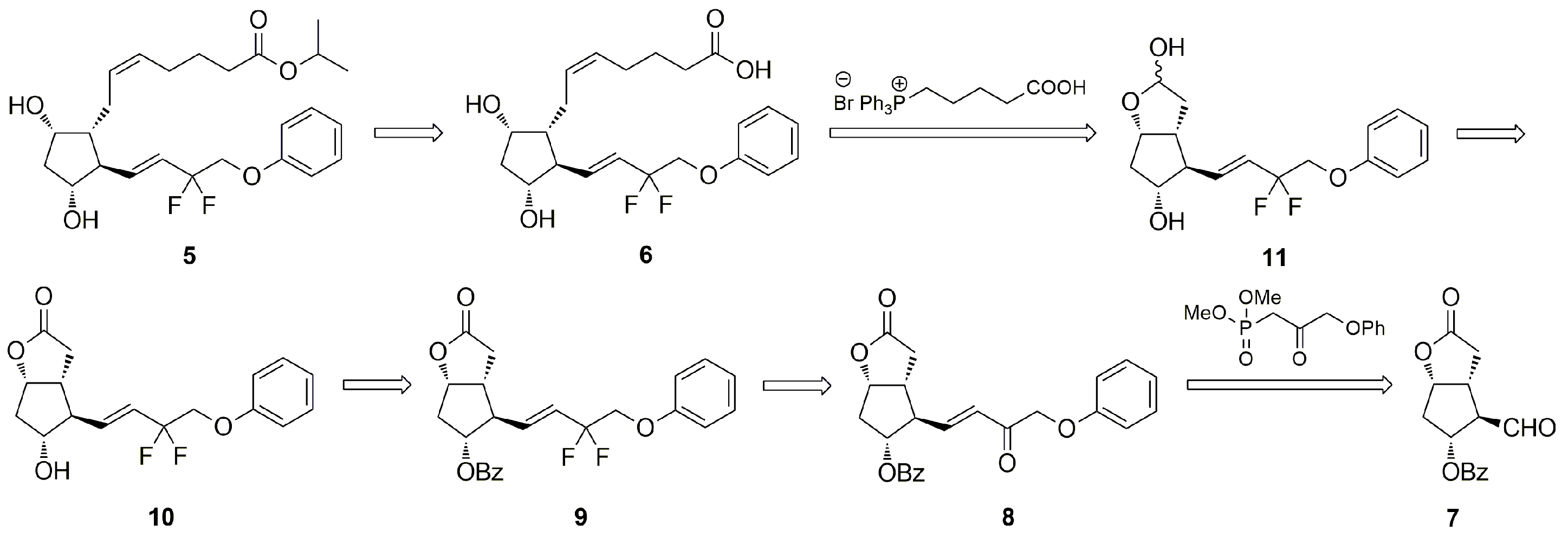
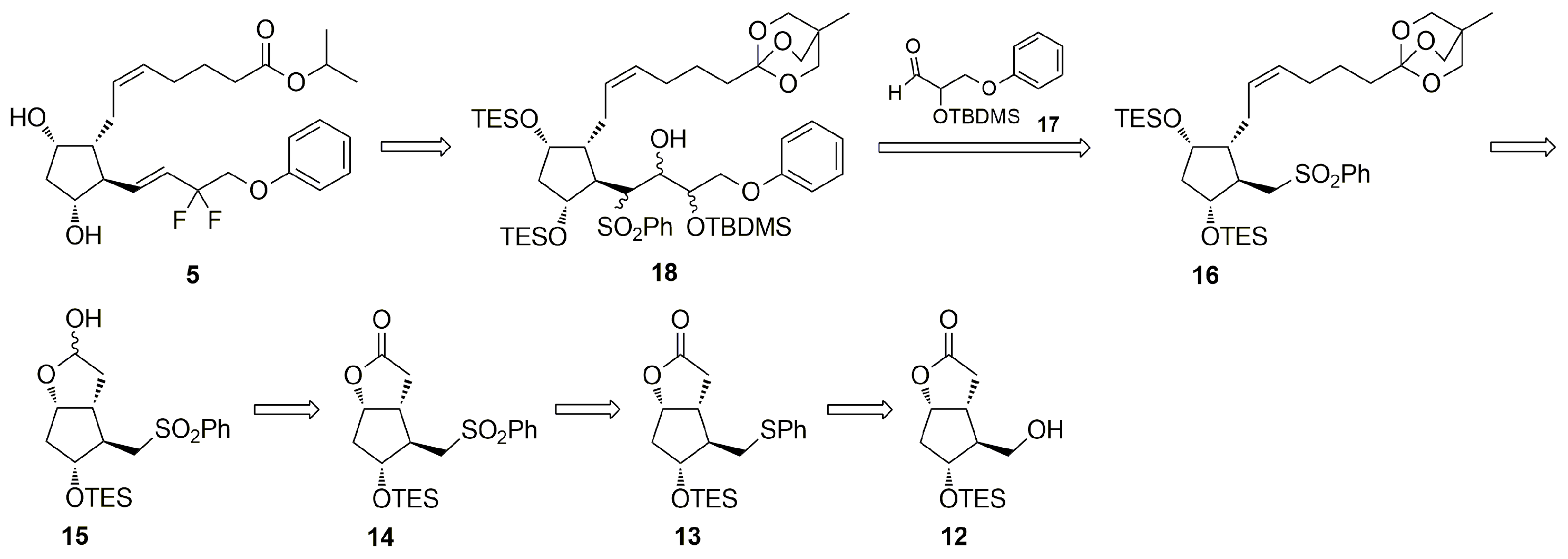
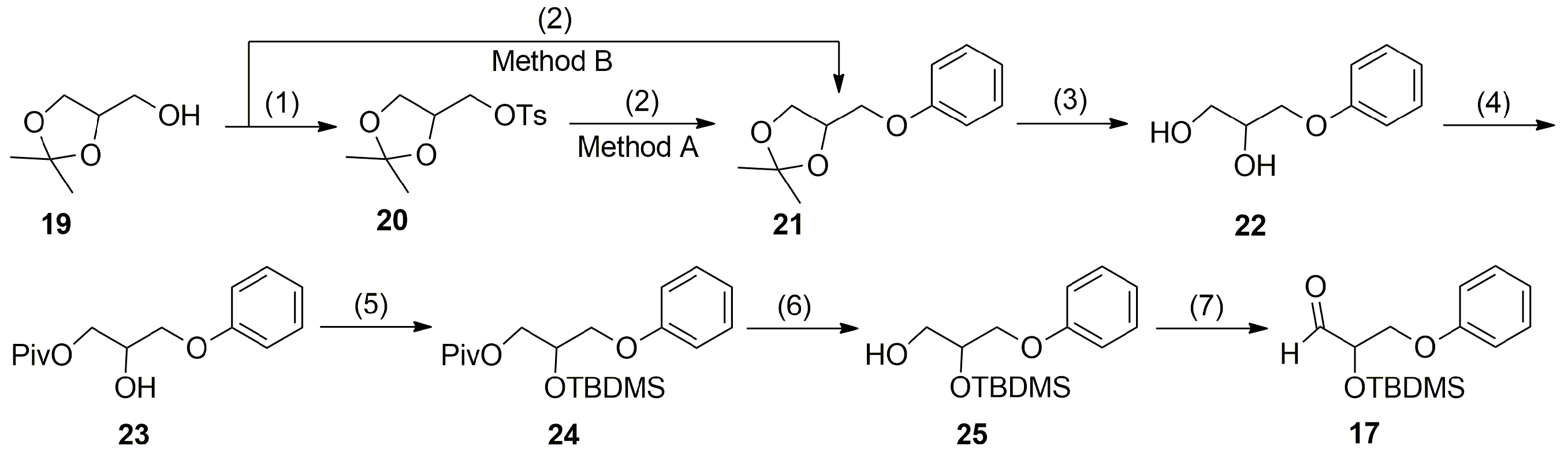
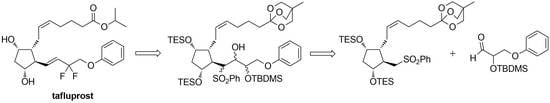
3.6. Syntheses
The synthesis of the aldehyde ω-chain synthon
17 from the commercially available
d,
l-1,2-isopropylidene glycerol (
19) is shown in
Scheme 3. The detailed descriptions of the preparation of compounds
20–
25 and
17 as well as their physical and spectroscopic data are provided in the
Supplementary Materials.
3.6.1. 1-{(Z)-6-[(1R,2R,3R,5S)-2-[(1R/1S,2R/2S,3R/3S)-3-(tert-Butyldimethylsilyloxy)-4-phenoxy-1-(phenylsulfonyl)butyl]-3,5-bis(triethylsilyloxy)cyklopentyl]hex-4-enyl}-4-methyl-2,6,7-trioxabicyclo-[2.2.2]octane (18)
n-BuLi (28.6 mL, 45.8 mmol, 1.6 M in hexanes) was added dropwise to a solution of diisopropylamine (7.96 mL, 45.8 mmol) in anhydrous THF (12 mL) at −78 °C under an argon atmosphere. After 15 min at −78 °C, the phenylsulfone 16 (21.0 g, 30.2 mmol) in anhydrous THF (40 mL) was added dropwise with vigorous stirring. The reaction mixture was stirred at −60 °C for 30 min and the aldehyde 17 (14.0 g, 50.0 mmol) in anhydrous THF (10 mL) was added dropwise. After being stirred at −60 °C for another 30 min, TLC analysis (6% MeCN/toluene) indicated disappearance of the starting phenylsulfone 16. The cold reaction was quenched with saturated aqueous NaCl solution (30 mL). The resulting layers were separated and the aqueous phase was extracted with THF (3 × 25 mL). The combined organic extracts were washed with