Solvent-Free Addition of Indole to Aldehydes: Unexpected Synthesis of Novel 1-[1-(1H-Indol-3-yl) Alkyl]-1H-Indoles and Preliminary Evaluation of Their Cytotoxicity in Hepatocarcinoma Cells
Abstract
:1. Introduction
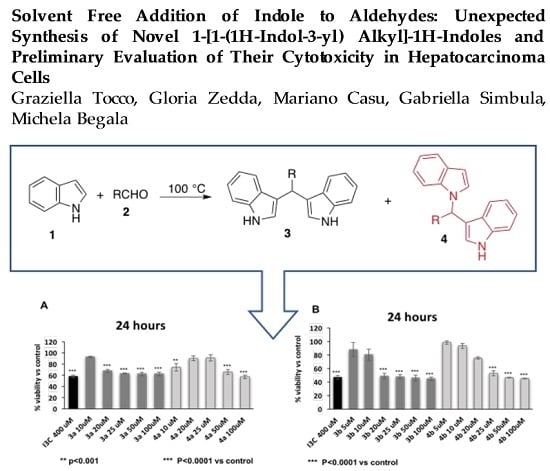
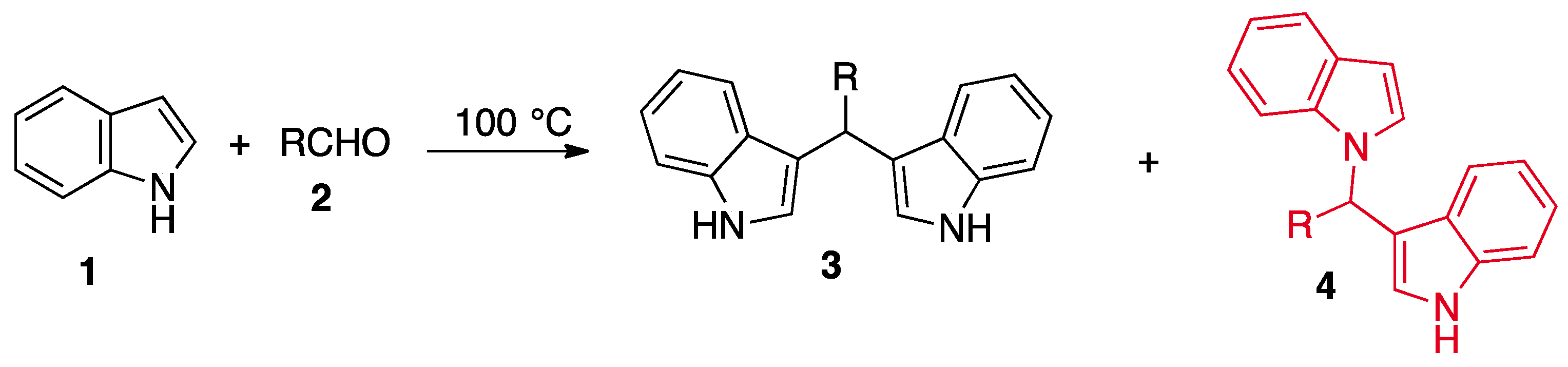
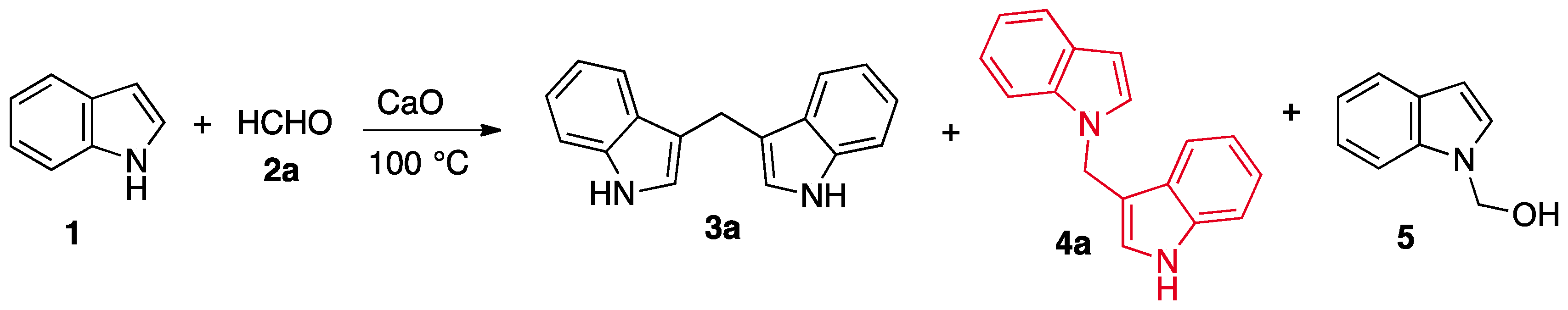
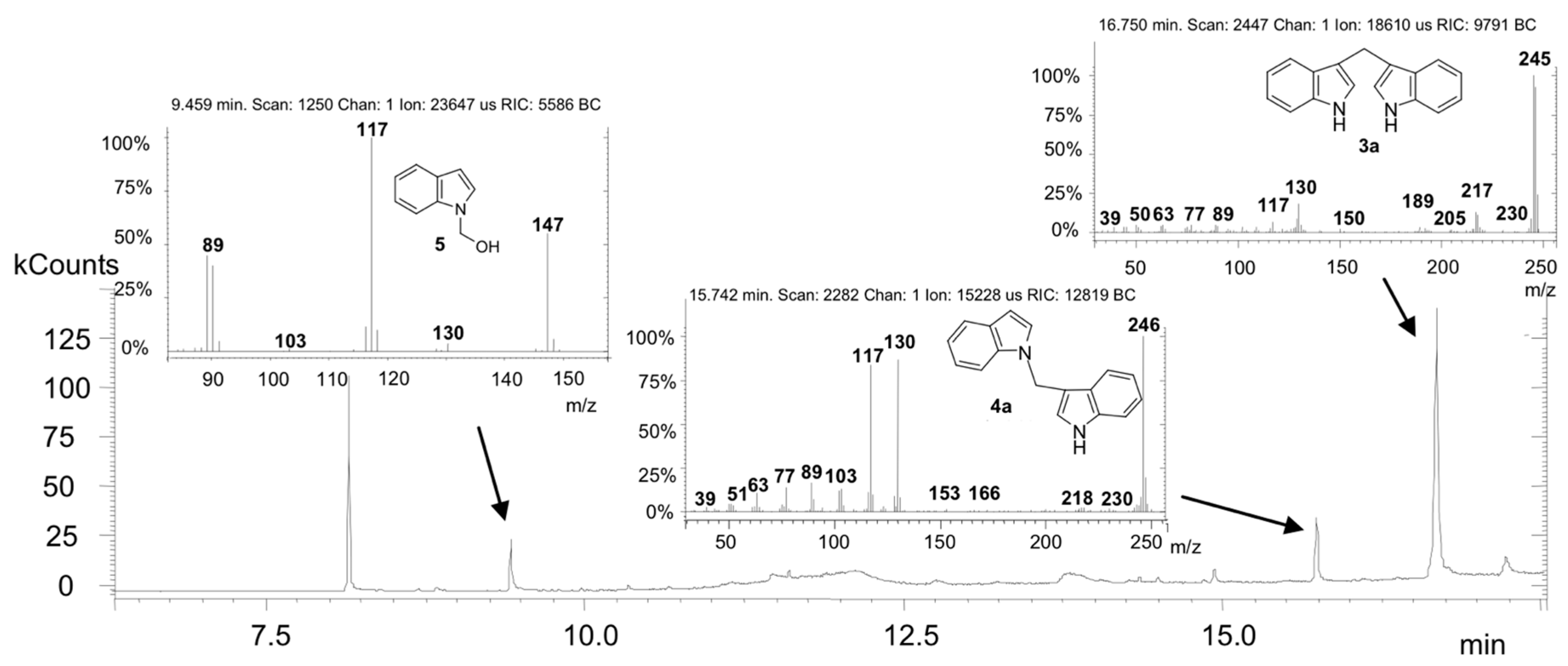
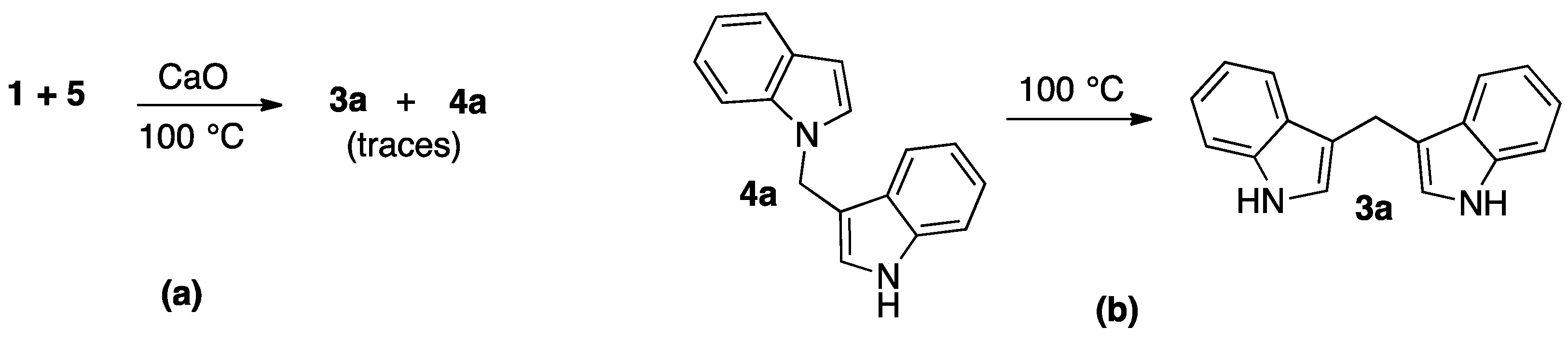
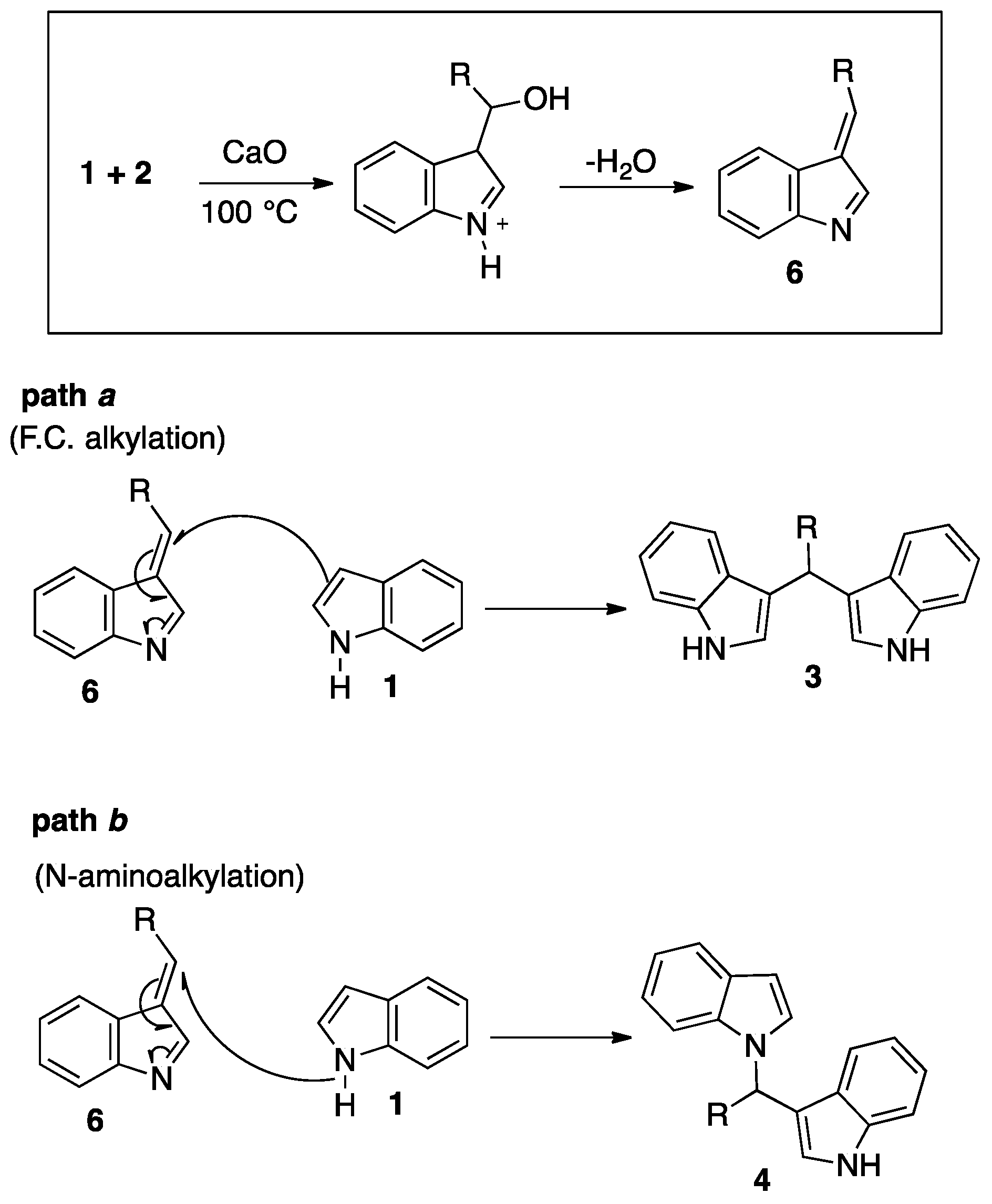
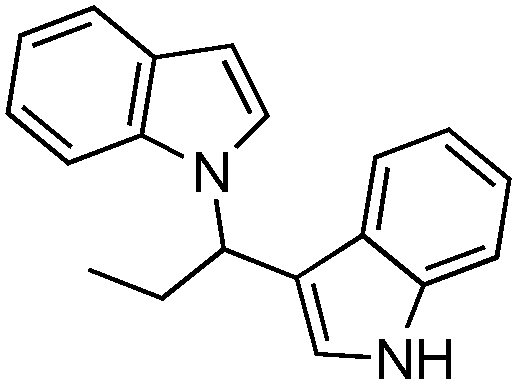
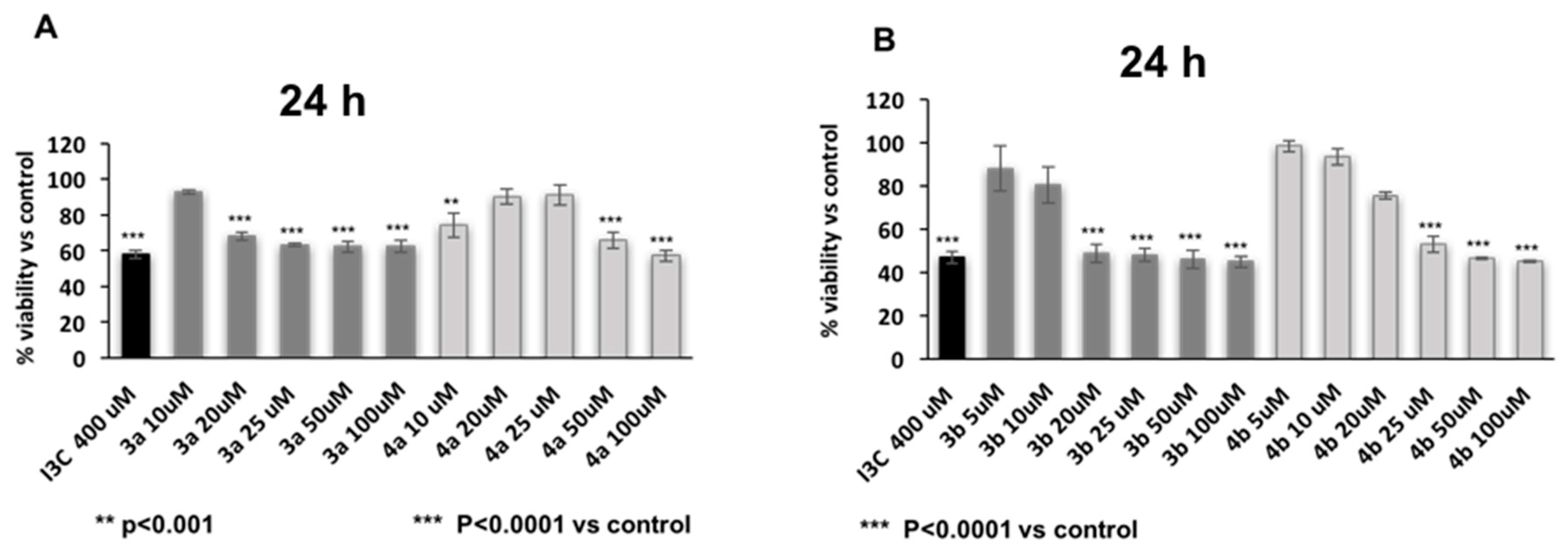
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.2. Experimental Procedures
Synthesis of 1-[1-(1H-indol-3-yl) methyl]-1H-indole (4a)
Typical procedure for 1-[1-(1H-indol-3-yl) alkyl]-1H-indoles (4b–e)
Synthesis of 3-(1-(1H-indol-3-yl) dodecyl)-1H-indole (3e)
3.3. Biology
Cell Line and Culture
Cell Viability
Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Joule, J.A. Indoles. In Science of Synthesis; Thomas, E.J., Ed.; Georg Thieme Verlag: Stuggart, Germany, 2011; Volume 10, p. 526. [Google Scholar]
- Bandini, M.; Eichholzer, A.; Umani-Ronchi, A. An Update on Catalytic Enantioselective Alkylations of Indoles. Mini Rev. Org. Chem. 2007, 4, 115–124. [Google Scholar] [CrossRef]
