Synthesis, Spectroscopic Characterization and Antimicrobial Potential of Certain New Isatin-Indole Molecular Hybrids
Abstract
:1. Introduction
2. Results and Discussion
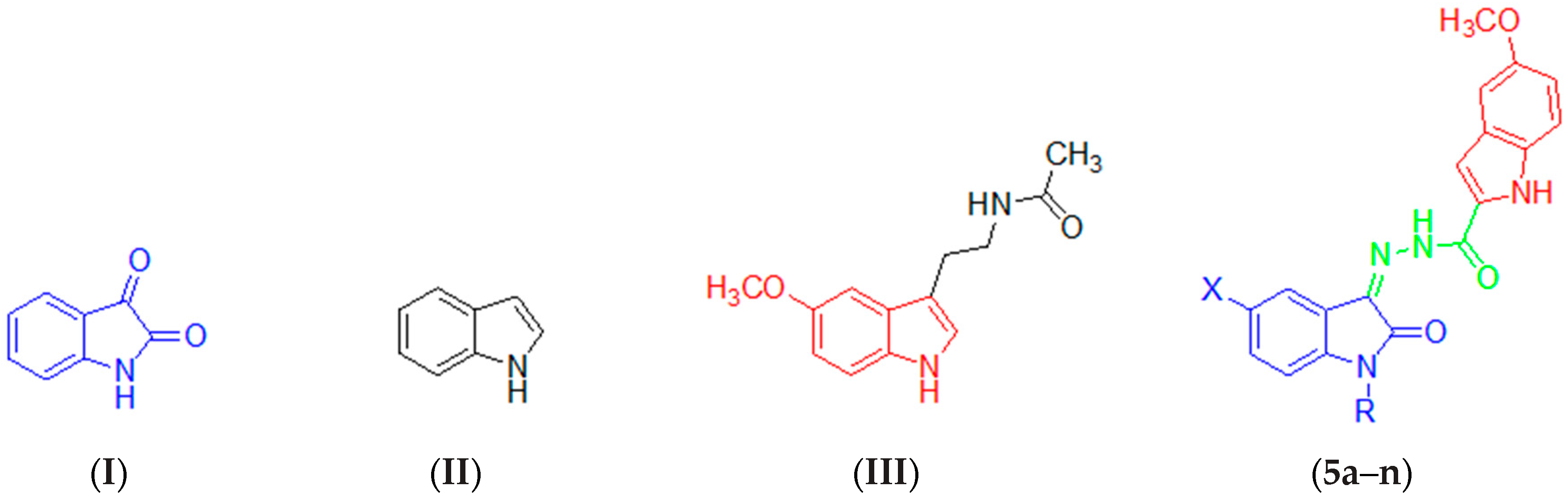
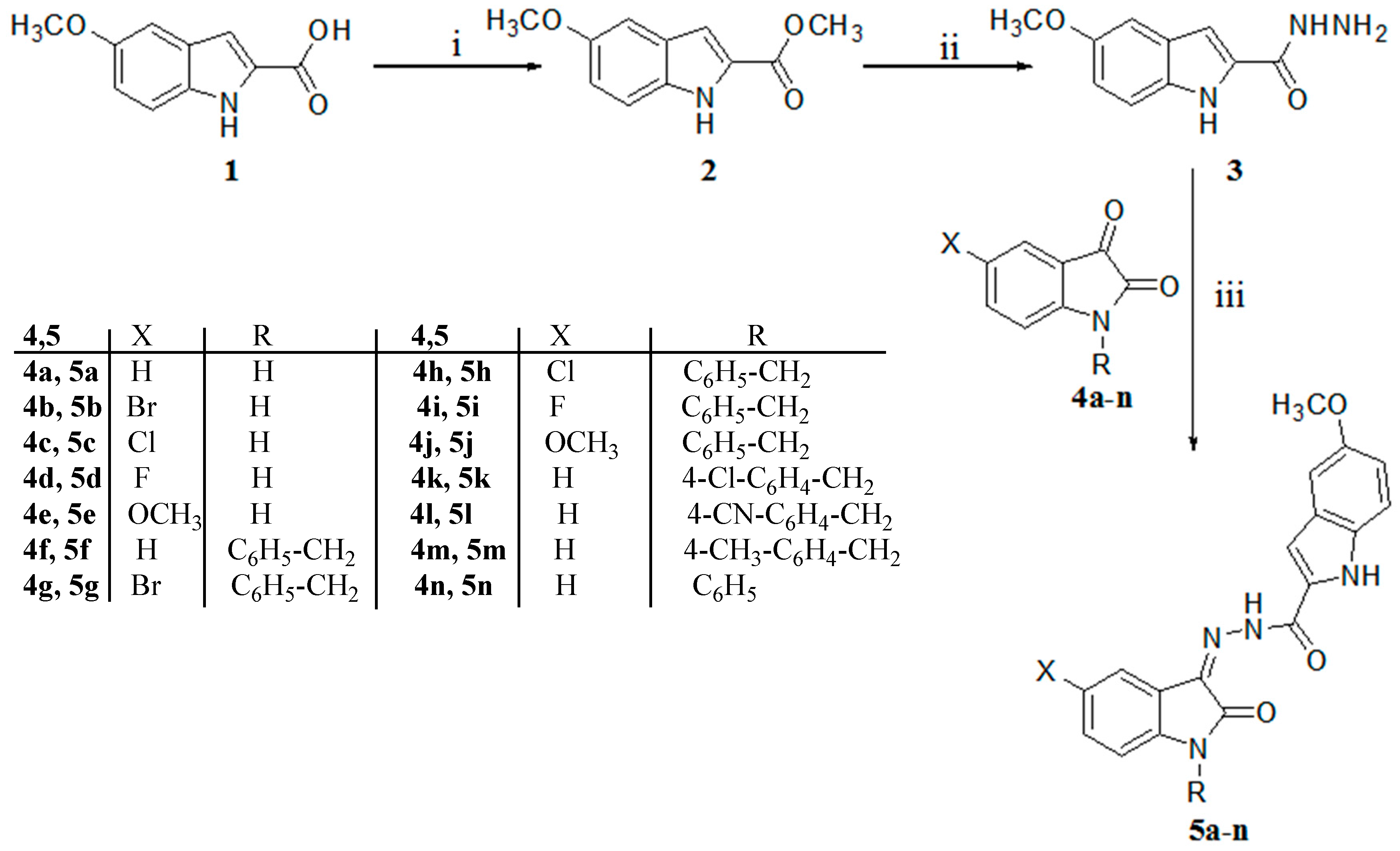
2.1. Chemistry
2.2. Antimicrobial Evaluation
2.2.1. Antimicrobial Susceptibility Testing
2.2.2. Scanning Electron Microscopy
3. Experimental
3.1. General
3.2. Chemistry
3.2.1. Synthesis of Methyl 5-Methoxy-1H-Indole-2-Carboxylate (2)
3.2.2. Synthesis of 5-Methoxy-1H-Indole-2-Carbohydrazide (3)
3.2.3. General Procedure of the Synthesis of the N-Benzylated isatins 4f–m
- 1-Benzyl-1H-indole-2,3-dione (4f): Orange powder; m.p. 138–140 °C [45].
- 1-Benzyl-5-bromo-1H-indole-2,3-dione (4g): Orange powder; m.p. 148–150 °C [46].
- 1-Benzyl-5-chloro-1H-indole-2,3-dione (4h): Orange powder; m.p. 138–140 °C [47].
- 1-Benzyl-5-fluoro-1H-indole-2,3-dione (4i): Light-red powder; m.p. 135–137 °C [48].
- 1-Benzyl-5-methoxy-1H-indole-2,3-dione (4j): Light brown powder; m.p. 123–125 °C [49].
- 1-(4-Chlorobenzyl)-1H-indole-2,3-dione (4k): Orange powder; m.p. 168–170 °C [50].
- 4-[(2,3-Dioxo-2,3-dihydro-1H-indol-1-yl)methyl]benzonitrile (4l): Orange powder; m.p. 217–219 °C [50].
