Zunyimycins B and C, New Chloroanthrabenzoxocinones Antibiotics against Methicillin-Resistant Staphylococcus aureus and Enterococci from Streptomyces sp. FJS31-2
Abstract
:1. Introduction
2. Results
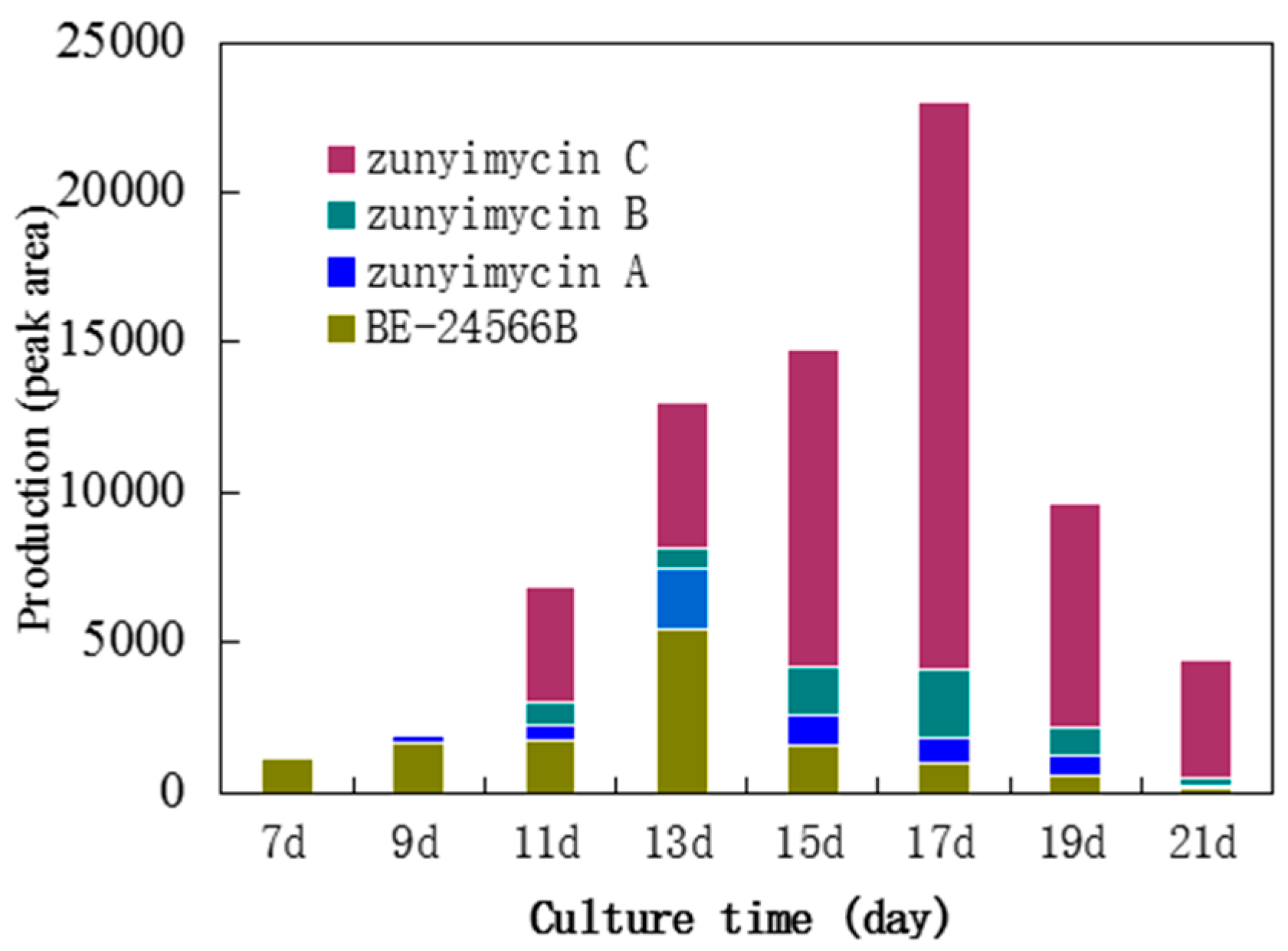
2.1. Biosynthesis of Zunyimycins
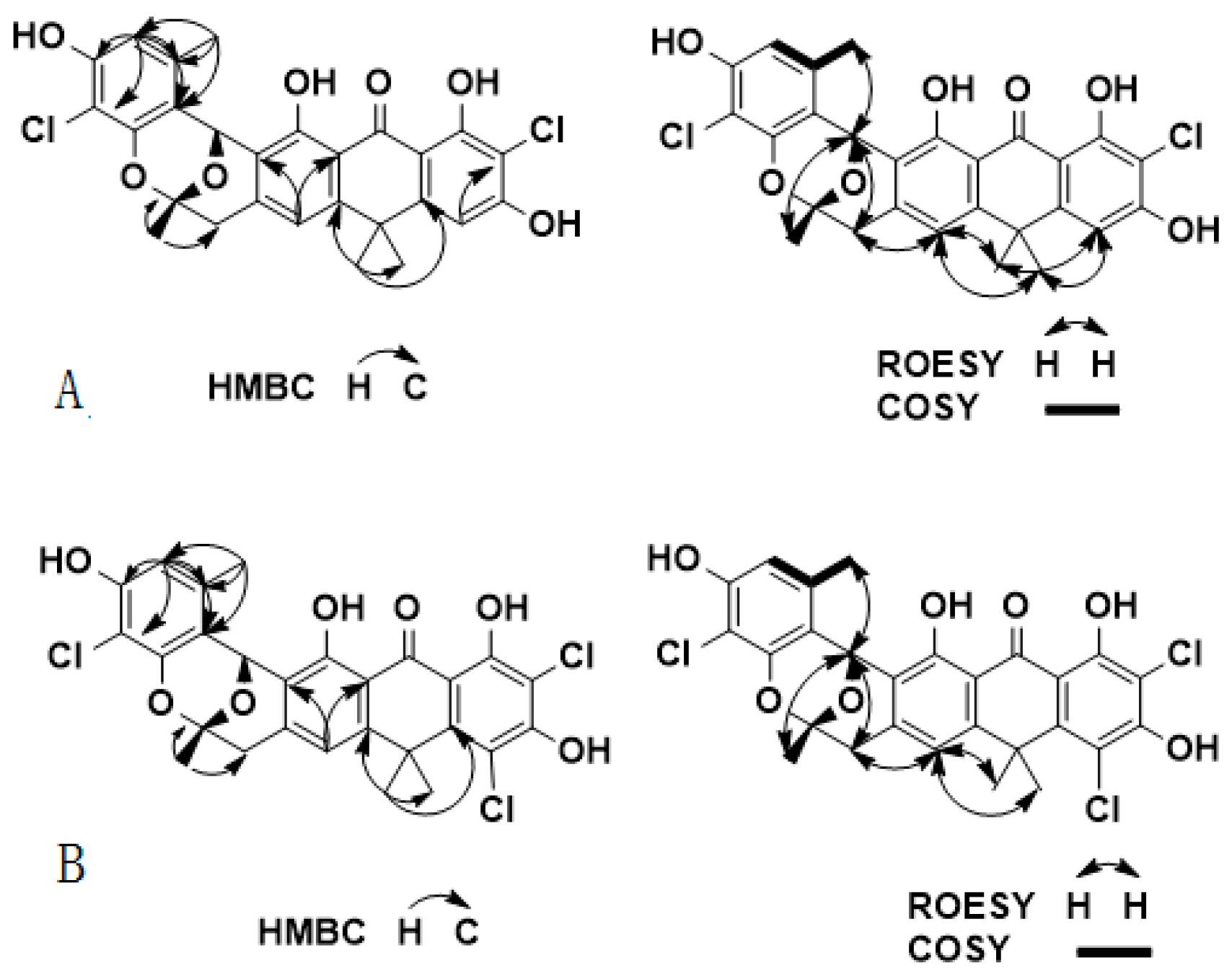
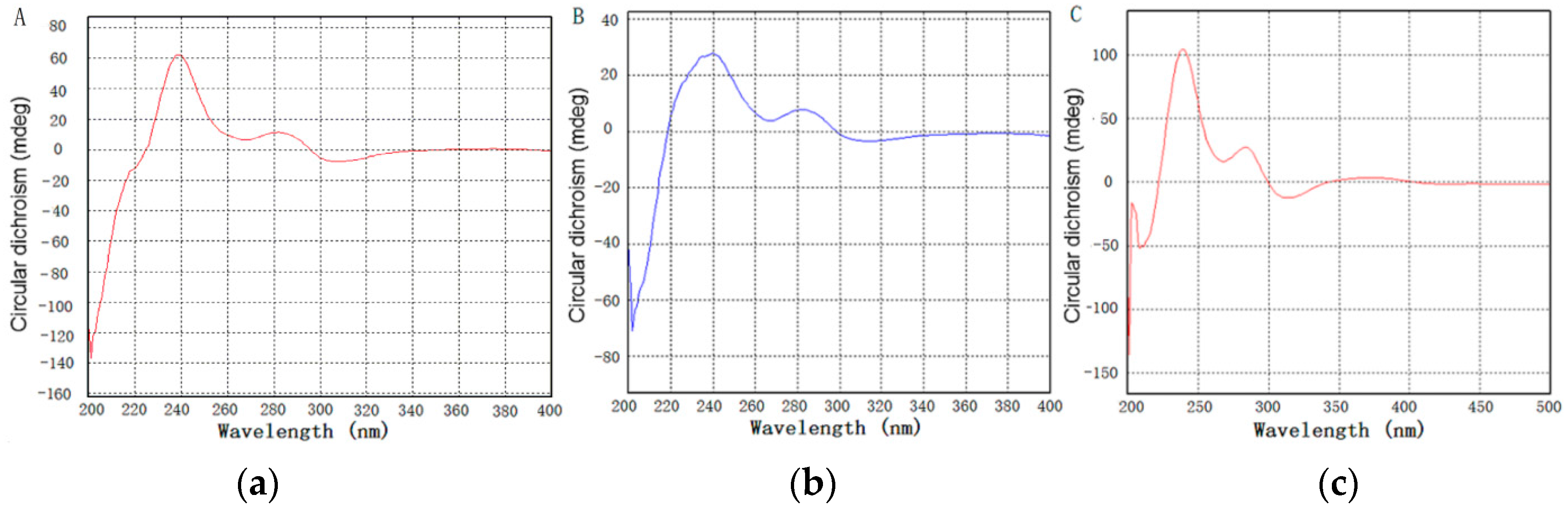
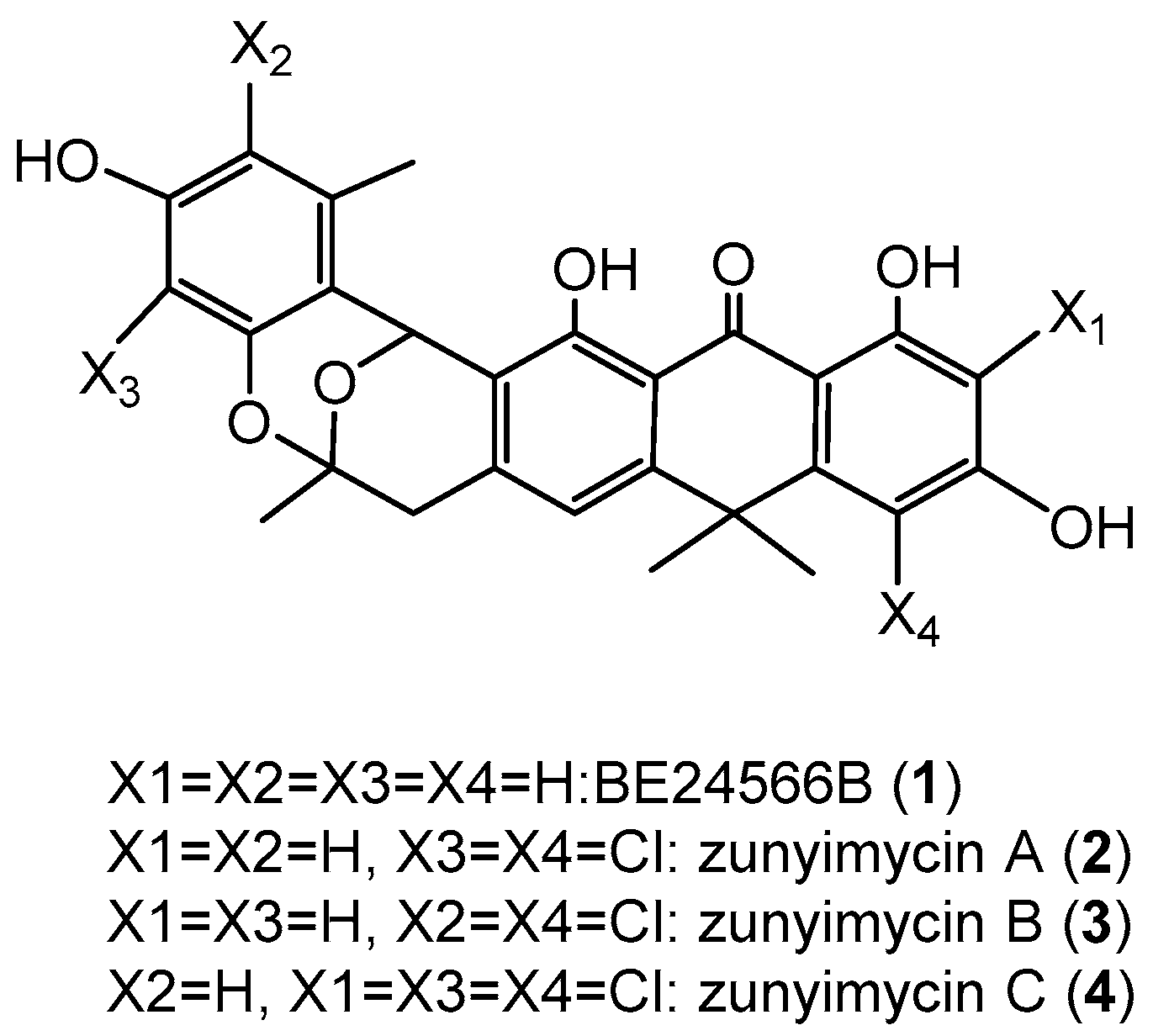
2.2. Chemical Identification of Zunyimycin B and C
2.3. Antibacterial Activity of Zunyimycins
3. Discussion
4. Materials and Methods
4.1. Strains and Medium
4.2. Fermentation, Isolation, and Chemical Identification of BE-24566B and Zunyimycins A, B, and C
4.3. Antibacterial Activity Assay
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gribble, G.W. Biological activity of recently discovered halogenated marine natural products. Mar. Drugs 2015, 13, 4044–4136. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wang, B.G. Independent evolution of six families of halogenating Enzymes. PLoS ONE 2016, 11, e0154619. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Chen, R.; Jiang, M.; Tian, X.; Liu, H.; Yu, Y.; Fan, C.; Chen, B. Bioprospecting potential of halogenases from Arctic marine actinomycetes. BMC Microbiol. 2016, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Purrello, S.M.; Garau, J.; Giamarellos, E.; Mazzei, T.; Pea, F.; Soriano, A.; Stefan, I.S. Methicillin-resistant Staphylococcus aureus infections: A review of the currently available treatment options. J. Glob. Antimicrob. Resist. 2016, 7, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, M.; Siricilla, S.; Mitachi, K. Advances in MRSA drug discovery: Where are we and where do we need to be? Expert Opin. Drug Discov. 2013, 8, 1095–10116. [Google Scholar] [CrossRef] [PubMed]
- Hoang, J.; Dersch-Mills, D.; Bresee, L.; Kraft, T.; Vanderkooi, O.G. Achieving therapeutic vancomycin levels in pediatric patients. Can. J. Hosp. Pharm. 2014, 67, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Reardon, J.; Lau, T.T.; Ensom, M.H. Vancomycin loading doses: A systematic review. Ann. Pharmacother. 2015, 49, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. ‘Game changer’ antibiotic and others in works for superbug. Nat. Med. 2011, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Escribano, J.P.; Alt, S.; Bibb, M.J. Next generation sequencing of actinobacteria for the discovery of novel natural products. Mar. Drugs 2016, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Niu, J.; Liu, N.; Lü, Y.; Liu, M.; Li, Y. Cloning and identification of the lobophorin biosynthetic gene cluster from marine Streptomyces olivaceus strain FXJ7.023. Pak. J. Pharm. Sci. 2016, 29 (Suppl. 1), 287–293. [Google Scholar] [PubMed]
- Walker, M.C.; Chang, M.C. Natural and engineered biosynthesis of fluorinated natural products. Chem. Soc. Rev. 2014, 43, 6527–6536. [Google Scholar] [CrossRef]
