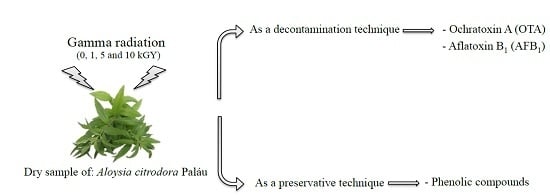
Is Gamma Radiation Suitable to Preserve Phenolic Compounds and to Decontaminate Mycotoxins in Aromatic Plants? A Case-Study with Aloysia citrodora Paláu
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Safety Considerations
3.2. Samples and Sample Preparation
3.3. Spiking with Mycotoxins
3.4. Irradiation Treatment
3.5. Phenolic Compounds Analysis
3.6. Cytotoxicity Evaluation in Porcine Liver Cells
3.7. Mycotoxin Analysis
3.7.1. Aflatoxin Extraction and Quantification
3.7.2. Ochratoxin A Extraction and Determination
3.7.3. In-House Method Validation
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lubbe, A.; Verpoortea, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Skotti, E.; Anastasaki, E.; Kanellou, G.; Polissiou, M.; Tarantilis, P.A. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind. Crops Prod. 2014, 53, 46–54. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Wan Ainiza, W.M.; Jinap, S.; Sanny, M. Simultaneous determination of aflatoxins and ochratoxin A in single and mixed spices. Food Control 2015, 50, 913–918. [Google Scholar] [CrossRef]
- The International Agency for Research on Cancer (IARC). Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; IARC Monographs on Evaluation of Carcinogenic Risk to Humans; IARC: Lyons, France, 2002. [Google Scholar]
- Rodrigues, P.; Venâncio, A.; Lima, N. Mycobiota and mycotoxins of almonds and chestnuts with special reference to aflatoxins. Food Res. Int. 2012, 48, 76–90. [Google Scholar] [CrossRef]
- Lee, D.; Lyu, J.; Lee, K.-G. Analysis of aflatoxins in herbal medicine and health functional foods. Food Control 2015, 48, 33–36. [Google Scholar] [CrossRef]
- Romagnoli, B.; Menna, V.; Gruppioni, N.; Bergamini, C. Aflatoxins in spices, aromatic herbs, herb-teas and medicinal plants marketed in Italy. Food Control 2007, 18, 697–701. [Google Scholar] [CrossRef]
- Harris, J.P.; Mantle, P.G. Biosynthesis of ochratoxins by Aspergillus ochraceus. Phytochemistry 2001, 58, 709–716. [Google Scholar] [CrossRef]
- Majeed, S.; Iqbal, M.; Asi, M.R.; Iqbal, S.Z. Aflatoxins and ochratoxin A contamination in rice, corn and corn products from Punjab, Pakistan. J. Cereal Sci. 2013, 58, 446–450. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Beszterda, M.; Bocianowski, J.; Golińnski, P. Natural occurrence of fumonisins and ochratoxin A in some herbs and spices commercialized in Poland analyzed by UPLCeMS/MS method. Food Microbiol. 2013, 36, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Ashiq, S.; Hussain, M.; Ahmad, B. Natural occurrence of mycotoxins in medicinal plants: A review. Fungal Genet. Biol. 2014, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Marín, S.; Sanchis, V.; Ramos, A.J. Screening of mycotoxin multi contamination in medicinal and aromatic herbs sampled in Spain. J. Sci. Food Agric. 2009, 89, 1802–1807. [Google Scholar] [CrossRef]
- Mizani, M.; Sheikh, N.; Ebrahimi, S.N.; Gerami, A.; Tavakoli, F.A. Effect of gamma irradiation on physico-mechanical properties of spice packaging films. Radiat. Phys Chem. 2009, 78, 806–809. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. Effects of radiation processing on phytochemicals and antioxidants in plant produce. Trends Food Sci. Technol. 2009, 20, 201–212. [Google Scholar] [CrossRef]
- Byun, M.W.; Yook, H.S.; Kim, K.S.; Chung, C.K. Effects of gamma irradiation on physiological effectiveness of Korean medicinal herbs. Radiat. Phys. Chem. 1999, 54, 291–300. [Google Scholar] [CrossRef]
- Sádecká, J. Irradiation of spices—A review. Czech J. Food Sci. 2007, 25, 231–242. [Google Scholar]
- Nagy, T.O.; Solar, S.; Sontag, G.; Koenig, J. Identification of phenolic components in dried spices and influence of irradiation. Food Chem. 2011, 128, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Mohácsi-Farkas, C. History and future of food irradiation. Trends Food Sci. Technol. 2011, 22, 121–126. [Google Scholar] [CrossRef]
- Directive 1999/3/EC of the European Parliament and of the Council of 22 February 1999 on the establishment of a Community list of foods and food ingredients treated with ionising radiation. Off. J. Eur. Communities 1999, L66, 24–25.