- 1-(4-Methylbenzyl)-1H-indole-2,3-dione (4m): Orange powder; m.p. 143–145 °C [51].
3.2.4. General Procedure for the Synthesis of the Target Compounds 5a–n
3.3. Antimicrobial Activity
3.3.1. Antimicrobial Agents
3.3.2. Media
3.3.3. Isolates
3.3.4. Culture Conditions
3.3.5. Growth of the Tested Microorganisms
3.3.6. Determination of Minimum Inhibitory Concentrations
3.3.7. Disk Diffusion Assay
3.3.8. Scanning Electron Microscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Canuto, M.M.; Rodero, F.G. Antifungal drug resistance to azoles and polyenes. Lancet Infect. Dis. 2002, 2, 550–563. [Google Scholar] [CrossRef]
- Sanglard, D.; Odds, F.C. Resistance of Candida species to antifungal agents: Molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2002, 2, 73–85. [Google Scholar] [CrossRef]
- Schillaci, D.; Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G.; Cascioferro, S.M. Pharmaceutical approaches to target antibiotic resistance mechanisms. J. Med. Chem. 2017, 60, 8268–8297. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, O.L. Untersuchungen über den Indigo. Arch. Pharm. 1840, 72, 253–285. [Google Scholar] [CrossRef]
- Laurent, A. Recherches sur l’indigo. Ann. Chim. Phys. 1840, 3, 393–434. [Google Scholar]
- Ama-Asamoah, R.; Kapadia, G.J.; Lloyd, H.A.; Sokoloski, E.A. Picratidine, a new indole alkaloid from Picralima nitida seeds. J. Nat. Prod. 1990, 53, 975–977. [Google Scholar] [CrossRef]
- Grafe, U.; Radics, L. Isolation and structure elucidation of 6-(3’-methylbuten-2’-yl)isatin, an unusual metabolite from Streptomyces albus. J. Antibiot. 1986, 39, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K.; Bremner, J.B.; Davis, A.R. Indole derivatives from the egg masses of muricid molluscs. Molecules 2001, 6, 70–78. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Smitha, S.; Jyoti, M.; Sridhar, S.K. Biological activities of isatin and its derivatives. Acta Pharm. 2005, 55, 27–46. [Google Scholar] [PubMed]
- Cerchiaro, G.; Ferreira, A.M.d.C. Oxindoles and copper complexes with oxindole-derivatives as potential pharmacological agents. J. Braz. Chem. Soc. 2006, 17, 1473–1485. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Sriram, D.; Nath, G.; DeClercq, E. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4′-chlorophenyl)thiazol-2-yl]thiosemicarbazide. Eur. J. Pharm. Sci. 1999, 9, 25–31. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Chakrabarti, A. Dose-related proconvulsant and anticonvulsant activity of isatin, a putative biological factor, in rats. Indian J. Exp. Biol. 1998, 36, 118–121. [Google Scholar] [PubMed]
- Pandeya, S.N.; Yogeeswari, P.; Ram, D.S.; Nath, G. Synthesis and antimicrobial activity of N-Mannich bases of 3-[N’-sulphadoximino]isatin and its methyl derivative. Boll. Chim. Farm. 1998, 137, 321–324. [Google Scholar]