- Kojiri, K.; Nakajima, S.; Fuse, A.; Suzuki, H.; Suda, H. BE-24566B, a new antibiotic produced by Streptomyces violaceusniger. J. Antibiot. (Tokyo) 1995, 48, 1506–1508. [Google Scholar] [CrossRef] [PubMed]
- Lü, Y.; Yue, C.; Shao, M.; Qian, S.; Liu, N.; Bao, Y.; Wang, M.; Liu, M.; Li, X.; Wang, Y.; et al. Molecular genetic characterization of an anthrabenzoxocinones gene cluster in Streptomyces sp. FJS31-2 for the biosynthesis of BE-24566B and zunyimycin A. Molecules 2016, 21, 711. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.J.; Vanderwal, C.D. Stereoselective halogenation in natural product synthesis. Angew. Chem. Int. Ed. Engl. 2016, 55, 4396–4434. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.; Galgoci, A.L.; Young, K.; Painter, R.; Silver, L.L.; Herath, K.B.; Singh, S.B.; Cully, D.; Barrett, J.F.; Schmatz, D.; et al. Determination of selectivity and efficacy of fatty acid synthesis inhibitors. J. Biol. Chem. 2005, 80, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Van Pée, K.H.; Milbredt, D.; Patallo, E.P.; Weichold, V.; Gajewi, M. Application and modification of flavin-dependent halogenases. Methods Enzymol. 2016, 575, 65–92. [Google Scholar] [PubMed]
- Herath, K.B.; Jayasuriya, H.; Guan, Z.; Schulman, M.; Ruby, C.; Sharma, N.; MacNaul, K.; Menke, J.G.; Kodali, S.; Galgoci, A.; et al. Anthrabenzoxocinones from Streptomyces sp.as liver X receptor ligands and antibacterial agents. J. Nat. Prod. 2005, 68, 1437–1440. [Google Scholar] [PubMed]
- Senn, H.M. Insights into enzymatic halogenation from computational studies. Front. Chem. 2014, 2, 98. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, H.; Guo, Z.; Liu, N.; Li, J.; Huang, Y.; Xiang, W.; Chen, Y. Discovery of pentangular polyphenols hexaricins A-C from marine Streptosporangium sp. CGMCC 4.7309 by genome mining. Appl. Microbiol. Biotechnol. 2016, 100, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard M7-A7; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Sample Availability: Samples of the compounds zunyimycins A, B and C are available from the authors.