- Pereira, E.; Antonio, A.L.; Barreira, J.C.M.; Barros, L.; Bento, A.; Ferreira, I.C.F.R. Gamma irradiation as a practical alternative to preserve the chemical and bioactive wholesomeness of widely used aromatic plants. Food Res. Int. 2015, 67, 338–348. [Google Scholar] [CrossRef]
- Bilia, A.R.; Giomi, M.; Innocenti, M.; Gallori, S.; Vincieri, F.F. HPLC–DAD–ESI–MS analysis of the constituents of aqueous preparations of verbena and lemon verbena and evaluation of the antioxidant activity. J. Pharm. Biomed. Anal. 2008, 46, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Funes, L.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. J. Chromatogr. A 2009, 1216, 5391–5397. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Characterization of phenolic and other polar compounds in a lemon verbena extract by capillary electrophoresis-electrospray ionization-mass spectrometry. J. Sep. Sci. 2010, 33, 2818–2827. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Grevenstuk, T.; Martins, N.; Romano, A. Antioxidant activity and verbascoside content in extracts from two uninvestigated endemic Plantago spp. Ind. Crops Prod. 2015, 65, 198–202. [Google Scholar] [CrossRef]
- Pereira, E.; Barros, L.; Antonio, A.L.; Cabo Verde, S.; Santos-Buelga, C.; Ferreira, I.C.F.R. Infusion from Thymus vulgaris L. treated at different gamma radiation doses: Effects on antioxidant activity and phenolic composition. LWT Food Sci. Technol. 2016, 74, 34–39. [Google Scholar] [CrossRef]
- Ramabulana, T.; Mavunda, R.D.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A.; Madala, N.E. Secondary metabolite perturbations in Phaseolus vulgaris leaves due to gamma radiation. Plant Physiol. Biochem. 2015, 97, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ramabulana, T.; Mavunda, R.D.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A.; Madala, N.E. Perturbation of pharmacologically relevant polyphenolic compounds in Moringa oleifera against photo-oxidative damages imposed by gamma radiation. J. Photochem. Photobiol. B 2016, 156, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Pimenta, A.I.; Calhelha, R.C.; Antonio, A.L.; Cabo Verde, S.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Effects of gamma irradiation on cytotoxicity and phenolic compounds of Thymus vulgaris L. and Mentha x piperita L. LWT Food Sci. Technol. 2016, 71, 370–377. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Communities 2006, L70, 12–34. [Google Scholar]
- Aziz, N.H.; Moussa, L.A.A. Reduction of fungi and mycotoxins formation in seeds by gamma-radiation. J. Food Saf. 2004, 24, 109–127. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Bhatti, I.A.; Asi, M.R.; Zuber, M.; Shahid, M.; Parveen, I. Effect of γ-irradiation on fungal load and aflatoxins reduction in red chillies. Radiat. Phys. Chem. 2003, 82, 80–84. [Google Scholar] [CrossRef]
- Jalili, M.; Jinap, S.; Noranizan, A. Effect of gamma radiation on reduction of mycotoxins in black pepper. Food Control 2010, 21, 1388–1393. [Google Scholar] [CrossRef]
- Jalili, M.; Jinap, S.; Noranizan, M.A. Aflatoxins and ochratoxin a reduction in black and white pepper by gamma radiation. Radiat. Phys. Chem. 2012, 81, 1786–1788. [Google Scholar] [CrossRef]
- Prado, G.; de Carvalho, E.P.; Oliveira, M.S.; Madeira, J.G.C.; Morais, V.D.; Correa, R.F.; Cardoso, V.N.; Soares, T.V.; da Silva, J.F.M.; Goncalves, R.C.P. Effect of gamma irradiation on the inactivation of aflatoxin B1 and fungal flora in peanut. Braz. J. Microbiol. 2003, 34, 138–140. [Google Scholar] [CrossRef]