- Banerjee, D.; Yogeeswari, P.; Bhat, P.; Thomas, A.; Srividya, M.; Sriram, D. Novel isatinyl thiosemicarbazones derivatives as potential molecule to combat HIV-TB co-infection. Eur. J. Med. Chem. 2011, 46, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Sarangapani, M.; Reddy, V. Synthesis and antimicrobial activity of 1-[(N,N-disubstituted amino) methyl]-3-[(2-phenyl-3, 4-dihydro-4-oxoquinazoline-3-yl]indole-2-one. Indian J. Heterocycl. Chem. 1994, 3, 257–260. [Google Scholar]
- Karki, S.S.; Kulkarni, A.A.; Kumar, S.; Veliyath, S.K.; De Clercq, E.; Balzarini, J. Synthesis and biological evaluation of 2-(5-substituted-1-((diethylamino)methyl)-2-oxoindolin-3-ylidene)-N-substituted-hydrazinecarbothioamides. Med. Chem. Res. 2013, 22, 2014–2022. [Google Scholar] [CrossRef]
- Sridhar, S.K.; Saravanan, M.; Ramesh, A. Synthesis and antibacterial screening of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur. J. Med. Chem. 2001, 36, 615–625. [Google Scholar] [CrossRef]
- Raj, R.; Singh, P.; Haberkern, N.T.; Faucher, R.M.; Patel, N.; Land, K.M.; Kumar, V. Synthesis of 1H-1,2,3-triazole linked β-lactam–isatin bi-functional hybrids and preliminary analysis of in vitro activity against the protozoal parasite Trichomonas vaginalis. Eur. J. Med. Chem. 2013, 63, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, A.; Nisha, N.; Bains, T.; Hahn, H.J.; Liu, N.; Tam, C.; Cheng, L.; Kim, J.H.; Debnath, A. Design, synthesis and preliminary antimicrobial evaluation of N-alkyl chain tethered C-5 functionalized bis-isatins. Med. Chem. Comm. 2017. In Press. [Google Scholar]
- Joshi, K.C.; Chand, P. Biologically active indole derivatives. Pharmazie 1982, 37, 1–12. [Google Scholar] [PubMed]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef] [PubMed]
- Fu, L. Advances in the total syntheses of complex indole natural products. In Heterocyclic Scaffolds II; Springer: Berlin, Germany, 2010; pp. 433–480. [Google Scholar]
- Lindel, T.; Marsch, N.; Adla, S.K. Indole prenylation in alkaloid synthesis. In Alkaloid Synthesis; Springer: Berlin, Germany, 2011; pp. 67–129. [Google Scholar]
- Rapolu, M.; Kumanan, R.; Duganath, N.; Murthy, M.; Ahmed, N.; Subramanyam, S. Synthesis, characterization and pharmacological Screening of 2-methyl-1H-indole-3-carboxylicacid [2-(2-substituted-phenyl)-4-oxothiazolidin-3-yl]amide derivatives. Int. J. Chem. Sci. Appl. 2011, 2, 91–99. [Google Scholar]
- Lau, C.K.; Black, W.C.; Belley, M.; Chan, C.; Charleson, S.; Denis, D.; Gauthier, J.Y.; Gordon, R.; Guay, D.; Hamel, P.; et al. From indomethacin to a selective COX-2 inhibitor. Development of indolalkanoic acids as potent and selective cyclooxygenase-2 inhibitors. Adv. Exp. Med. Biol. 1997, 407, 73–78. [Google Scholar] [PubMed]
- Flynn, B.L.; Hamel, E.; Jung, M.K. One-pot synthesis of benzo[b]furan and indole inhibitors of tubulin polymerization. J. Med. Chem. 2002, 45, 2670–2673. [Google Scholar] [CrossRef] [PubMed]
- Leboho, T.C.; Michael, J.P.; van Otterlo, W.A.; van Vuuren, S.F.; de Koning, C.B. The synthesis of 2- and 3-aryl indoles and 1,3,4,5-tetrahydropyrano[4,3-b]indoles and their antibacterial and antifungal activity. Bioorg. Med. Chem. Lett. 2009, 19, 4948–4951. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Guo, Z.; Chu, F.; Bai, A.; Yi, X.; Cheng, G.; Li, J. Synthesis and biological evaluation of substituted 2-sulfonyl-phenyl-3-phenyl-indoles: A new series of selective COX-2 inhibitors. Bioorg. Med. Chem. 2003, 11, 1153–1160. [Google Scholar] [CrossRef]