- Chao, W-R.; Yean, D.; Amin, K.; Green, C.; Jong, L. Computer-Aided Rational Drug Design: A Novel Agent (SR13668) Designed to Mimic the Unique Anticancer Mechanisms of Dietary Indole-3-Carbinol to Block Akt Signaling. J. Med. Chem. 2007, 50, 3412. [Google Scholar] [CrossRef] [PubMed]
- Jamsheena, V.; Shilpa, G.; Saranya, J.; Harry, N.A.; Lankalapalli, R.S.; Priya, S. Anticancer activity of synthetic bis(indolyl)methane-ortho-biaryls against human cervical cancer (HeLa) cell. Chem. Biol. Interact. 2016, 247, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-R.; Tsai, C.-H.; Kulp, S.K.; Chen, C.S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008, 262, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Azizian, J.; Teimouri, F.; Mohammadizadeh, M.R. Ammonium chloride catalyzed one-pot synthesis of diindolylmethas unender solvent-free conditions. Catal. Commun. 2007, 8, 1117–1121. [Google Scholar] [CrossRef]
- Babu, G.; Sridher, N.; Perumal, P.T. A convenient method of synthesis of Bis-Indolylmethanes: Indium trichloride catalyzed reactions of indole with aldehydes and schiff’s bases. Synth. Commun. 2000, 30, 1609–1614. [Google Scholar] [CrossRef]