- Akueche, E.C.; Anjorin, S.T.; Harcourt, B.I.; Kana, D.; Adeboye, E.; Shehu, I.; Akande, R.; Adeleke, A.T.; Shonowo, O.A.; Adesanmi, C.A. Studies on fungal load, total aflatoxins and ochratoxin A contents of gamma-irradiated and non-irradiated Sesamum indicum grains from Abuja markets, Nigeria. Kasetsart J. Nat. Sci. 2012, 46, 371–382. [Google Scholar]
- Herzallah, S.; Alshawabkeh, K.; Al Fataftah, A. Aflatoxin decontamination of artificially contaminated feeds by sunlight, gamma-radiation, and microwave heating. J. Appl. Poult Res. 2008, 17, 515–521. [Google Scholar] [CrossRef]
- Hooshmand, H.; Kloperstein, C.F. Effects of gamma irradiation on mycotoxin disappearance and amino acid contents of corn, wheat, and soybeans with different moisture contents. Plant Foods Hum. Nutr. 1995, 47, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kunwar, A.; Gautam, S.; Sharma, A. Inactivation of A. ochraceus spores and detoxification of ochratoxin A in coffee beans by gamma irradiation. J. Food Sci. 2012, 77, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Vita, D.S.; Rosa, P.; Giuseppe, A. Effect of Gamma Irradiation on Aflatoxins and Ochratoxin A Reduction in Almond Samples. J. Food Res. 2014, 3, 113–118. [Google Scholar] [CrossRef]
- Calado, T.; Venâncio, A.; Abrunhosa, L. Irradiation for mold and mycotoxin control: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1049–1061. [Google Scholar] [CrossRef] [Green Version]
- Rustom, I.Y.S. Aflatoxin in food and feed: Occurrence, legislation and inactivation by physical methods. Food Chem. 1997, 59, 57–67. [Google Scholar] [CrossRef]
- Fan, X.; Sokorai, K.J.B. Retention of quality and nutritional value of 13 fresh-cut vegetables treated with low-dose radiation. J. Food Sci. 2008, 73, S367–S372. [Google Scholar] [CrossRef] [PubMed]
- Koike, A.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Villavicencio, A.L.C.H.; Ferreira, I.C.F.R. Irradiation as a novel approach to improve quality of Tropaeolum majus L. flowers: Benefits in phenolic profiles and antioxidant activity. Innov. Food Sci. Emerg. Technol. 2015, 30, 138–144. [Google Scholar] [CrossRef]
- Rezende, A.C.B.; Igarashi, M.C.; Destro, M.T.; Franco, B.D.G.M.; Landgraf, M. Effect of Gamma Radiation on the Reduction of Salmonella strains, Listeria monocytogenes, and Shiga Toxin-Producing Escherichia coli and Sensory Evaluation of Minimally Processed Spinach (Tetragonia expansa). J. Food Prot. 2014, 10, 1768–1772. [Google Scholar] [CrossRef] [PubMed]
- Castegnaro, M.; Hunt, S.C.; Sansone, E.B.; Schuller, P.L.; Siriwardana, B.G.; Telling, G.M.; van Egmond, H.P.; Walker, E.A. Laboratory decontamination and destruction of aflatoxins B1, B2, G1 and G2 in laboratory wastes. In IARC Publications No. 37; International Agency for Research on Cancer: Lyon, France, 1980. [Google Scholar]
- Pereira, E.; Barros, L.; Dueñas, M.; Antonio, A.L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Gamma irradiation improves the extractability of phenolic compounds in Ginkgo biloba L. Ind. Crops Prod. 2015, 74, 144–149. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Abreu, R.M.V.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Yuan, Y.; Zhang, X.; Yue, T. Identification of ochratoxin A in Chinese spices using HPLC fluorescent detectors with immunoaffinity column cleanup. Food Control 2014, 46, 332–337. [Google Scholar] [CrossRef]
- Arita, C.; Calado, T.; Venâncio, A.; Lima, N.; Rodrigues, P. Description of a strain from an atypical population of Aspergillus parasiticus that produces aflatoxins B only, and the impact of temperature on fungal growth and mycotoxin production. Eur. J. Plant Pathol. 2014, 139, 655–661. [Google Scholar]
- Sample Availability: Samples are available from the authors.