brine (150 mL), dried over Na2SO4, filtered and concentrated in vacuo. The crude product was carefully dried in vacuo to yield a mixture of diastereoisomeric hydroxysulfones 18 (38.2 g), as a yellow oil. The hydroxysulfones 18 were directly used for the next step without further purification.
3.6.2. 1-{(4Z)-6-[(1R,2R,3R,5S)-2-[(1E,3R/3S)-3-(tert-Butyldimethylsilyloxy)-4-(phenoxy)but-1-enyl]-3,5-bis(triethylsilyloxy)cyclopentyl]hex-4-enyl}-4-methyl-2,6,7-trioxabicyclo[2.2.2] octane (26)
The crude mixture of diastereoisomeric hydroxysulfones 18 (38.2 g) was dissolved in THF (25 mL) and saturated methanolic solution of Na2HPO4 (120 mL) was added in one portion. On cooling to 0 °C under an argon atmosphere, sodium amalgam (20%, 25.0 g, 217.4 mmol Na) was added portionwise over 60 min with vigorous stirring. The cooling bath was removed and the resulting suspension was stirred at room temperature for 16 h to disappearance of the starting hydroxysulfones 18 (TLC, MeCN/toluene 1:1). The solution was decanted from the remaining amalgam and concentrated under reduced pressure. The residue was diluted with H2O (60 mL) and AcOEt (60 mL). The resulting layers were separated and the aqueous phase was extracted with AcOEt (3 × 50 mL). The combined organic extracts were washed with brine (150 mL) and dried over anhydrous Na2SO4. Filtration and evaporation in vacuo furnished the crude product 26 (38.1 g) as a light yellow oil. The crude olefin 26 was directly used for the next step without further purification.
3.6.3. 2,2-Bis(hydroxymethyl)propyl (5Z)-7-{(1R,2R,3R,5S)-2-[(1E,3R/3S)-3-(tert-butyldimethylsilylo-xy)-4-(phenoxy)but-1-enyl]-3,5-dihydroxycyclopentyl}hept-5-enoate (27a/b)
PPTS (28.0 g, 111.42 mmol) was added to a solution of the crude silyl protected prostaglandin derivatives 26 (38.1 g) in a mixture of CH2Cl2-MeOH (1:1, 300 mL). The resulting brown solution was stirred for 3 h, whereupon brine (150 mL) and saturated aqueous solution NaHCO3 (150 mL) were added. After being stirred for 15 min, the organic solvents were evaporated under reduced pressure. The residue was extracted with AcOEt (3 × 100 mL). The combined organic extracts were dried over anhydrous Na2SO4. Filtration and evaporation in vacuo furnished the crude tetraol (24.8 g). Purification by silica gel flash chromatography with gradient elution 1%–6% MeOH-CH2Cl2 afforded a sample of prostaglandin analog (+)-27a (84 mg) and an inseparable mixture of diastereoisomeric tetraols 27a/b (14.5 g, 79% yield from phenylsulfone 16) as a light yellow viscous oil. (+)-27a: Rf = 0.42 (50% CH3CN/toluene). = +17.72 (c 1.0, CH2Cl2). FT-IR (thin film) ν (cm−1): 3393, 2946, 2929, 2899, 2857, 1731, 1717, 1600, 1587, 1497, 1471, 1388, 1301, 1248, 1172, 1143, 1044, 974, 837, 810, 779, 754, 733, 692, 596, 509. 1H-NMR (CDCl3, 600 MHz) δ (ppm): 0.10 (two s, 6H, (CH3)2Si), 0.84 (s, 3H, (-C(CH3)(CH2OH)2), 0.91 (s, 9H, (CH3)3C-Si), 1.57 (m, 1H, cyclopentane CH-1), 1.71 (m, 2H, α-chain CH2-3), 1.81 (m, 1H, one of the cyclopentane CH2-4 group), 2.05–2.20 (m, 4H, α-chain CH2-4, one of the α-chain CH2-7 group, one of the cyclopentane CH2-4 group), 2.32 (m, 1H, one of the α-chain CH2-7 group), 2.33–2.40 (m, 3H, α-chain CH2-2 and cyclopentane CH-2), 3.52 (d, J = 11.5 Hz, 2H, -CH2OH), 3.57 (d, J = 11.5 Hz, 2H, -CH2OH), 3.86 (d, J = 6.0 Hz, 2H, ω-chain CH2-4), 3.99 (m, 1H, cyclopentane CH-3), 4.15–4.20 (m, 3H, -OCH2C(CH3)(CH2OH)2 and cyclopentane CH-5), 4.51 (m, 1H, ω-chain CH-3), 5.36 (m, 1H, α-chain CH-5), 5.42 (m, 1H, α-chain CH-6), 5.62–5.68 (m, 2H, ω-chain CH-1 and CH-2), 6.88 (m, 2H, aromatic CH-2 and CH-6), 6.93 (m, 1H, aromatic CH-4), 7.27 (m, 2H, aromatic CH-3 and CH-5). 13C-NMR (150 MHz, CDCl3) δ (ppm): −4.7 (CH3-Si), −4.6 (CH3-Si), 16.9 (-C(CH3)(CH2OH)2), 18.4 ((CH3)3C-Si), 24.8 (α-chain C-3), 25.8 (3C, (CH3)3C-Si), 26.1 (α-chain C-7), 26.6 (α-chain C-4), 33.6 (α-chain C-2), 40.6 (-C(CH3)(CH2OH)2), 42.8 (cyclopentane C-4), 50.6 (cyclopentane C-1), 56.0 (cyclopentane C-2), 66.5 (-OCH2C(CH3)(CH2OH)2), 67.5 (2C, -OCH2C(CH3)(CH2OH)2), 71.6 (ω-chain C-3), 72.2 (ω-chain C-4), 73.6 (cyclopentane C-5), 78.4 (cyclopentane C-3), 114.4 (2C, aromatic C-2 and C-6), 120.7 (aromatic C-4), 129.3 (α-chain CH-6), 129.4 (2C, aromatic C-3 and C-5), 129.5 (α-chain CH-5), 131.0 (ω-chain C-2), 133.0 (ω-chain C-1), 158.8 (aromatic C-1), 174.7 (C=O). HRMS (ESI): calcd. for C33H54O8NaSi [M + Na]+ 629.3486; found 629.3474.
3.6.4. 2,2-Bis(acetoxymethyl)propyl (5Z)-7-{(1R,2R,3R,5S)-3,5-diacetoxy-2-[(1E,3R/3S)-3-(tert-butyldi-methylsilyloxy)-4-(phenoxy)but-1-enyl]cyclopentyl}hept-5-enoate (28a/b)
TEA (23.7 mL, 173.3 mmol), DMAP (0.2 g) and Ac2O (8.2 mL, 86.5 mol) were added to a solution of the diastereoisomeric prostaglandin derivatives 27a/b (10.5 g, 17.3 mmol) in CH2Cl2 (100 mL). The mixture was stirred for 1 h at room temperature prior the addition of saturated aqueous solution NH4Cl (100 mL). The resulting layers were separated and the aqueous phase was extracted with CH2Cl2 (3 × 50 mL). The combined organic extracts were washed with saturated aqueous solution NaHCO3 (50 mL) and dried over anhydrous MgSO4. Filtration and evaporation in vacuo furnished the crude product, which was purified by silica gel flash chromatography with gradient elution 5%–10% AcOEt-hexanes to give a sample of ester (+)-28a (70 mg) and an inseparable mixture of diastereoisomeric esters 28a/b (12.97 g, yield 97% yield). (+)-28a: Rf = 0.35 (25% AcOEt/hexanes). = +23.72 (c 1.0, CH2Cl2). FT-IR (thin film) ν (cm−1): 2954, 2932, 2896, 2857, 1740, 1717, 1600, 1587, 1497, 1472, 1375, 1240, 1173, 1150, 1043, 974, 917, 838, 811, 779, 756, 692, 605, 510. 