- Jafarpour, M.; Rezaeifard, A.; Golshani, T. A new catalytic method for ecofriendly synthesis of bis-and trisindolylmethanes by zirconyldodecylsulfate under mild conditions. J. Heterocycl. Chem. 2009, 46, 535–539. [Google Scholar] [CrossRef]
- Ji, S-J.; Wang, S-Y.; Zhang, Y.; Loh, T-P. Facile synthesis of bis (indolyl) methanes using catalytic amount of iodine at room temperature under solvent-free conditions. Tetrahedron 2004, 60, 2051–2055. [Google Scholar] [CrossRef]
- Karthik, M.; Magesh, C.J.; Perumal, P.T.; Palanichamy, M.; Arabindoo, B.; Murugesan, V. Zeolite-catalyzed ecofriendly synthesis of vibrindole A and bis (indolyl) methanes. Appl. Catal. A: Gen. 2005, 286, 137–141. [Google Scholar] [CrossRef]
- Chakrabarty, M.; Ghosh, N.; Basak, R.; Harigaya, Y. Dry reaction of indoles with carbonyl compounds on montmorillonite K10 clay: A mild, expedient synthesis of diindolylalkanes and vibrindole A. Tetrahedron Lett. 2002, 43, 4075–4078. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M. Synthesis of Bis (indolyl) methanes using a Catalytic Amount of ZnO under Solvent-Free Conditions. Synth. Commun. 2008, 38, 832–840. [Google Scholar] [CrossRef]
- Azizi, N.; Torkian, L.; Saidi, M.R. Highly efficient synthesis of bis (indolyl) methanes in water. J. Mol. Catal. A Chem. 2007, 275, 109–112. [Google Scholar] [CrossRef]