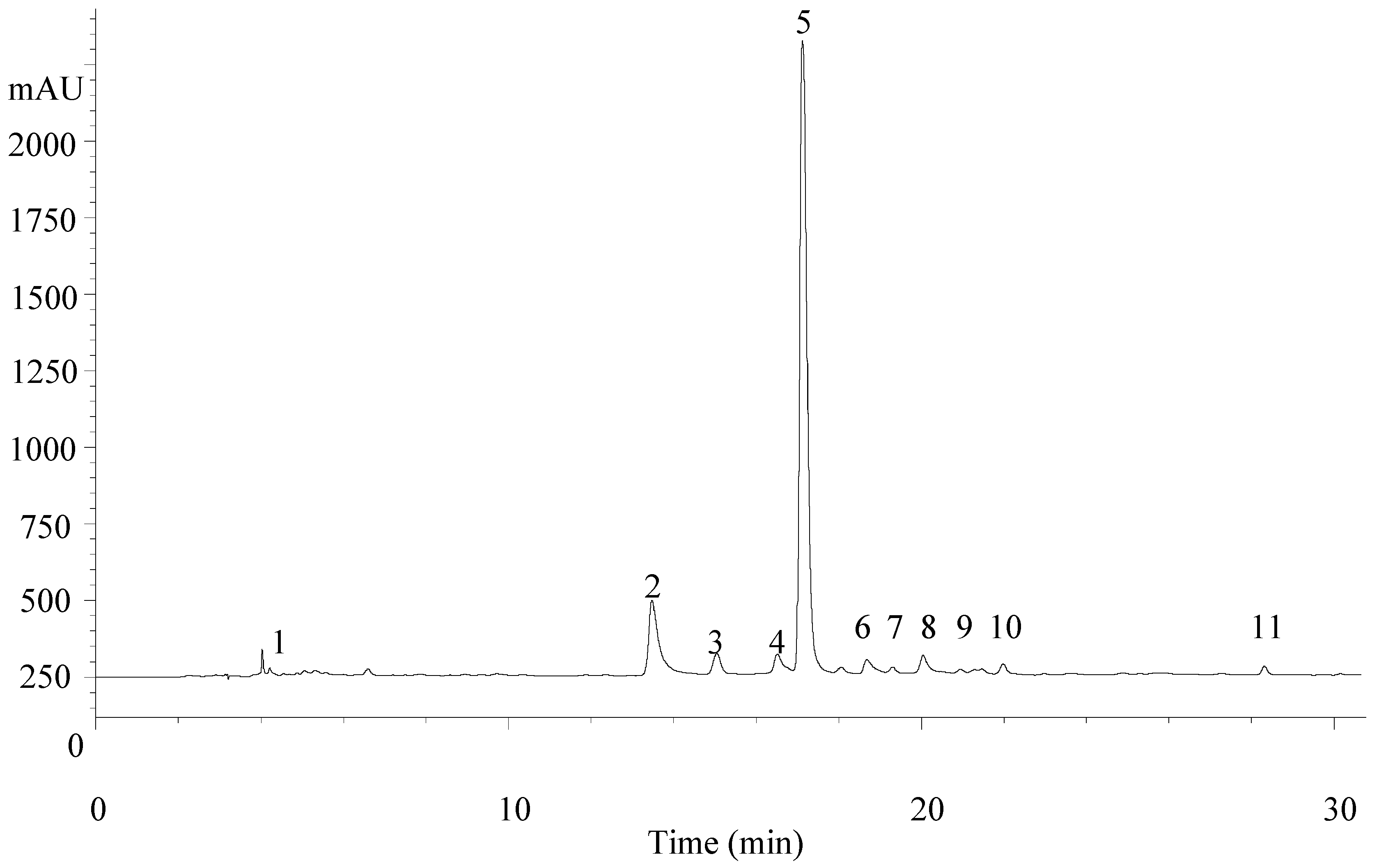
| Peak | Rt (min) | λmax (nm) | Molecular Ion [M − H]− (m/z) | MS2 (m/z) | Tentative Identification | References |
|---|---|---|---|---|---|---|
| 1 | 4.5 | 280 | 461 | 315 (8), 135 (28) | Verbasoside | [23,24] |
| 2 | 15.1 | 344 | 637 | 351 (100), 285 (89) | Luteolin-7-O-diglucuronide | [22,23,24] |
| 3 | 16.8 | 314 | 163 | 119 (100) | p-Coumaric acid | - |
| 4 | 17.7 | 338 | 621 | 351 (100), 269 (20) | Apigenin-7-O-diglucuronide | [22] |
| 5 | 18.2 | 330 | 623 | 461 (18), 315 (5) | Verbascoside | [22,23,24] |
| 6 | 20.3 | 350 | 651 | 351 (100), 299 (5) | Chrysoeriol-7-O-diglucuronide | [23,24] |
| 7 | 20.6 | 330 | 623 | 461 (18), 315 (5) | Isoverbascoside | [22] |
| 8 | 21.3 | 330 | 623 | 461 (15), 315 (10) | Forsythoside | [23] |
| 9 | 21.8 | 350 | 491 | 315 (100), 300 (23) | Isorhamnetin-3-O-glucuronide | - |
| 10 | 23.2 | 330 | 637 | 491 (5), 461 (60), 315 (13) | Eukovoside | [22,23,24] |
| 11 | 29.2 | 330 | 651 | 505 (7), 475 (22) | Martinoside | [23,24] |
| Peak | Phenolic Compounds | 0 kGy | 1 kGy | 5 kGy | 10 kGy |
|---|---|---|---|---|---|
| 1 | Verbasoside 1 | 0.118 ± 0.001a | 0.110 ± 0.01a | 0.125 ± 0.02a | 0.140 ± 0.03a |
| 2 | Luteolin-7-O-diglucuronide 2 | 18.9 ± 0.08b | 19.1 ± 0.02a | 18.6 ± 0.05c | 18.1 ± 0.8d |
| 3 | p-Coumaric acid 3 | 1.14 ± 0.01b | 1.07 ± 0.03c | 1.13 ± 0.03b | 1.20 ± 0.04a |
| 4 | Apigenin-7-O-diglucuronide 4 | 1.79 ± 0.03ab | 1.81 ± 0.04a | 1.71 ± 0.04b | 1.61 ± 0.05c |
| 5 | Verbascoside 1 | 71.6 ± 0.24a | 69 ± 0.95b | 69 ± 0.71b | 69.5 ± 0.47b |
| 6 | Chrysoeriol-7-O-diglucuronide 4 | 2.93 ± 0.01c | 3.27 ± 0.05a | 3.04 ± 0.04b | 2.80 ± 0.04d |
| 7 | Isoverbascoside 1 | 0.74 ± 0.03a | 0.79 ± 0.04a | 0.73 ± 0.04a | 0.67 ± 0.02a |
| 8 | Forsythoside 1 | 1.67 ± 0.03a | 1.65 ± 0.20a | 1.71 ± 0.15a | 1.76 ± 0.10a |
| 9 | Isorhamnetin-3-O-glucuronide 5 | 1.63 ± 0.03b | 1.75 ± 0.06a | 1.51 ± 0.05c | 1.27 ± 0.04d |
| 10 | Eukovoside 1 | 1.00 ± 0.03a | 1.00 ± 0.04a | 1.05 ± 0.06a | 1.11 ± 0.09a |
| 11 | Martinoside 1 | 0.57 ± 0.01a | 0.56 ± 0.04a | 0.62 ± 0.08a | 0.67 ± 0.11a |
| TCP | 75.8 ± 0.2a | 73 ± 1b | 73 ± 1b | 73.8 ± 0.2b | |
| TPA | 1.14 ± 0.01b | 1.07 ± 0.03c | 1.13 ± 0.03b | 1.20 ± 0.04a | |
| TF | 25.22 ± 0.03b | 25.96 ± 0.09a | 24.9 ± 0.1c | 23.7 ± 0.1d | |
| TPC | 102.1 ± 0.2a | 100 ± 1b | 99.3 ± 0.6b | 98.8 ± 0.2b |
| Standard | AFB1 | OTA | |
|---|---|---|---|
| Rt (retention time) | Min | 6.79 | 2.20 |
| CV, % (n = 11) | 0.76 | 2.45 | |
| Calibration curve | y = 312.36x − 27.24 | y = 362.40x − 31.13 | |
| Correlation coefficient (R2) | 0.999 | 0.999 | |
| Linearity range (ng/mL) | 20 to 0.05 | 20 to 0.05 | |
| Limits | LOD a (ng/mL) | 0.6 | 0.5 |
| LOQ b (ng/mL) | 1.9 | 1.7 | |
| AFB1 | OTA | |||
|---|---|---|---|---|
| 10 ng/g | 30 ng/g | 10 ng/g | 30 ng/g | |
| Mean Recovery (%) | 88.3 | 88.9 | 76.4 | 92.0 |
| RSDr (%) a | 8.3–14.4 | 0.1 | 2.5–9.3 | 5.1 |
| RSDR (%) b | 3.3 | - | 5.6 | - |