- Samosorn, S.; Bremner, J.B.; Ball, A.; Lewis, K. Synthesis of functionalised 2-aryl-5-nitro-1H-indoles and their activity as bacterial NorA efflux pump inhibitors. Bioorg. Med. Chem. 2006, 14, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Falcó, J.L.; Piqué, M.; González, M.; Buira, I.; Méndez, E.; Terencio, J.; Pérez, C.; Príncep, M.; Palomer, A.; Guglietta, A. Synthesis, pharmacology and molecular modeling of N-substituted 2-phenyl-indoles and benzimidazoles as potent GABAA agonists. Eur.J. Med.Chem. 2006, 41, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Mazzon, E.; Muia, C.; Bramanti, P.; De Sarro, A.; Cuzzocrea, S. Attenuation in the evolution of experimental spinal cord trauma by treatment with melatonin. J. Pineal Res. 2005, 38, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.F. Melatonin, the pineal gland and their implications for headache disorders. Cephalalgia 2005, 25, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E.; Sauer, L.A.; Dauchy, R.T. Melatonin as a chronobiotic/anticancer agent: Cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr. Top. Med. Chem. 2002, 2, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.I.; Julius, J.; Witt-Enderby, P.A.; Zlotos, D.P. Synthesis and pharmacological evaluation of 6a,7-dihydro-6H,13H-pyrazino [1,2-a;4,5-a′]diindole analogs as melatonin receptor ligands. Tetrahedron 2007, 63, 754–760. [Google Scholar] [CrossRef]
- Attia, M.I.; Witt-Enderby, P.A.; Julius, J. Synthesis and pharmacological evaluation of pentacyclic 6a,7-dihydrodiindole and 2,3-dihydrodiindole derivatives as novel melatoninergic ligands. Bioorg. Med. Chem. 2008, 16, 7654–7661. [Google Scholar] [CrossRef] [PubMed]
- Markl, C.; Attia, M.I.; Julius, J.; Sethi, S.; Witt-Enderby, P.A.; Zlotos, D.P. Synthesis and pharmacological evaluation of 1,2,3,4-tetrahydropyrazino[1,2-a]indole and 2-[(phenylmethyl amino)methyl]-1H-indole analogues as novel melatoninergic ligands. Bioorg. Med. Chem. 2009, 17, 4583–4594. [Google Scholar] [CrossRef] [PubMed]
- Markl, C.; Clafshenkel, W.P.; Attia, M.I.; Sethi, S.; Witt-Enderby, P.A.; Zlotos, D.P. N-Acetyl-5-arylalkoxytryptamine analogs: Probing the melatonin receptors for MT1-selectivity. Arch. Pharm. 2011, 344, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Mayur, Y.C.; Peters, G.J.; Prasad, V.V.; Lemo, C.; Sathish, N.K. Design of new drug molecules to be used in reversing multidrug resistance in cancer cells. Curr. Cancer Drug Targets 2009, 9, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Khalil, N.A.; Ahmed, E.M. Synthesis of novel isatin-thiazoline and isatin-benzimidazole conjugates as anti-breast cancer agents. Arch. Pharm. Res. 2011, 34, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.; Eldehna, W.M.; Abou-Seri, S.M.; Abdel-Aziz, H.A.; Aly, M.H.; Tolba, M.F. Design, synthesis and in vitro antiproliferative activity of novel isatin-quinazoline hybrids. Arch. Pharm. 