- Barluenga, J.; Fernández, A.; Rodríguez, F.; Fañańas, F.J. Synthesis of bis (indolyl) alkanes by a site-selective gold-catalyzed addition of indoles to butynol derivatives. J. Organomet. Chem. 2009, 694, 546–550. [Google Scholar] [CrossRef]
- Yihong, W.; Rui, S.; Yang, Z.; Li, G.; Mei, G.; Yong, W. Graphene oxide: An efficient recyclable solid acid for the synthesis of bis (indolyl) methanes from aldehydes and indoles in water. Catal. Commun. 2017, 89, 138–142. [Google Scholar]
- Zolfigol, M.A.; Ayazi-Nasrabadi, R. Synthesis of the first magnetic nanoparticles with a thiourea dioxide-based sulfonic acid tag: Application in the one-pot synthesis of 1,1,3-tri(1H-indol-3-yl) alkanes under mild and green conditions. RSC Adv. 2016, 6, 69595–69604. [Google Scholar] [CrossRef]
- Losada, E.; Rajabi, F.; Feiz, A.; Luque, R. An Efficient and Reusable Cobalt Nanocatalyst for the Synthesis of Bis (indolyl) methanes under Solvent-Free Conditions. Curr. Org. Synth. 2016, 13, 888–892. [Google Scholar] [CrossRef]
- Tong, J.; Yang, C.; Xu, D. Ionic Liquid [DABCO-H][HSO4] as a Highly Efficient and Recyclable Catalyst for Friedel–Crafts Alkylation in the Synthesis of Bis (naphthol) methane and Bis (indolyl) methane Derivatives. Synthesis 2016, 48, 3559–3566. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Wilson, Z.E.; Roy, S.; Ley, S.V. Utilization of flow chemistry in catalysis: New avenues for the selective synthesis of Bis (indolyl) methanes. Tetrahedron 2017, 73, 1812–1819. [Google Scholar] [CrossRef]
- Thesing, J.; Klüssendorf, S.; Ballach, P.; Mayer, H. Synthesen mit Mannich-Basen des Indols. Chem. Ber. 1955, 88, 1295. [Google Scholar] [CrossRef]
- Kametani, T.; Suzuki, T.; Ogasawara, K. Potassium ferricyanide oxidation of N-methyltryptamine in liquid ammonia. J. Chem. Soc. C 1968, 2965–2968. [Google Scholar] [CrossRef]
- Carter, M.D.; Hadden, M.; Weaver, D.F.; Jacobo, S.M.H.; Lu, E. Treatment of Protein Folder Disorders. Patent WO 2006/125324 A1, 30 November 2006. [Google Scholar]
- Colombo, F.; Cravotto, G.; Palmisano, G.; Penoni, A.; Sisti, M. Three-Component Indium-Mediated Domino Allylation of 1H-Indole-3-carbaldehyde with Electron-Rich (Hetero)arenes: Highly Efficient Access to Variously Functionalized Indolylbutenes. Eur. J. Org. Chem. 2008, 16, 2801–2804. [Google Scholar] [CrossRef]