| Recommended Range (European Regulation No. 401/2006) | ||||
| Recovery (%) | 70–110 | |||
| RSDr (%) | <21 | <22 | <21 | <22 |
| RSDR (%) | <32 | <34 | <32 | <34 |
| Irradiation Dose | Mycotoxin Decrease (ng/g) | |
|---|---|---|
| AFB1 | OTA | |
| 0 kGy | 21.9 ± 3.5 a | 22.6 ± 0.8 a |
| 1 kGy | 20.7 ± 0.4 a | 21.5 ± 1.0 a |
| 5 kGy | 19.8 ± 1.2 a | 21.2 ± 1.5 a |
| 10 kGy | 20.4 ± 1.4 a | 21.4 ± 0.7 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, E.; Barros, L.; Antonio, A.L.; Cabo Verde, S.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Rodrigues, P. Is Gamma Radiation Suitable to Preserve Phenolic Compounds and to Decontaminate Mycotoxins in Aromatic Plants? A Case-Study with Aloysia citrodora Paláu. Molecules 2017, 22, 347. https://doi.org/10.3390/molecules22030347
Pereira E, Barros L, Antonio AL, Cabo Verde S, Santos-Buelga C, Ferreira ICFR, Rodrigues P. Is Gamma Radiation Suitable to Preserve Phenolic Compounds and to Decontaminate Mycotoxins in Aromatic Plants? A Case-Study with Aloysia citrodora Paláu. Molecules. 2017; 22(3):347. https://doi.org/10.3390/molecules22030347
Chicago/Turabian StylePereira, Eliana, Lillian Barros, Amilcar L. Antonio, Sandra Cabo Verde, Celestino Santos-Buelga, Isabel C. F. R. Ferreira, and Paula Rodrigues. 2017. "Is Gamma Radiation Suitable to Preserve Phenolic Compounds and to Decontaminate Mycotoxins in Aromatic Plants? A Case-Study with Aloysia citrodora Paláu" Molecules 22, no. 3: 347. https://doi.org/10.3390/molecules22030347