1H-NMR (CDCl3, 600 MHz) δ (ppm): 0.08 (two s, 6H, (CH3)2Si), 0.89 (s, 9H, (CH3)3C-Si), 1.00 (s, 3H, (-C(CH3)(CH2OH)2), 1.64 (m, 3H, one of the cyclopentane CH2-4 group and α-chain CH2-3), 1.68 (m, 1H, cyclopentane CH-1), 1.95–2.16 (three s and two m, 16H, four -COOCH3 groups, α-chain CH2-4 and α-chain CH2-7), 2.28 (t, J = 7.7 Hz, 2H, α-chain CH2-2), 2.55–2.65 (m, 2H, one proton of the cyclopentane CH2-4 group and cyclopentane CH-2), 3.83 (dd, J = 9.4 and 5.2 Hz, 1H, one of the ω-chain CH2-4 group), 3.89 (dd, J = 9.4 and 6.9 Hz, 1H, one of the ω-chain CH2-4 group), 3.99 (s, 6H, -OCH2C(CH3)(CH2OH)2), 4.50 (m, 1H, ω-chain CH-3), 4.90 (m, 1H, cyclopentane CH-3), 5.09 (m, 1H, cyclopentane CH-5), 5.30–5.38 (m, 2H, α-chain CH-5 and α-chain CH-6), 5.62 (dd, J = 8.3 and 15.3 Hz, 1H, ω-chain CH-1), 5.67 (dd, J = 5.6 and 15.3 Hz, 1H, ω-chain CH-2), 6.87 (m, 2H, aromatic CH-2 and CH-6), 6.93 (m, 1H, aromatic CH-4), 7.27 (m, 2H, aromatic CH-3 and CH-5). 13C-NMR (150 MHz, CDCl3) δ (ppm): −4.7 (CH3-Si), −4.6 (CH3-Si), 17.0 (-C(CH3)(CH2OH)2), 18.3 ((CH3)3C-Si), 20.7, 21.0 and 21.2 (4C, four -COOCH3 groups), 24.7 (α-chain C-3), 24.9 (α-chain C-7), 25.8 (3C, (CH3)3C-Si), 26.6 (α-chain C-4), 33.5 (α-chain C-2), 38.2 (–C(CH3)(CH2OH)2), 39.0 (cyclopentane C-4), 47.3 (cyclopentane C-1), 51.8 (cyclopentane C-2), 65.6 (-OCH2C(CH3)(CH2OH)2), 65.7 (2C, -OCH2C(CH3)(CH2OH)2), 71.7 (ω-chain C-3), 72.1 (ω-chain C-4), 74.3 (cyclopentane C-5), 77.8 (cyclopentane C-3), 114.4 (2C, aromatic C-2 and C-6), 120.9 (aromatic C-4), 128.0 (α-chain CH-6), 129.4 (2C, aromatic C-3 and C-5), 129.7 (α-chain CH-5), 131.4 (ω-chain C-1), 133.2 (ω-chain C-2), 158.8 (aromatic C-1), 170.4, 170.6 and 170.7 (4C, four –COOCH3 groups). 173.1 (-CH2COOCH2-). HRMS (ESI): calcd. for C41H62O12NaSi [M + Na]+ 797.3908; found 797.3898.
3.6.5. 2,2-Bis(acetoxymethyl)propyl (5Z)-7-{(1R,2R,3R,5S)-3,5-Diacetoxy-2-[(1E,3R/3S)-4-phenoxy-3-hydroxybut-1-enyl]cyclopentyl}hept-5-enoate (29a/b)
Camphorosulfonic acid (1.12 g, 4.81 mmol) was added to a solution of diastereoisomeric esters 28a/b (11.3 g, 14.58 mmol) in a mixture of MeOH-CH2Cl2 (1:1, 100 mL). The resulting solution was stirred for 7 h, whereupon NaHCO3 (0.47 g) was added. After being stirred for 30 min, the mixture was filtered through a Büchner funnel and the filtrate was concentrated under reduced pressure. The crude product was purified by silica gel flash chromatography with gradient elution 5%–40% AcOEt-hexanes to give a sample of alcohol (+)-29a (59 mg) and an inseparable mixture of diastereoisomeric alcohols 29a/b (6.94 g, 82% yield). (+)-29a: Rf = 0.37 (50% AcOEt/hexanes). = +32.46 (c 1.0, MeOH). FT-IR (thin film) ν (cm−1): 3501, 2940, 1740, 1600, 1497, 1378, 1243, 1173, 1152, 1042, 975, 915, 814, 757, 693, 606, 512. 1H-NMR (CDCl3, 600 MHz) δ (ppm): 1.00 (s, 3H, (-C(CH3)(CH2OH)2), 1.60–1.74 (m, 4H, one of the cyclopentane CH2-4 group, cyclopentane CH-1, α-chain CH2-3), 1.95–2.10 (three s and two m, 14H, four -COOCH3 groups and α-chain CH2-4), 2.14 (m, 2H, α-chain CH2-7), 2.30 (t, J = 7.7 Hz, 2H, α-chain CH2-2), 2.55 (m, 1H, one of the cyclopentane CH2-4 group), 2.62 (m, 1H, cyclopentane CH-2), 3.91 (dd, J = 7.2 and 9.0 Hz, 1H, one of the α-chain CH2-4), 3.99 (m, 7H, -OCH2C(CH3)(CH2OH)2 and one of the ω-chain CH2-4 group), 4.54 (m, 1H, ω-chain CH-3), 4.94 (m, 1H, cyclopentane CH-3), 5.11 (m, 1H, cyclopentane CH-5), 5.30–5.38 (m, 2H, α-chain CH-5 and α-chain CH-6), 5.71 (dd, J = 5.4 and 15.6 Hz, 1H, ω-chain CH-1), 5.74 (dd, J = 7.2 and 15.6 Hz, 1H, ω-chain CH-2), 6.92 (m, 2H, aromatic CH-2 and CH-6), 6.98 (m, 1H, aromatic CH-4), 7.29 (m, 2H, aromatic CH-3 and CH-5). 13C-NMR (150 MHz, CDCl3) δ (ppm): 17.0 (-C(CH3)(CH2OH)2), 20.7, 21.1 and 21.2 (4C, four -COOCH3 groups), 24.6 (α-chain C-3), 24.8 (α-chain C-7), 26.6 (α-chain C-4), 33.5 (α-chain C-2), 38.2 (–C(CH3)(CH2OH)2), 39.0 (cyclopentane C-4), 47.3 (cyclopentane C-1), 52.0 (cyclopentane C-2), 65.6 (-OCH2C(CH3)(CH2OH)2), 65.7 (2C, -OCH2C(CH3)(CH2OH)2), 70.5 (ω-chain C-3), 71.7 (ω-chain C-4), 74.3 (cyclopentane C-5), 77.8 (cyclopentane C-3), 114.6 (2C, aromatic C-2 and C-6), 121.2 (aromatic C-4), 128.1 (α-chain CH-6), 129.5 (2C, aromatic C-3 and C-5), 129.8 (α-chain CH-5), 131.1 (ω-chain C-2), 132.6 (ω-chain C-1), 158.5 (aromatic C-1), 170.4, 170.6 and 170.7 (4C, four -COOCH3 groups). 173.2 (-CH2COOCH2-). HRMS (ESI): calcd. for C35H48O12Na [M + Na]+ 683.3043; found 683.3040.
3.6.6. 2,2-Bis(acetoxymethyl)propyl (5Z)-7-{(1R,2R,3R,5S)-3,5-Diacetoxy-2-[(1E)-4-(phenoxy)-3-oxo-but-1-enyl]cyclopentyl}hept-5-enoate (30)
Dess-Martin periodinane (4.48 g, 10.56 mmol) was added portionwise to a suspension of diastereoisomeric alcohols 29a/b (5.8 g, 8.8 mmol) and dry NaHCO3 (2.22 g, 26.4 mmol) in anhydrous CH2Cl2 (50 mL). After being stirred for 1 h at room temperature, TLC analysis (AcOEt/hexanes, 1:1) indicated disappearance of the starting alcohols 29a/b. Saturated aqueous NaHCO3 (100 mL) and Na2SO3 (8.87 g, 70.4 mmol) were then added simultaneously and the mixture was stirred at room temperature for 30 min The resulting layers were separated and the aqueous phase was extracted with CH2Cl2 (3 × 50 mL). The combined extracts were washed with water brine (100 mL) and dried over Na2SO4. Filtration and evaporation in vacuo furnished the crude product as a light yellow oil, which was purified by flash column chromatography (silica gel, 2%–20% AcOEt/hexanes) to give the ketone 30 (5.76 g, 99% yield) as a colorless oil. Rf = 0.54 (50% AcOEt/hexanes). = +40.67 (c 1.0, CH2Cl2). FT-IR (thin film) ν (cm−1): 2944, 1740, 1697, 1626, 1600, 1589, 1496, 1474, 1435, 1376, 1239, 1174, 1043, 984, 917, 785, 757, 693, 645, 606, 509. 1H-NMR (CDCl3, 600 MHz) δ (ppm): 1.01 (s, 3H, -C(CH3)(CH2OH)2), 1.64 (m, 2H, α-chain CH2-3), 1.73 (ddd, J = 1.3, 4.2 and 15.8 Hz, 1H, one of the cyclopentane CH2-4 group), 1.84 (m, 1H, cyclopentane CH-1), 1.92–2.10 (three s and two m, 15H, four -COOCH3 groups, α-chain CH2-4 and one of the α-chain CH2-7 group), 2.15 (m, 1H, one of the α-chain CH2-7 group), 2.29 (t, J = 7.6 Hz, 2H, α-chain CH2-2 group), 2.58 (ddd, J = 5.6, 9.1 and 15.8 Hz, 1H, one of the cyclopentane CH2-4 group), 2.76 (m, 1H, cyclopentane CH-2 group), 3.99 (m, 6H, -OCH2C(CH3)(CH2OH)2), 4.72 (two d, J = 16.4 Hz, ω-chain CH2-4), 4.98 (ddd, J = 4.2, 7.4 and 11.7 Hz, 1H, cyclopentane CH-3), 5.12 (m, 1H, cyclopentane CH-5), 5.29 (m, 1H, α-chain CH-6), 5.34 (m, 1H, α-chain CH-5), 6.51 (dd, J = 0.8 and 15.7 Hz, 1H, ω-chain CH-2), 6.90 (m, 2H, aromatic CH-2 and CH-6), 6.92 (dd, J = 9.1 and 15.7 Hz, 1H, ω-chain CH-1), 6.99 (m, 1H, aromatic CH-4), 7.29 (m, 2H, aromatic CH-3 and CH-5). 13C-NMR (150 MHz, CDCl3) δ (ppm): 17.0 (-C(CH3)(CH2OH)2), 20.7, 21.1 and 21.2 (4C, four -COOCH3 groups), 24.6 (α-chain C-3), 25.0 (α-chain C-7), 26.6 (α-chain C-4), 33.5 (α-chain C-2), 38.2 (-C(CH3)(CH2OH)2), 39.1 (cyclopentane C-4), 47.7 (cyclopentane C-1), 52.4 (cyclopentane C-2), 65.6 (-OCH2C(CH3)(CH2OH)2), 65.7 (2C, -OCH2C(CH3)(CH2OH)2), 72.0 (ω-chain C-4), 74.3 (cylopentane C-5), 77.2 (cyclopentane C-3), 114.6 (2C, aromatic C-2 and C-6), 121.7 (aromatic C-4), 127.2 (ω-chain CH-2), 127.4 (α-chain CH-6), 129.6 (2C, aromatic C-3 and C-5), 130.3 (α-chain CH-5), 147.7 (ω-chain C-1), 157.8 (aromatic C-1), 170.2, 170.4 and 170.7 (4C, four -COOCH3 groups), 173.1 (-CH2COOCH2), 195.5 (ω-chain C-3). HRMS (ESI): calcd. for C35H46O12Na [M + Na]+ 681.2887; found 681.2877.