2015, 348, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Haress, N.G.; Ghabbour, H.A.; Almutairi, M.S.; Fun, H.-K.; Attia, M.I. Crystal structure of 5-methoxy-N′-[(3Z)-5-chloro-1-(4-fluorobenzyl)-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1H-indole-2-carbohydrazide-DMSO (1/1), C25H18ClFN4O3·C2H6OS. Z. Krist. New Crys. Struct. 2016, 231, 1021–1023. [Google Scholar] [CrossRef]
- Coowar, D.; Bouissac, J.; Hanbali, M.; Paschaki, M.; Mohier, E.; Luu, B. Effects of indole fatty alcohols on the differentiation of neural stem cell derived neurospheres. J. Med. Chem. 2004, 47, 6270–6282. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.S.; Xavier, S.; Sathish, M.; Ghabbour, H.A.; Sebastian, S.; Periandy, S.; Al-Wabli, R.I.; Attia, M.I. Spectroscopic (FT-IR, FT-Raman, UV, 1H and 13C NMR) profiling and computational studies on methyl 5-methoxy-1H-indole-2-carboxylate: A potential precursor to biologically active molecules. J. Mol. Struct. 2017, 1133, 199–210. [Google Scholar] [CrossRef]
- Bhat, G.; Siddappa, S. Synthesis of indole-2-carbaldehydes, 2-(2-aminoethyl)-and 2-(2-amino propyl)indoles. J. Chem. Soc. C 1971, 178–181. [Google Scholar] [CrossRef]
- Schutte, M.; Visser, H.; Roodt, A.; Braband, H. N-Benzylisatin. Acta Crystallogr. Sect. E 2012, 68, o777. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, K.; Mimura, S.; Numata, Y.; Mikami, K. Palladium-Catalyzed enantioselective ene and Aldol reactions with isatins, keto esters, and diketones: Reliable approach to chiral tertiary alcohols. Eur. J. Org. Chem. 2011, 2011, 62–65. [Google Scholar] [CrossRef]
- Kamal, A.; Mahesh, R.; Nayak, V.L.; Babu, K.S.; Kumar, G.B.; Shaik, A.B.; Kapure, J.S.; Alarifi, A. Discovery of pyrrolospirooxindole derivatives as novel cyclin dependent kinase 4 (CDK4) inhibitors by catalyst-free, green approach. Eur. J. Med. Chem. 2016, 108, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Senwar, K.R.; Jeengar, M.K.; Reddy, T.S.; Naidu, V.; Kamal, A.; Shankaraiah, N. H2O-mediated isatin spiro-epoxide ring opening with NaCN: Synthesis of novel 3-tetrazolylmethyl-3-hydroxy-oxindole hybrids and their anticancer evaluation. Eur. J. Med. Chem. 2015, 104, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Babu, K.S.; Vardhan, M.V.; Hussaini, S.A.; Mahesh, R.; Shaik, S.P.; Alarifi, A. Sulfamic acid promoted one-pot three-component synthesis and cytotoxic evaluation of spirooxindoles. Bioorg. Med. Chem. Lett. 2015, 25, 2199–2202. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.-T.; Lee, W.-C.; Liao, J.-H.; Cheng, J.-J.; Lin, L.-C.; Chen, C.-Y.; Song, J.-S.; Wu, M.-H.; Shia, K.-S.; Li, W.-T. Synthesis and evaluation of 3-ylideneoxindole acetamides as potent anticancer agents. Eur. J. Med. Chem. 2015, 98, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vintonyak, V.V.; Warburg, K.; Over, B.; Hübel, K.; Rauh, D.; Waldmann, H. Identification and further development of thiazolidinones spiro-fused to indolin-2-ones as potent and selective inhibitors of Mycobacterium tuberculosis protein tyrosine phosphatase B. Tetrahedron 2011, 67, 6713–6729. [Google Scholar] [CrossRef]
- Garcia, L.S. Clinical Microbiology Procedures Handbook; American Society for Microbiology Press: Washington, DC, USA, 2010. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Abbreviated Identification of Bacteria and Yeas, 2nd ed.; CLSI Document M35-A2; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; NCCLS Document M26-A; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 1999. [Google Scholar]
- Clinical Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; CLSI Document M07-A10; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2015. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard-Third Edition CLSI document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard CLSI Document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- National Committee Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests; M100-S21; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011; Volume 31. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests, 12th ed.; CLSI Document M02-A12; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Stadtländer, C. Scanning electron microscopy and transmission electron microscopy of mollicutes: Challenges and opportunities. Mod. Res. Edu. Top. Microsc. 2007, 1, 122–131. [Google Scholar]
Sample Availability: Samples of the synthesized compounds are available from the corresponding author. |

| Comp. No. | DIZ in mm ± S.D. * | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | ||||||||||||
| Gram-Negative Bacteria | Gram-Positive Bacteria | Fungi | ||||||||||
| E. coli | Ps.aeruginosa | P. vulgaris | K. pneumonia | S. enteridis | S. aureus | MRSA | E. fecalis | B. subtilis | C. albicans | A. niger | P. notatum | |
| 5a | 14 ± 0.6 | 8 ± 0.8 | −ve | −ve | −ve | 14 ± 0.8 | −ve | −ve | −ve | −ve | −ve | 11 ± 0.3 |
| 5b | 10 ± 1.0 | 8 ± 0.4 | −ve | −ve | −ve | 18 ± 0.4 | 18 ± 0.5 | −ve | 18 ± 1.2 | 12 ± 0.7 | −ve | 13 ± 0.0 |
| 5c | 12 ± 2.0 | 8 ± 0.4 | −ve | −ve | −ve | 18 ± 0.4 | 18 ± 0.0 | −ve | 12 ± 1.6 | 10 ± 0.0 | −ve | −ve |
| 5d | 10 ± 0.5 | 10 ± 0.0 | −ve | −ve | −ve | 12 ± 0.0 | −ve | −ve | 11 ± 0.9 | 12 ± 0.0 | −ve | −ve |
| 5e | −ve | 9 ± 0.6 | −ve | −ve | 9 ± 0.3 | 9 ± 0.0 | 9 ± 0.0 | 9 ± 0.2 | 13 ± 0.4 | 20 ± 0.2 | 18 ± 0.4 | 15 ± 0.9 |
| 5f | 10 ± 0.8 | 10 ± 1.0 | −ve | 12 ± 1.9 | −ve | 20 ± 1.0 | 14 ± 0.7 | −ve | 14 ± 0.6 | 10 ± 0.0 | 11 ± 0.0 | 9 ± 0.6 |
| 5g | 20 ± 0.9 | 14 ± 1.8 | 12 ± 0.7 | −ve | 10 ± 0.4 | 22 ± 0.43 | −ve | 12 ± 0.0 | 12 ± 0.0 | 18 ± 0.0 | 16 ± 0.5 | 13 ± 0.0 |
| 5h | 12 ± 1.2 | 18 ± 0.4 | −ve | 12 ± 1.76 | −ve | 18 ± 0.4 | 12 ± 0.3 | −ve | 12 ± 0.0 | 18 ± 0.5 | 11 ± 0.0 | 11 ± 0.12 |
| 5i | 10 ± 1.4 | 16 ± 0.9 | −ve | 12 ± 1.6 | −ve | 18 ± 0.9 | 14 ± 0.6 | −ve | 12 ± 1.0 | 14 ± 0.7 | −ve | −ve |
| 5j | −ve | 11 ± 0.4 | −ve | −ve | 9 ± 0.1 | 15 ± 0.6 | 9 ± 0.0 | 9 ± 0.5 | 18 ± 0.8 | 29 ± 0.0 | 15 ± 1.1 | 14 ± 0.2 |
| 5k | −ve | 9 ± 0.0 | −ve | −ve | 11 ± 0.0 | 15 ± 0.0 | 9 ± 0.6 | 9 ± 0.0 | 16 ± 0.12 | 18 ± 1.0 | 18 ± 0.0 | −ve |