| Medium | Production (Peak Height, mAU) | |||
|---|---|---|---|---|
| BE24566B | Zunyimycin A | Zunyimycin B | Zunyimycin C | |
| 1 | ||||
| 2 | 107 | |||
| 3 | ||||
| 4 | ||||
| 5 | 50 | |||
| 6 | 320 | 135 | 190 | |
| 7 | ||||
| 8 | 124 | |||
| 9 | 122 | |||
| 10 | 200 | 52 | 24 | |
| 11 | 90 | |||
| 12 | 75 | |||
| 13 | ||||
| 14 | 150 | |||
| 15 | 115 | |||
| 16 | 110 | |||
| 17 | ||||
| 18 | 100 | 108 | ||
| 19 | 99 | |||
| 20 | 160 | |||
| 21 | ||||
| 22 | 525 | 250 | 526 | 824 |
| 23 | 117 | 150 | ||
| Position | 1H | 13C | ||
|---|---|---|---|---|
| Zunyimycin B | Zunyimycin C | Zunyimycin B | Zunyimycin C | |
| 1 | 133.4 | 133.4 | ||
| 2 | 6.17 (1H, s) | 6.27 (1H, s) | 101.7 | 101.7 |
| 3 | 150.7 | 150.7 | ||
| 4 | 113.6 | 113.6 | ||
| 4a | 152.6 | 152.6 | ||
| 5 | ||||
| 6 | 98.4 | 98.3 | ||
| 7 | 3.04 (1H, d, J = 11.3) 2.93 (1H, d, J = 17.5) | 3.04–3.11 (2H, m) | 39.6 | 39.7 |
| 7a | 141.2 | 142.4 | ||
| 8 | 6.82 (1H, s) | 6.86 (1H, s) | 117.2 | 117.9 |
| 8a | 150.7 | 146.4 | ||
| 9 | 38.1 | 39.4 | ||
| 9a | 150.4 | 153.0 | ||
| 10 | 6.57 (1H, s) | 107.2 | 107.9 | |
| 11 | 160.6 | 159.6 | ||
| 12 | 106.3 | 107.8 | ||
| 13 | 151.8 | 157.4 | ||
| 13a | 113.6 | 109.7 | ||
| 14 | 189.7 | 190.8 | ||
| 14a | 111.2 | 112.3 | ||
| 15 | 157.7 | 157.1 | ||
| 15a | 122.5 | 122.1 | ||
| 16 | 6.10 (1H, s) | 6.10 (1H, s) | 65.8 | 65.7 |
| 16a | 115.7 | 115.6 | ||
| 1-CH3 | 2.52 (3H, s) | 2.49 (3H, s) | 15.6 | 15.8 |
| 6-CH3 | 1.51 (3H, s) | 1.60 (3H, s) | 26.2 | 26.2 |
| 9-CH3 | 1.36 (3H, s) | 1.79 (3H, s) | 33.0 | 27.5 |
| 9-CH3 | 1.45 (3H, s) | 1.69 (3H, s) | 32.6 | 27.8 |
| Strains | Ampicillin | Zunyimycin A | Zunyimycin B | Zunyimycin C |
|---|---|---|---|---|
| S. aureus (ATCC: 29213) | 1.05 | 3.44 | 3.94 | 0.94 |
| MRSA clinical isolates (08301) | >100 | 6.89 | 7.88 | 3.75 |
| MRSA clinical isolates (161222330) | >100 | 16.71 | 25.62 | 8.14 |
| MRSA clinical isolates (161231380) | >100 | 8.36 | 12.81 | 4.07 |
| MRSA clinical isolates (170108317) | >100 | 16.71 | 25.62 | 4.07 |
| MRSA clinical isolates (161231350) | >100 | 16.71 | 25.62 | 4.07 |
| E. faecalis (ATCC: 29212) | >100 | 13.78 | 15.75 | 7.50 |
| E. faecalis clinical isolates (160803348) | >100 | 16.71 | 12.81 | 4.07 |