3.6.7. 2,2-Bis(acetoxymethyl)propyl (5Z)-7-{(1R,2R,3R,5S)-3,5-diacetoxy-2-[(1E)-3,3-difluoro-4-(phe-noxy)-but-1-enyl]cyclopentyl}hept-5-enoate (31)
Deoxo-Fluor (39.1 mL, 91.2 mmol, 50% in THF) was added dropwise to a stirred solution of ketone 30 (5.0 g, 7.6 mmol) in CH2Cl2 (60 mL) at 0 °C under an argon atmosphere. The resulting mixture was refluxed for 24 h, cooled to 0–5 °C, whereupon saturated aqueous solution of NaHCO3 (200 mL) was added dropwise. After being stirred for 30 min at room temperature, the layers were separated and the aqueous phase was extracted with CH2Cl2 (3 × 50 mL). The combined extracts were washed with brine (100 mL) and dried over Na2SO4. Filtration and evaporation in vacuo furnished the crude product as a light yellow oil, which was purified by flash column chromatography (silica gel, 5%–20% AcOEt/hexanes) to give the difluoro derivative 31 (4.03 g, 78% yield) as a colorless oil. Rf = 0.64 (50% AcOEt/hexanes). = +27.75 (c 1.0, MeOH). FT-IR (thin film) ν (cm−1): 2945, 1739, 1599, 1496, 1378, 1239, 1159, 1046, 977, 849, 757, 693, 606, 509. 1H-NMR (CDCl3, 600 MHz) δ (ppm): 1.00 (s, 3H, -C(CH3)(CH2OH)2), 1.64 (m, 2H, α-chain CH2-3), 1.70 (ddd, J = 1.3, 4.5 and 15.8 Hz, 1H, one proton of the cyclopentane CH2-4 group), 1.77 (m, 1H, cyclopentane CH-1), 1.95–2.10 (three s and two m, 14H, four -COOCH3 groups and α-chain CH2-4), 2.14 (m, 2H, α-chain CH2-7), 2.28 (t, J = 7.6 Hz, 2H, α-chain CH2-2), 2.58 (ddd, J = 5.8, 9.0 and 15.8 Hz, 1H, one proton of the cyclopentane CH2-4), 2.68 (m, 1H, cyclopentane CH-2), 4.00 (m, 6H, -OCH2C(CH3)(CH2OH)2), 4.19 (m, 2H, ω-chain CH2-4), 4.95 (ddd, J = 4.5, 7.7 and 11.9 Hz, 1H, cyclopentane CH-3), 5.12 (m, 1H, cyclopentane CH-5), 5.32–5.40 (m, 2H, α-chain CH-5 and α-chain CH-6), 5.84 (m, 1H, ω-chain CH-2), 6.11 (m, 1H, ω-chain CH-1), 6.91 (m, 2H, aromatic CH-2 and CH-6), 7.00 (m, 1H, aromatic CH-4), 7.30 (m, 2H, aromatic CH-3 and CH-5). 13C-NMR (150 MHz, CDCl3) δ (ppm): 17.0 (-C(CH3)(CH2OH)2), 20.8, 21.1 and 21.2 (4C, four -COOCH3 groups), 24.6 (α-chain C-3), 24.8 (α-chain C-7), 26.6 (α-chain C-4), 33.5 (α-chain C-2), 38.2 (-C(CH3)(CH2OH)2), 39.0 (cyclopentane C-4), 47.4 (cyclopentane C-1), 51.8 (cyclopentane C-2), 65.6 (-OCH2C(CH3)(CH2OH)2), 65.8 (2C, -OCH2C(CH3)(CH2OH)2), 69.5 (2JC-F = 35.0 Hz, ω-chain C-4), 74.2 (cyclopentane C-5), 77.2 (cyclopentane C-3), 114.7 (2C, aromatic C-2 and C-6), 118.0 (1JC-F = 240.5 Hz, ω-chain C-3), 121.8 (aromatic C-4), 125.2 (2JC-F = 25.0 Hz, ω-chain C-2), 127.6 (α-chain CH-6), 129.6 (2C, aromatic C-3 and C-5), 130.1 (α-chain CH-5), 136.8 (3JC-F = 8.8 Hz, ω-chain C-1), 157.9 (aromatic C-1), 170.3, 170.6 and 170.8 (4C, four -COOCH3 groups), 173.1 (-CH2COOCH2). HRMS (ESI): calcd. for C35H46O11NaF2 [M + Na]+ 703.2906; found 703.2900.
3.6.8. Methyl (5Z)-7-{(1R,2R,3R,5S)-2-[(1E)-3,3-difluoro-4-(phenoxy)but-1-enyl]-3,5-dihydroxycyclo-pentyl}hept-5-enoate (32)
K2CO3 (0.46 g, 3.3 mmol) was added to a solution of ester 31 (0.75 g, 1.1 mmol) in MeOH (25 mL). The resulting suspension was stirred for 24 h at room temperature and then concentrated under reduced pressure. The residue was diluted with H2O (50 mL) and the product was extracted with AcOEt (3 × 25 mL). The combined extracts were washed with brine (100 mL) and dried over Na2SO4. Filtration and evaporation in vacuo furnished the crude product as a light yellow oil, which was purified by flash column chromatography (silica gel, 10%–40% AcOEt/hexanes) to give the methyl ester 32 (0.35 g, 76% yield) as a colorless oil. Rf = 0.48 (75% AcOEt/hexanes). = +23.97 (c 1.0, MeOH). FT-IR (thin film) ν (cm−1): 3412, 3009, 2935, 1733, 1676, 1599, 1497, 1456, 1438, 1303, 1250, 1155, 1055, 975, 935, 848, 756, 692, 509. 1H-NMR (CDCl3, 600 MHz) δ (ppm): 1.62 (m, 1H, cyclopentane CH-1), 1.68 (m, 2H, α-chain CH2-3), 1.85 (m, 1H, one proton of the cyclopentane CH2-4 group), 2.07 (m, 1H, one of the α-chain CH2-4 group), 2.10–2.18 (m, 3H, one of the α-chain CH2-4 group, one of the α-chain CH2-7 group and one of the cyclopentane CH2-4 group), 2.18–2.26 (br s, 2H, two -OH), 2.27–2.36 (m, 3H, one of the α-chain CH2-7 group and α-chain CH2-2), 2.47 (m, 1H, cyclopentane CH-2), 3.66 (s, 3H, -COOCH3), 4.03 (m, 1H, cyclopentane CH-3), 4.17–4.23 (m, 2H, cyclopentane CH-5 and α-chain CH2-4), 5.33–5.42 (m, 2H, α-chain CH-5 and α-chain CH-6), 5.80 (m, 1H, ω-chain CH-2), 6.11 (m, 1H, ω-chain CH-1), 6.91 (m, 2H, aromatic CH-2 and CH-6), 7.00 (m, 1H, aromatic CH-4), 7.30 (m, 2H, aromatic CH-3 and CH-5). 13C-NMR (150 MHz, CDCl3) δ (ppm): 24.7 (α-chain C-3), 25.7 (α-chain C-7), 26.6 (α-chain C-4), 33.3 (α-chain C-2), 43.0 (cyclopentane C-4), 50.5 (cyclopentane C-1), 51.6 (-COOCH3), 55.8 (cyclopentane C-2), 69.5 (2JC-F = 35.0 Hz, ω-chain C-4), 73.3 (cyclopentane C-5), 78.0 (cyclopentane C-3), 114.7 (2C, aromatic C-2 and C-6), 118.1 (1JC-F = 241.0 Hz, ω-chain C-3), 121.8 (aromatic C-4), 123.6 (2JC-F = 24.9 Hz, ω-chain C-2), 128.6 (α-chain CH-6), 129.6 (2C, aromatic C-3 and C-5), 130.0 (α-chain CH-5), 138.6 (3JC-F = 9.1 Hz, ω-chain C-1), 157.9 (aromatic C-1), 174.3 (C=O). HRMS (ESI): calcd. for C23H30O5NaF2 [M + Na]+ 447.1959; found 447.1960.
3.6.9. (5Z)-7-{(1R,2R,3R,5S)-2-[(1E)-3,3-difluoro-4-(phenoxy)but-1-enyl]-3,5-dihydroxycyclopentyl}-hept-5-enoic acid (6)
LiOH·H2O (1.4 g, 33.36 mmol) was added in one portion to a solution of ester 31 (2.84 g, 4.17 mmol) in MeOH (50 mL). The resulting suspension was stirred overnight resulting in disappearance of the starting material 31 (TLC, MeOH/AcOEt 1:6). After cooling and evaporating the solvent, the residual solid was dissolved in water (150 mL) and washed with Et2O (25 mL) to remove organic impurities. The water layer was acidified with citric acid to pH 4–5 and the product was extracted with AcOEt (3 × 50 mL). The combined organic extracts were dried over anhydrous Na2SO4, filtered and evaporated in vacuo to afford the crude acid. Purification by silica gel flash chromatography with gradient elution 10%–50% MeOH/AcOEt afforded the pure tafluprost acid (6) (1.69 g, 98% yield) as a thick pale yellow oil. Rf = 0.49 (15% MeOH/AcOEt). = +17.98 (c 1.0, MeOH). FT-IR (thin film) ν (cm−1): 3370, 2933, 1709, 1599, 1497, 1457, 1406, 1302, 1249, 1155, 1055, 974, 934, 849, 755, 692, 509. 1H-NMR (CDCl3, 600 MHz) δ (ppm): 1.59 (m, 1H, cyclopentane CH-1), 1.66 (m, 2H, α-chain CH2-3), 1.84 (m, 1H, one of the cyclopentane CH2-4), 2.00–2.20 (m, 3H, one of the α-chain CH2-4, one of the α-chain CH2-7 and one of the cyclopentane CH2-4 group), 2.28–2.36 (m, 3H, one of the α-chain CH2-7 and α-chain CH2-2), 2.47 (m, 1H, cyclopentane CH-2), 4.03 (m, 1H, cyclopentane CH-3), 4.17–4.22 (m, 3H, cyclopentane CH-5 and ω-chain CH2-4), 5.33–5.42 (m, 2H, α-chain CH-5 and α-chain CH-6), 5.80 (m, 1H, ω-chain CH-2), 6.10 (m, 1H, ω-chain CH-1), 6.91 (m, 2H, aromatic CH-2 and CH-6), 6.99 (m, 1H, aromatic CH-4), 7.29 (m, 2H, aromatic CH-3 and CH-5). 13C-NMR (150 MHz, CDCl3) δ (ppm): 24.5 (α-chain C-3), 25.7 (α-chain C-7), 26.4 (α-chain C-4), 33.2 (α-chain C-2), 42.8 (cyclopentane C-4), 50.4 (cyclopentane C-1), 55.5 (cyclopentane C-2), 69.5 (2JC-F = 34.7 Hz, ω-chain C-4), 73.4 (cyclopentane C-5), 77.8 (cyclopentane C-3), 114.8 (2C, aromatic C-2 and C-6), 118.2 (1JC-F = 240.0 Hz, ω-chain C-3), 121.8 (aromatic C-4), 123.7 (2JC-F = 25.0 Hz, ω-chain C-2), 128.8 (α-chain CH-6), 129.6 (2C, aromatic C-3 and C-5), 129.9 (α-chain CH-5), 138.6 (3JC-F = 8.7 Hz, ω-chain C-1), 158.0 (aromatic C-1), 178.8 (C=O). HRMS (ESI): calcd. for C22H28O5NaF2 [M + Na]+ 433.1803; found 433.1801.
3.6.10. Isopropyl (5Z)-7-{(1R,2R,3R,5S)-2-[(1E)-3,3-difluoro-4-(phenoxy)but-1-enyl]-3,5-dihydroxycy-clopentyl}hept-5-enoate (5)
DBU (3.4 mL, 22.82 mmol) was added dropwise to a stirred solution of acid
6 (1.34 g, 3.26 mmol) in anhydrous Me
2CO (20 mL) at 0 °C under an argon atmosphere. The mixture was allowed to warm to room temperature, whereupon 2-iodopropane (2.3 mL, 22.82 mmol) was added dropwise. The resulting solution was stirred for 21 h resulting in disappearance of the starting acid
6 (TLC, MeOH/AcOEt 1:6). The reaction was quenched with AcOEt (100 mL). The resulting white solid was filtered off and washed with AcOEt (3 × 15 mL). The filtrate and washings were combined and acidified with 3% citric acid solution to pH 5–6. The resulting layers were separated and the aqueous phase was extracted with AcOEt (3 × 15 mL). The combined extracts were washed with saturated NaHCO
3 (100 mL), brine (100 mL) and dried over anhydrous Na
2SO
4. Filtration and evaporation in vacuo furnished the crude product, which was purified by silica gel flash chromatography with gradient elution 10%–45% AcOEt/hexanes to afford the title compound
5 (1.23 g, 83% yield) as a pale yellow oil. This sample was further purified by preparative HPLC on silica gel to give pharmaceutical grade tafluprost (
5) as a thick colorless oil.
= +22.46 (
c 1.0, MeOH). Lit.
= +21.6 (
c 1.0, CHCl
3) [
45].
Rf = 0.48 (75% AcOEt/hexanes). FT-IR (thin film) ν (cm
−1): 3408, 2980, 2935, 1727, 1599, 1497, 1456, 1375, 1303, 1250, 1154, 1108, 1055, 973, 936, 848, 821, 755, 691, 509.
1H-NMR (CDCl
3, 600 MHz) δ (ppm): 1.22 (d, 6H, -CH(C
H3)
2), 1.61 (m, 1H, cyclopentane CH-1), 1.67 (m, 2H, α-chain CH
2-3), 1.85 (m, 1H, one of the cyclopentane CH
2-4 group), 2.06 (m, 1H, one of the cyclopentane CH
2-4 group), 2.09–2.16 (m, 3H, one of the α-chain CH
2-4, one of the α-chain CH
2-7 group and one of the cyclopentane CH
2-4 group), 2.24–2.36 (m, 3H, α-chain CH
2-2 and one of the α-chain CH
2-7), 2.47 (m, 1H, cyclopentane CH-2), 4.02 (m, 1H, cyclopentane CH-3), 4.17–4.22 (m, 2H, cyclopentane CH-5 and ω-chain CH
2-4), 5.00 (sept,
J = 6.30 Hz, 1H, -C
H(CH
3)
2), 5.33–5.42 (m, 2H, α-chain CH-5 and α-chain CH-6), 5.80 (m, 1H, ω-chain CH-2), 6.10 (m, 1H, ω-chain CH-1), 6.92 (m, 2H, aromatic CH-2 and CH-6), 7.00 (m, 1H, aromatic CH-4), 7.30 (m, 2H, aromatic CH-3 and CH-5).
13C-NMR (150 MHz, CDCl
3) δ (ppm): 21.8 (2s, 2C, -CH(
CH
3)
2), 24.8 (α-chain C-3), 25.7 (α-chain C-7), 26.6 (α-chain C-4), 34.0 (α-chain C-2), 42.9 (cyclopentane C-4), 50.5 (cyclopentane C-1), 55.7 (cyclopentane C-2), 67.7 (-
CH(CH
3)
2), 69.5 (
2JC-F = 34.9 Hz, ω-chain C-4), 73.3 (cyclopentane C-5), 77.9 (cyclopentane C-3), 114.8 (2C, aromatic C-2 and C-6), 118.2 (
1JC-F = 240.0 Hz, ω-chain C-3), 121.8 (aromatic C-4), 123.6 (
2JC-F = 25.0 Hz, ω-chain C-2), 128.6 (α-chain CH-6), 129.6 (2C, aromatic C-3 and C-5), 130.1 (α-chain CH-5), 138.6 (
3JC-F = 8.8 Hz, ω-chain C-1), 157.9 (aromatic C-1), 173.5 (C=O).
19F-NMR (470 MHz, CDCl
3) δ (ppm): −102.6 (
2JF-F = 255.8 Hz), −103.1 (
2JF-F = 255.8 Hz). HRMS (ESI): calcd. for C
25H
34O
5NaF
2 [M + Na]
+ 475.2272; found 475.2272.
3.6.11. (5Z)-N-Ethyl-7-{(1R,2R,3R,5S)-2-[(1E)-3,3-difluoro-4-(phnoxy)but-1-enyl]-3,5-dihydroxycy-clopentyl}hept-5-enamide (33)
EtNH2 (50 mL, 629.0 mmol, 70% in water) was added in one portion to the tafluprost 5 (0.8 g, 1.77 mmol). The resulting solution was stirred for 60 h to disappearance of the starting material 5 (TLC, 7% MeOH/CH2Cl2). The excess of EtNH2 was then removed by evaporation under reduced pressure and the aqueous residue was diluted with brine (50 mL) and AcOEt (50 mL). The resulting layers were separated and the aqueous phase was extracted with AcOEt (3 × 25 mL). The combined extracts were dried over anhydrous Na2SO4. Filtration and evaporation in vacuo furnished the crude product, which was purified by silica gel flash chromatography with gradient elution from 1%–5% MeOH/CH2Cl2 to afford the title compound 33 (0.66 g, 85% yield) as colorless oil. Rf = 0.34 (7% MeOH/CH2Cl2). = +27.55 (c 1.0, MeOH). FT-IR (thin film) ν (cm−1): 3308, 3095, 2934, 1645, 1599, 1553, 1497, 1456, 1294, 1249, 1155, 1055, 974, 933, 848, 755, 691, 509. 1H-NMR (CDCl3, 600 MHz) δ (ppm): 1.12 (t, 3H, -CH2CH3), 1.58 (m, 1H, cyclopentane CH-1), 1.67 (m, 2H, α-chain CH2-3), 1.87 (m, 1H, one of the cyclopentane CH2-4 group), 2.00 (m, 1H, one of the α-chain CH2-4 group), 2.05–2.22 (m, 5H, one of the α-chain CH2-4 group, one of the α-chain CH2-7 group, one of the cyclopentane CH2-4 group and α-chain CH2-2), 2.38 (m, 1H, one of the α-chain CH2-7 group), 2.49 (m, 1H, cyclopentane CH-2), 2.94 (br s, 2H, two -OH), 3.26 (m, 2H, -CH2CH3), 4.04 (m, 1H, cyclopentane CH-3), 4.16–4.22 (m, 2H, cyclopentane CH-5 and ω-chain CH2-4), 5.33–5.42 (m, 2H, α-chain CH-5 and α-chain CH-6), 5.69 (br s, 1H, -NH-), 5.79 (m, 1H, ω-chain CH-2), 6.11 (m, 1H, ω-chain CH-1), 6.91 (m, 2H, aromatic CH-2 and CH-6), 7.00 (m, 1H, aromatic CH-4), 7.30 (m, 2H, aromatic CH-3 and CH-5). 13C-NMR (150 MHz, CDCl3) δ (ppm): 14.8 (-CH2CH3), 25.6 (α-chain C-3), 25.7 (α-chain C-7), 26.6 (α-chain C-4), 34.4 (-CH2CH3), 35.8 (α-chain C-2), 43.1 (cyclopentane C-4), 50.7 (cyclopentane C-1), 55.7 (cyclopentane C-2), 69.5 (2JC-F = 34.7 Hz, ω-chain C-4), 73.1 (cyclopentane C-5), 78.0 (cyclopentane C-3), 114.8 (2C, aromatic C-2 and C-6), 118.2 (1JC-F = 240.0 Hz, ω-chain C-3), 121.8 (aromatic C-4), 123.3 (2JC-F = 24.8 Hz, ω-chain C-2), 128.9 (α-chain CH-6), 129.6 (2C, aromatic C-3 and C-5), 130.0 (α-chain CH-5), 138.9 (3JC-F = 8.8 Hz, ω-chain C-1), 158.0 (aromatic C-1), 173.2 (C=O). 19F-NMR (470 MHz, CDCl3) δ (ppm): −102.4 (2JF-F = 255.8 Hz), −103.1 (2JF-F = 255.8 Hz). HRMS (ESI): calcd. for C24H33NO4NaF2 [M + Na]+ 460.2275; found 460.2274.