| 5l | −ve | 9 ± 0.0 | −ve | −ve | 11 ± 0.1 | 21 ± 0.5 | 9 ± 0.2 | 14 ± 0.5 | 11 ± 0.8 | 16 ± 0.0 | 8 ± 0.0 | −ve |
| 5m | −ve | 9 ± 0.0 | −ve | −ve | 11 ± 0.0 | 18 ± 1.1 | 9 ± 0.0 | 9 ± 0.0 | 20 ± 0.6 | 18 ± 0.0 | 14 ± 0.0 | −ve |
| 5n | −ve | 12 ± 0.4 | −ve | −ve | 11 ± 0.3 | 18 ± 0.4 | 16 ± 0.6 | 9 ± 0.6 | 16 ± 1.0 | 18 ± 0.0 | 18 ± 0.0 | −ve |
| AMP | 30 ± 0.0 | −ve | 36 ± 0.7 | −ve | 45 ± 1.0 | 32 ± 0.4 | 18 ± 0.4 | 35 ± 1.0 | 30 ± 0.5 | ND | ND | ND |
| FLC | ND | ND | ND | ND | ND | ND | ND | ND | ND | 21 ± 0.5 | 16 ± 0.8 | 15 ± 0.0 |
| MIC Values (µg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp. No. | Strain | |||||||||||
| Gram-Negative Bacteria | Gram-Positive Bacteria | Fungi | ||||||||||
| E. coli | Ps. aeruginosa | P. vulgaris | K. pneumonia | S. enteridis | S. aureus | MRSA | E. fecalis | B. subtilis | C. albicans | A. niger | P. notatum | |
| 5a | 250 | 500 | 500 | 500 | 500 | 1000 | 1000 | 1000 | 250 | 125 | 500 | 125 |
| 5b | 250 | 500 | 500 | 500 | 500 | 15.6 | 31.25 | 125 | 15.6 | 7.8 | >1000 | 31.25 |
| 5c | 500 | 500 | 500 | 500 | 500 | 15.6 | 15.6 | 62.5 | 15.6 | 31.25 | >1000 | 31.25 |
| 5d | 250 | 500 | 500 | 500 | 500 | 1000 | 500 | 500 | 500 | 7.8 | 500 | 62.5 |
| 5e | 250 | 125 | 250 | 500 | 500 | 1000 | 1000 | 500 | 500 | 15.6 | 15.6 | 62.5 |
| 5f | 250 | 500 | 500 | 500 | 500 | >1000 | >1000 | 500 | 125 | 31.25 | 31.25 | 15.6 |
| 5g | 500 | 125 | 125 | 500 | 1000 | 500 | 500 | 500 | 250 | 7.8 | 15.6 | 7.8 |
| 5h | 250 | 500 | 250 | 500 | 500 | 500 | 500 | >1000 | 250 | 31.25 | 31.25 | 7.8 |
| 5i | 250 | 62.5 | 125 | 1000 | 500 | 500 | 500 | >1000 | 250 | 62.5 | 62.5 | 31.25 |
| 5j | 250 | 250 | 250 | 500 | 1000 | 500 | >1000 | 1000 | 250 | 3.9 | 31.25 | 62.5 |
| 5k | 250 | 250 | 1000 | 500 | 500 | 250 | 500 | 250 | 250 | 62.5 | 62.5 | 125 |
| 5l | 250 | 250 | 1000 | 500 | >1000 | 62.5 | 500 | 125 | 250 | 125 | 250 | 250 |
| 5m | 250 | 500 | 1000 | 1000 | 500 | 125 | 500 | 250 | 500 | 62.5 | 125 | 250 |
| 5n | 250 | 250 | 250 | 500 | 500 | 250 | 1000 | 250 | 250 | 62.5 | 62.5 | 250 |
| AMP | 15.6 | >1000 | <7.8 | >1000 | <7.8 | 250 | 500 | 3.9 | 1000 | ND | ND | ND |
| FLC | ND | ND | ND | ND | ND | ND | ND | ND | ND | 15.6 | 31.25 | 250 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wabli, R.I.; Zakaria, A.S.; Attia, M.I. Synthesis, Spectroscopic Characterization and Antimicrobial Potential of Certain New Isatin-Indole Molecular Hybrids. Molecules 2017, 22, 1958. https://doi.org/10.3390/molecules22111958
Al-Wabli RI, Zakaria AS, Attia MI. Synthesis, Spectroscopic Characterization and Antimicrobial Potential of Certain New Isatin-Indole Molecular Hybrids. Molecules. 2017; 22(11):1958. https://doi.org/10.3390/molecules22111958
Chicago/Turabian StyleAl-Wabli, Reem I., Azza S. Zakaria, and Mohamed I. Attia. 2017. "Synthesis, Spectroscopic Characterization and Antimicrobial Potential of Certain New Isatin-Indole Molecular Hybrids" Molecules 22, no. 11: 1958. https://doi.org/10.3390/molecules22111958





