulated β-Galactosidase | ||
|---|---|---|---|---|
| Specific Activity (U/g Support) | Residual Activity (%) | Specific Activity (U/g Support ) | Residual Activity (%) | |
| 1 | 1.45 × 106 | 100.0% | 58,864.0 | 100.0% |
| 2 | 1.45 × 106 | 100.0% | 58,864.0 | 100.0% |
| 3 | 1.44 × 106 | 99.3% | 58,864.0 | 100.0% |
| 4 | 1.44 × 106 | 99.3% | 58,864.0 | 100.0% |
| 5 | 1.43 × 106 | 98.6% | 58,864.0 | 100.0% |
| 6 | 1.43 × 106 | 98.6% | 58,801.6 | 99.9% |
| 7 | 1.42 × 106 | 97.9% | 58,801.6 | 99.9% |
| 8 | 1.41 × 106 | 97.2% | 58,801.6 | 99.9% |
| 9 | 1.41 × 106 | 97.2% | 58,749.6 | 99.8% |
| 10 | 1.40 × 106 | 96.6% | 58,749.6 | 99.8% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, S.; Tian, P.; Wu, Z.; Li, Z. Prevention of Bacterial Contamination of a Silica Matrix Containing Entrapped β-Galactosidase through the Action of Covalently Bound Lysozymes. Molecules 2017, 22, 377. https://doi.org/10.3390/molecules22030377
Li H, Li S, Tian P, Wu Z, Li Z. Prevention of Bacterial Contamination of a Silica Matrix Containing Entrapped β-Galactosidase through the Action of Covalently Bound Lysozymes. Molecules. 2017; 22(3):377. https://doi.org/10.3390/molecules22030377
Chicago/Turabian StyleLi, Heng, Shuai Li, Pu Tian, Zhuofu Wu, and Zhengqiang Li. 2017. "Prevention of Bacterial Contamination of a Silica Matrix Containing Entrapped β-Galactosidase through the Action of Covalently Bound Lysozymes" Molecules 22, no. 3: 377. https://doi.org/10.3390/molecules22030377