- Safe, S.; Papineni, S.; Chintharlapalli, S. Cancer chemotherapy with indole-3-carbinol, bis (3′-indolyl) methane and synthetic analogs. Cancer Lett. 2008, 269, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Tou, J.C.; Hong, C.; Kim, H.A.; Riby, J.E.; Firestone, G.L.; Bjeldanes, L.F. 3,3′-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis 2005, 26, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Kim, H.K.; Kim, J.H.; Choi, E.S.; Shin, J.A.; Lee, S.O.; Chintharlapalli, S.; Safe, S.; Abdelrahim, M.; Kong, G.; et al. The p38 MAPK pathway is critical for 5,5′-dibromodiindolylmethane-induced apoptosis to prevent oral squamous carcinoma cells. Eur. J. Cancer Prev. 2010, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.R.; Tsai, C.H.; Kulp, S.K.; Wang, D.; Lin, C.H.; Yang, H.C.; Ma, Y.; Sargeant, A.; Chiu, C.F.; Tsai, M.H.; et al. A potent indole-3-carbinol–derived antitumor agent with pleiotropic effects on multiple signaling pathways in prostate cancer cell. Cancer Res. 2007, 67, 7815–7824. [Google Scholar] [CrossRef] [PubMed]
- Zedda, G.; Simbula, G.; Begala, M.; Pibiri, M.; Floris, C.; Casu, M.; Casu, L.; Tocco, G. Mild Synthetic Approach to Novel Indole-1-Carbinols and Preliminary Evaluation of Their Cytotoxicity in Hepatocarcinoma Cells. Arch. Pharm. Pharm. Med. Chem. 2012, 345, 195–202. [Google Scholar] [CrossRef]
- Sharghi, H.; