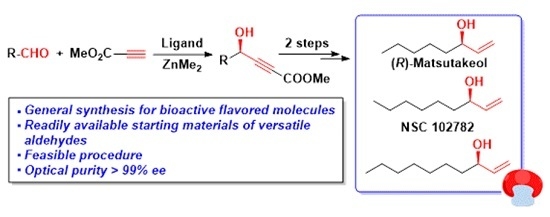
A General Asymmetric Synthesis of (R)-Matsutakeol and Flavored Analogs
Abstract
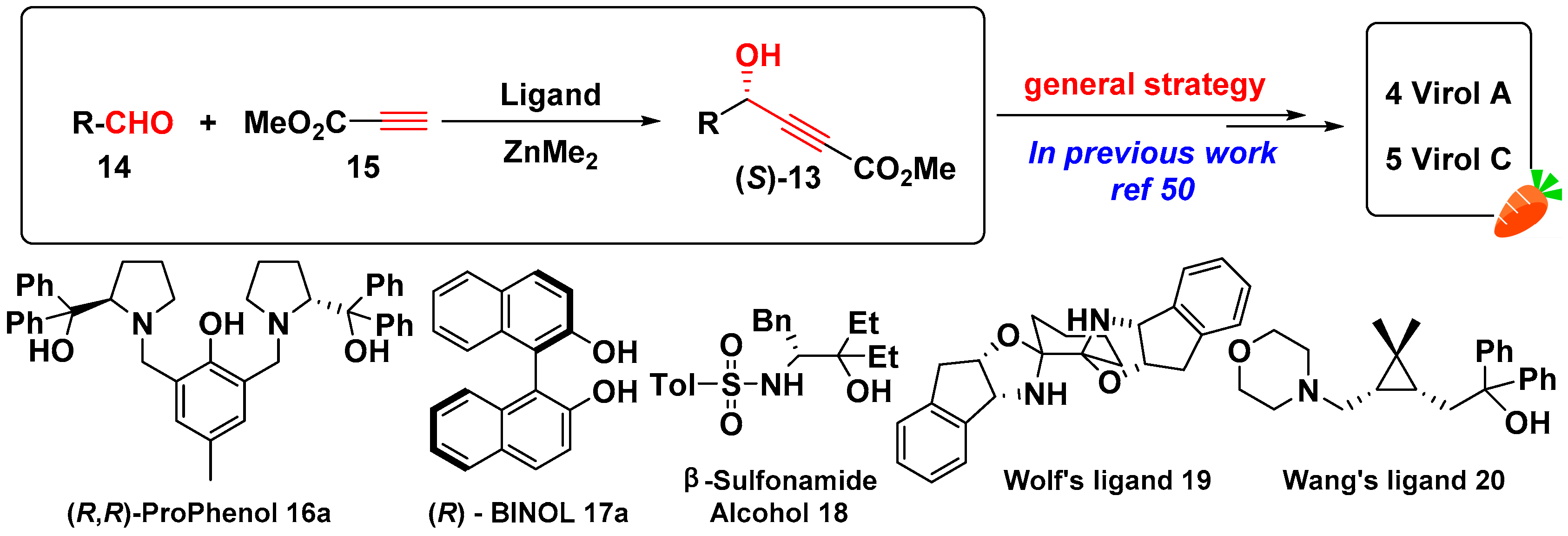
:1. Introduction
2. Results and Discussion
3. Materials and Methods
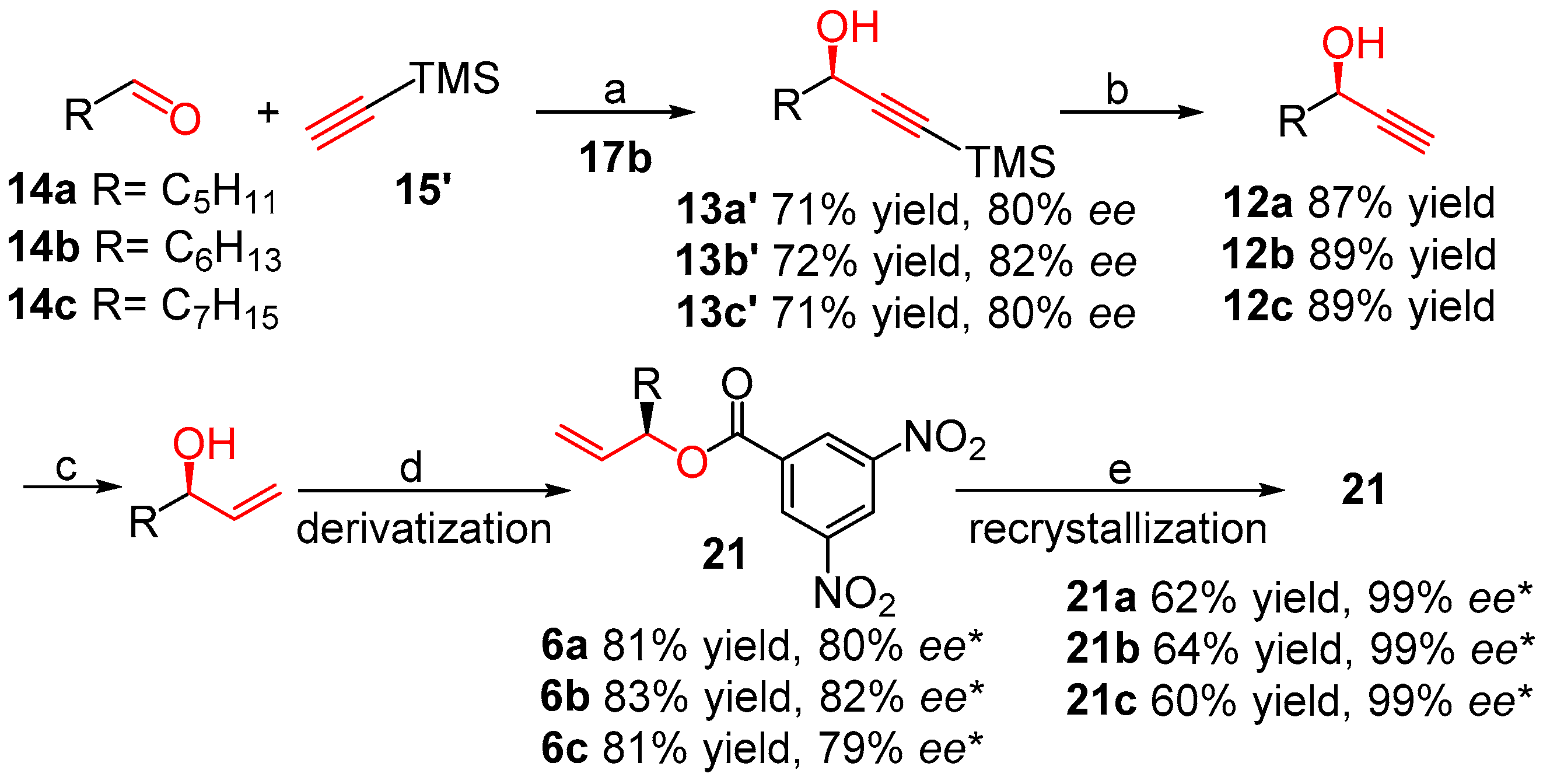
3.1. General Procedure of Asymmetric Addition of Methyl Propiolate to Aliphatic Aldehydes (Table 1)
3.1.1. Synthesis of (R)-Methyl-4-hydroxynon-2-ynoate: (R)-13a
3.1.2. Synthesis of (R)-Methyl-4-hydroxydec-2-ynoate: (R)-13b
3.1.3. Synthesis of (R)-Methyl-4-hydroxyundec-2-ynoate: (R)-13c
3.1.4. Synthesis of (R)-Methyl-4-hydroxypent-2-ynoate: (R)-13d
3.1.5. Synthesis of Methyl (R)-Methyl-4-hydroxyhex-2-ynoate: (R)-13e
3.1.6. Synthesis of (R)-Methyl-4-hydroxyhept-2-ynoate: (R)-13f
3.1.7. Synthesis of (R)-Methyl-4-hydroxyoct-2-ynoate: (R)-13g
3.1.8. Synthesis of (R)-Methyl-4-hydroxy-5-methylhex-2-ynoate: (R)-13h
3.1.9. Synthesis of (R)-Methyl-4-hydroxyoct-7-en-2-ynoate: (R)-13i
3.2. General Procedure for the Synthesis of Chiral Alkynols 12 from Propargyl Alcohols 13
3.2.1. Synthesis of (R)-Oct-1-yn-3-ol: (R)-12a
3.2.2. Synthesis of (R)-Non-1-yn-3-ol: (R)-12b
3.2.3. Synthesis of (R)-Dec-1-yn-3-ol: (R)-12c
3.3. General Procedure for the Selective Reduction of the Chiral Alkynols 12
3.3.1. Synthesis of (R)-Oct-1-en-3-ol: (R)-6a
3.3.2. Synthesis of (R)-Non-1-en-3-ol: (R)-6b
3.3.3. Synthesis of (R)-Dec-1-en-3-ol: (R)-6c
3.4. General Procedure for the Esterification Reaction and Determination of Enantiomeric Excess by HPLC
3.4.1. Synthesis of (R)-Oct-1-en-3-yl-3,5-dinitrobenzoate: (R)-21a
3.4.2. Synthesis of (R)-Non-1-en-3-yl-3,5-dinitrobenzoate: (R)-21b
3.4.3. Synthesis of (R)-Dec-1-en-3-yl-3,5-dinitrobenzoate: (R)-21c
3.5. General Procedure of Asymmetric Addition of Ethynyltrimethylsilane to Aliphatic Aldehydes
3.5.1. Synthesis of (R)-1-(Trimethylsilyl)oct-1-yn-3-ol: (R)-13a’
3.5.2. Synthesis of (R)-1-(Trimethylsilyl)non-1-yn-3-ol: (R)-13b’
3.5.3. Synthesis of (R)-1-(Trimethylsilyl)dec-1-yn-3-ol: (R)-13c’
3.6. General Procedure for the Synthesis of chIral Alkynols 12 from Propargyl Alcohols 13’
3.6.1. Synthesis of (R)-Oct-1-yn-3-ol: (R)-12a
3.6.2. Synthesis of (R)-Non-1-yn-3-ol: (R)-12b
3.6.3. Synthesis of (R)-Dec-1-yn-3-ol: (R)-12c
3.7. General Procedure for the Selective Reduction of the Chiral Alkynols 12
3.7.1. Synthesis of (R)-Oct-1-en-3-ol: (R)-6a
3.7.2. Synthesis of (R)-Non-1-en-3-ol: (R)-6b
3.7.3. Synthesis of (R)-Dec-1-en-3-ol: (R)-6c
3.8. General Procedure for the Esterification Reaction and Determination of Enantiomeric Excess by HPLC
3.8.1. Synthesis of (R)-Oct-1-en-3-yl-3,5-dinitrobenzoate: (R)-21a
3.8.2. Synthesis of (R)-Non-1-en-3-yl-3,5-dinitrobenzoate: (R)-21b
3.8.3. Synthesis of (R)-Dec-1-en-3-yl-3,5-dinitrobenzoate: (R)-21c
3.9. General Procedure for the Recrystallization to Improve Optical Purity
3.9.1. Recrystallization of (R)-Oct-1-en-3-yl-3,5-dinitrobenzoate: (R)-21a
3.9.2. Recrystallization of (R)-Non-1-en-3-yl-3,5-dinitrobenzoate: (R)-21b
3.9.3. Recrystallization of (R)-Dec-1-en-3-yl-3,5-dinitrobenzoate: (R)-21c
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Negri, R. Polyacetylenes from terrestrial plants and fungi: Recent phytochemical and biological advances. Fitoterapia 2015, 106, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Konovalov, D.A. Polyacetylene compounds of plants of the Asteraceae Family (Review). Pharm. Chem. J. 2014, 48, 613–631. [Google Scholar] [CrossRef]
- Finimundy, T.C.; Dillon, A.J.P.; Henriques, J.A.P.; Ely, M.R. A review on general nutritional compounds and pharmacological properties of the Lentinula edodes mushroom. Food Nutr. Sci. 2014, 5, 1095–1105. [Google Scholar] [CrossRef]
- Swift, K.A.D. Current Topics in Flavors and Fragrances: Towards a New Millennium of Discovery; Springer Science + Business Media B.V.: GX Dordrecht, The Netherlands, 1999; p. 235. [Google Scholar]
- Mizuno, T. Bioactive biomolecules of mushrooms: Food function and medicinal effect of mushroom fungi. Food Rev. Int. 1995, 11, 7–21. [Google Scholar] [CrossRef]
- Murahashi, S. The odor of matsutake (Armillaria matsutake Ito et Imai Agaricaceae). II. Sci. Papers Inst. Phys. Chem. Res. 1938, 34, 155–172. [Google Scholar]
- Hung, R.; Lee, S.; Bennett, J.W. The effects of low concentrations of the enantiomers of mushroom alcohol (1-octen-3-ol) on Arabidopsis thaliana. Mycology 2014, 5, 73–80. [Google Scholar] [CrossRef] [PubMed]
- McMeniman, C.J.; Corfas, R.A.; Matthews, B.J.; Ritchie, S.A.; Vosshall, L.B. Multimodal Integration of Carbon Dioxide and Other Sensory Cues Drives Mosquito Attraction to Humans. Cell 2014, 156, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Sikuljak, T.; Mazuir, F.; Arevalo, A.; Menon, A. Pesticidal Mixture Comprising a Carboxamide Compound and a Biopesticide. WO2016142456 A1, 10 March 2016. [Google Scholar]
- Combet, E.; Henderson, J.; Eastwood, D.C.; Burton, K.S. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Helmchen, G.; Ihrig, K.; Schindler, H. EPC-syntheses via asymmetric Diels-Alder reactions/retro Diels-Alder reactions. I. (R)- and (S)-Matsutake alcohol, (R)- and (S)-sarcomycin methyl ester. Tetrahedron Lett. 1987, 28, 183–186. [Google Scholar] [CrossRef]
- Takano, S.; Yanase, M.; Takahashi, M.; Ogasawara, K. Enantiodivergent synthesis of both enantiomers of sulcatol and matsutake alcohol from (R)-epichlorohydrin. Chem. Lett. 1987, 16, 2017–2020. [Google Scholar] [CrossRef]
- Kitamura, M.; Kasahara, I.; Manabe, K.; Noyori, R.; Takaya, H. Kinetic resolution of racemic allylic alcohols by BINAP-ruthenium(II) catalyzed hydrogenation. J. Org. Chem. 1988, 53, 708–710. [Google Scholar] [CrossRef]
- Oppolzer, W.; Radinov, R.N. Enantioselective synthesis of sec-allyl alcohols by catalytic asymmetric addition of divinylzinc to aldehydes. Tetrahedron Lett. 1988, 29, 5645–5648. [Google Scholar] [CrossRef]
- Kusuda, S.; Ueno, Y.; Toru, T. Vinyl anion equivalent V. Asymmetric synthesis of allylic alcohols using chiral 2-(trialkylsilyl)ethyl sulfoxides. Tetrahedron 1994, 50, 1045–1062. [Google Scholar] [CrossRef]
- Bisogno, F.R.; Rioz-Martinez, A.; Rodriguez, C.; Lavandera, I.; de Gonzalo, G.; Torres Pazmino, D.E.; Fraaije, M.W.; Gotor, V. Oxidoreductases Working Together: Concurrent Obtaining of Valuable Derivatives by Employing the PIKAT Method. ChemCatChem 2010, 2, 946–949. [Google Scholar] [CrossRef]
- Rej, R.K.; Nanda, S. Chemoenzymic Asymmetric Total Synthesis of Nonanolide (Z)-Cytospolides D, E and Their Stereoisomers. Eur. J. Org. Chem. 2014, 2014, 860–871. [Google Scholar] [CrossRef]
- Rej, R.K.; Kumar, R.; Nanda, S. Asymmetric synthesis of cytospolide C and cytospolide D through successful exploration of stereoselective Julia-Kocienski olefination and Suzuki reaction followed by macrolactonization. Tetrahedron 2015, 71, 3185–3194. [Google Scholar] [CrossRef]
- Lee, W.H.; Bae, I.H.; Kim, B.M.; Seu, Y.-B. New Synthetic Approach to Optically Activate Matsutakeol, the Major Flavor Component of Tricholoma matsutake, from L-Tartaric Acid. Bull. Korean Chem. Soc. 2016, 37, 1910–1911. [Google Scholar] [CrossRef]
- Huang, J.; Wei, S.; Wang, L.; Zhang, C.; Li, S.; Liu, P.; Du, X.; Wang, Q. Highly enantioselective catalytic methyl propiolate addition to both aromatic and aliphatic aldehydes. Tetrahedron Asymmetry 2016, 27, 428–435. [Google Scholar] [CrossRef]
- Funes-Maldonado, M.; Sieng, B.; Amedjkouh, M. Enabling Asymmetric Alkynylation of Azaaryl Aldehydes with Soai Autocatalyst. Eur. J. Org. Chem. 2015, 2015, 4081–4086. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Li, S.-N.; Zhong, J.-C.; Zhou, Y.; Zeng, H.-Z.; Duan, H.-J.; Bian, Q.-H.; Wang, M. Total synthesis of each enantiomer of falcarinol and panaxjapyne A via asymmetric catalytic alkynylation of an aldehyde. Tetrahedron Asymmetry 2015, 26, 361–366. [Google Scholar] [CrossRef]
- Trost, B.M.; Quintard, A. Asymmetric Catalytic Alkynylation of Acetaldehyde: Application to the Synthesis of (+)-Tetrahydropyrenophorol. Angew. Chem. Int. Ed. 2012, 51, 6704–6708. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Burns, A.C.; Bartlett, M.J.; Tautz, T.; Weiss, A.H. Thionium Ion Initiated Medium-Sized Ring Formation: The Total Synthesis of Asteriscunolide D. J. Am. Chem. Soc. 2012, 134, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Bartlett, M.J.; Weiss, A.H.; von Wangelin, A.J.; Chan, V.S. Development of Zn-ProPhenol-Catalyzed Asymmetric Alkyne Addition: Synthesis of Chiral Propargylic Alcohols. Chem. Eur. J. 2012, 18, 16498–16509. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Bartlett, M.J. Transition-Metal-Catalyzed Synthesis of Aspergillide B: An Alkyne Addition Strategy. Org. Lett. 2012, 14, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Na, R.; Wang, B.; Liu, H.; Bian, Q.; Zhong, J.; Wang, M.; Guo, H. Synthesis and application of novel dithiane alcohol ligand based on chiral cyclopropane-backbone for asymmetric ethylation of aromatic aldehydes. Lett. Org. Chem. 2012, 9, 622–627. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Wang, L.; Yu, X.-Q.; Huang, D.-S.; Pu, L. Synthesis of a C1 symmetric BINOL-terpyridine ligand and highly enantioselective methyl propiolate addition to aromatic aldehydes. Tetrahedron 2010, 66, 1990–1993. [Google Scholar] [CrossRef]
- Ito, J.-I.; Asai, R.; Nishiyama, H. Asymmetric Direct Alkynylation Catalyzed by Chiral Ru-Bis(oxazolinyl)phenyl Complexes. Org. Lett. 2010, 12, 3860–3862. [Google Scholar] [CrossRef] [PubMed]
- Turlington, M.; DeBerardinis, A.M.; Pu, L. Highly Enantioselective Catalytic Alkyl Propiolate Addition to Aliphatic Aldehydes. Org. Lett. 2009, 11, 2441–2444. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhao, Q.; Li, A.N.; Ren, F.; Yang, F.; Wang, R. Enantioselective synthesis of Anomala osakana pheromone and Janus integer pheromone: A flexible approach to chiral γ-butyrolactones. Org. Biomol. Chem. 2009, 7, 3663–3665. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wang, Q.; Lin, L.; Liu, X.; Jiang, X.; Zhao, Q.; Hu, G.; Wang, R. Highly enantioselective addition of terminal alkynes to aldehydes catalyzed by a new chiral β-sulfonamide alcohol/Ti(OiPr)4/Et2Zn/R3N catalyst system. Chirality 2009, 21, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.-C.; Hou, S.-C.; Bian, Q.-H.; Yin, M.-M.; Na, R.-S.; Zheng, B.; Li, Z.-Y.; Liu, S.-Z.; Wang, M. Highly enantioselective zinc/amino alcohol-catalyzed alkynylation of aldehydes. Chem. Eur. J. 2009, 15, 3069–3071. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Turlington, M.; Yu, X.-Q.; Pu, L. 3,3′-Anisyl-Substituted BINOL, H4BINOL, and H8BINOL Ligands: Asymmetric Synthesis of Diverse Propargylic Alcohols and Their Ring-Closing Metathesis to Chiral Cycloalkenes. J. Org. Chem. 2009, 74, 8681–8689. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, N.; Ding, Z.; Wang, T.; Mao, J.; Zhang, Y. L-Proline-derived tertiary amino alcohol as a new chiral ligand for enantioselective alkynylation of aldehydes. Tetrahedron Lett. 2009, 50, 926–929. [Google Scholar] [CrossRef]
- Yang, F.; Xi, P.; Yang, L.; Lan, J.; Xie, R.; You, J. Facile, Mild, and Highly Enantioselective Alkynylzinc Addition to Aromatic Aldehydes by BINOL/N-Methylimidazole Dual Catalysis. J. Org. Chem. 2007, 72, 5457–5460. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Jiang, X.; Liu, W.; Qiu, L.; Xu, Z.; Xu, J.; Chan, A.S.C.; Wang, R. Highly Enantioselective Synthesis of γ-Hydroxy-α,β-acetylenic Esters Catalyzed by a β-Sulfonamide Alcohol. Org. Lett. 2007, 9, 2329–2332. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Weiss, A.H. Catalytic Enantioselective Synthesis of Adociacetylene B. Org. Lett. 2006, 8, 4461–4464. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Weiss, A.H.; von Wangelin, A.J. Dinuclear Zn-Catalyzed Asymmetric Alkynylation of Unsaturated Aldehydes. J. Am. Chem. Soc. 2006, 128, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Wang, Q.; Yu, X.-Q.; Xie, R.-G.; Pu, L. Highly enantioselective synthesis of γ-hydroxy-α,β-acetylenic esters by asymmetric alkyne addition to aldehydes. Angew. Chem. Int. Ed. 2006, 45, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Liu, S. Bisoxazolidine-catalyzed enantioselective alkynylation of aldehydes. J. Am. Chem. Soc. 2006, 128, 10996–10997. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, D.P.G.; Hems, W.P.; Davis, B.G. Carbohydrate-Derived Amino-Alcohol Ligands for Asymmetric Alkynylation of Aldehydes. Org. Lett. 2006, 8, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, P.G.; Rudolph, J.; Bolm, C.; Norrby, P.-O.; Tomasini, C. Me2Zn-Mediated Addition of Acetylenes to Aldehydes and Ketones. J. Org. Chem. 2005, 70, 5733–5736. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Yamada, K.-i.; Tomioka, K. Catalytic asymmetric addition of terminal alkynes to aldehydes mediated by (1R,2R)-2-(dimethylamino)-1,2-diphenylethanol. Adv. Synth. Catal. 2005, 347, 1649–1652. [Google Scholar] [CrossRef]
- Jiang, B.; Si, Y.-G. The first highly enantioselective alkynylation of chloral: A practical and efficient pathway to chiral trichloromethyl propargyl alcohols. Adv. Synth. Catal. 2004, 346, 669–674. [Google Scholar] [CrossRef]
- Dahmen, S. Enantioselective Alkynylation of Aldehydes Catalyzed by [2.2]Paracyclophane-Based Ligands. Org. Lett. 2004, 6, 2113–2116. [Google Scholar] [CrossRef] [PubMed]
- Stefani, H.A.; Menezes, P.H.; Costa, I.M.; Silva, D.O.; Petragnani, N. Use of chiral sulfoxide in the asymmetric synthesis of (+)-virol C. Synlett 2002, 8, 1335–1337. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, Z.; Xiong, W. Highly enantioselective alkynylation of aldehydes catalyzed by a readily available chiral amino alcohol-based ligand. Chem. Commun. (Cambridge, UK) 2002, 14, 1524–1525. [Google Scholar] [CrossRef]
- Rotticci, D.; Haeffner, F.; Orrenius, C.; Norin, T.; Hult, K. Molecular recognition of sec-alcohol enantiomers by Candida antarctica lipase B. J. Mol. Catal. B Enzym. 1998, 5, 267–272. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.-L.; Guo, X.-R.; Zhou, L.; Wang, Y.; Duan, Y.-N.; Wang, M.-Z.; Na, R.-S.; Yu, B. A general strategy toward the total synthesis of C17 polyacetylenes virols A and C. Tetrahedron 2016, 72, 6603–6610. [Google Scholar] [CrossRef]
- Morandi, S.; Palate, F.; Benvenuti, S.; Prati, F. Total synthesis of a dienynone from Echinacea pallida. Tetrahedron 2008, 64, 6324–6328. [Google Scholar] [CrossRef]
- Zheng, M.; Wu, F.; Chen, K.; Zhu, S. Styrene as 4π-Component in Zn (II)-Catalyzed Intermolecular Diels–Alder/Ene Tandem Reaction. Org. Lett. 2016, 18, 3554–3557. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.S.; Elliott, R.; Elliott, J.D. Asymmetric synthesis via acetal templates. 4. Reactions with silylacetylenic compounds. Formation of chiral propargylic alcohols. J. Am. Chem. Soc. 1983, 105, 2904–2905. [Google Scholar] [CrossRef]
- Jiang, X.; Fu, C.; Ma, S. Highly stereoselective iodolactonization of 4,5-allenoic acids—An efficient synthesis of 5-(1′-iodo-1′(Z)-alkenyl)-4,5-dihydro-2(3H)-furanones. Chemistry 2008, 14, 9656–9664. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Chen, J.; Guo, L.-C.; Ye, J.-L.; Ruan, Y.-P.; Huang, P.-Q. Asymmetric Total Synthesis of (−)-Awajanomycin. Org. Lett. 2009, 11, 5242–5245. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Nakayama, J.; Toru, T. Asymmetric Reduction of α-(Trimethylsilyl)methyl-β-ketosulfoxide with DIBAL under Basic Conditions. J. Org. Chem. 2003, 68, 5766–5768. [Google Scholar] [CrossRef] [PubMed]
- Bessodes, M.; Abushanab, E.; Antonakis, K. Enantiospecific synthesis of the immunopotentiators erythro-9-(2-hydroxy-3-nonyl)hypoxanthines and the threo-diastereomers. Tetrahedron Lett. 1984, 25, 5899–5902. [Google Scholar] [CrossRef]
- Gartner, M.; Mader, S.; Seehafer, K.; Helmchen, G. Enantio- and Regioselective Iridium-Catalyzed Allylic Hydroxylation. J. Am. Chem. Soc. 2011, 133, 2072–2075. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of (R)-Matsutakeol and compounds 13a–i are available from the authors.




| Entry | Ligand | Ligand/% | Temp/°C | Yield/% c | ee/% d |
|---|---|---|---|---|---|
| 1 a | 16b | 20 | −10 | 70 | 75 |
| 2 a | 16b | 40 | −10 | 72 | 78 |
| 3 a | 16b | 40 | 25 | 74 | 76 |
| 4 b | 17b | 40 | −10 | -- | -- |
| 5 b | 17b | 20 | 25 | 52 | 70 |
| 6 b | 17b | 40 | 25 | 71 | 80 |
| 7 b | 17b | 60 | 25 | 67 | 81 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, H.; Zheng, C.; Lu, S.; Guo, X.; Yin, X.; Na, R.; Yu, B.; Wang, M. A General Asymmetric Synthesis of (R)-Matsutakeol and Flavored Analogs. Molecules 2017, 22, 364. https://doi.org/10.3390/molecules22030364
Liu J, Li H, Zheng C, Lu S, Guo X, Yin X, Na R, Yu B, Wang M. A General Asymmetric Synthesis of (R)-Matsutakeol and Flavored Analogs. Molecules. 2017; 22(3):364. https://doi.org/10.3390/molecules22030364
Chicago/Turabian StyleLiu, Jia, Honglian Li, Chao Zheng, Shichao Lu, Xianru Guo, Xinming Yin, Risong Na, Bin Yu, and Min Wang. 2017. "A General Asymmetric Synthesis of (R)-Matsutakeol and Flavored Analogs" Molecules 22, no. 3: 364. https://doi.org/10.3390/molecules22030364