| E. faecalis clinical isolates (160804314) | >100 | 16.71 | 12.81 | 8.14 |
| E. faecalis clinical isolates (161222328) | >100 | 33.43 | 12.81 | 4.07 |
| E. faecalis clinical isolates (170106034) | >100 | 16.71 | 25.62 | 8.14 |
| B. subtilis (CGMCC: 1.2428) | 0.71 | 13.78 | 15.75 | 3.75 |
| Medium Components (g/L) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Medium | CaCO3 | Glucose | Malt Exact | Yeast Exact | Mannitol | NH4NO3 | Humic Acid A | Humic Acid B |
| 1 | 2 | 10 | 4 | 4 | ||||
| 2 | 2 | 10 | 4 | 4 | ||||
| 3 | 2 | 10 | 1 | 4 | ||||
| 4 | 2 | 10 | 2 | 4 | 2 | |||
| 5 | 2 | 1 | 10 | 4 | ||||
| 6 | 2 | 1 | 10 | 4 | ||||
| 7 | 2 | 1 | 10 | 1 | ||||
| 8 | 2 | 1 | 10 | 2 | 2 | |||
| 9 | 2 | 4 | 10 | 4 | ||||
| 10 | 2 | 4 | 10 | 4 | ||||
| 11 | 2 | 4 | 10 | 1 | ||||
| 12 | 2 | 4 | 10 | 2 | 2 | |||
| 13 | 2 | 2 | 10 | 2 | 4 | |||
| 14 | 2 | 2 | 10 | 4 | 2 | |||
| 15 | 2 | 2 | 10 | 1 | 2 | |||
| 16 | 2 | 2 | 10 | 2 | 2 | 2 | ||
| 17 | 2 | 1 | 1 | |||||
| 18 | 2 | 4 | 10 | 4 | 0.5 | |||
| 19 | 2 | 4 | 10 | 4 | 1. | |||
| 20 | 2 | 4 | 10 | 4 | 1.5 | |||
| 21 | 2 | 4 | 10 | 4 | 0.5 | |||
| 22 | 2 | 4 | 10 | 4 | 1.0 | |||
| 23 | 2 | 4 | 10 | 4 | 1.5 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lü, Y.; Shao, M.; Wang, Y.; Qian, S.; Wang, M.; Wang, Y.; Li, X.; Bao, Y.; Deng, C.; Yue, C.; et al. Zunyimycins B and C, New Chloroanthrabenzoxocinones Antibiotics against Methicillin-Resistant Staphylococcus aureus and Enterococci from Streptomyces sp. FJS31-2. Molecules 2017, 22, 251. https://doi.org/10.3390/molecules22020251
Lü Y, Shao M, Wang Y, Qian S, Wang M, Wang Y, Li X, Bao Y, Deng C, Yue C, et al. Zunyimycins B and C, New Chloroanthrabenzoxocinones Antibiotics against Methicillin-Resistant Staphylococcus aureus and Enterococci from Streptomyces sp. FJS31-2. Molecules. 2017; 22(2):251. https://doi.org/10.3390/molecules22020251
Chicago/Turabian StyleLü, Yuhong, Meiyun Shao, Yinyin Wang, Shengyan Qian, Miao Wang, Yingquan Wang, Xiaoqian Li, Yuxin Bao, Chengmin Deng, Changwu Yue, and et al. 2017. "Zunyimycins B and C, New Chloroanthrabenzoxocinones Antibiotics against Methicillin-Resistant Staphylococcus aureus and Enterococci from Streptomyces sp. FJS31-2" Molecules 22, no. 2: 251. https://doi.org/10.3390/molecules22020251






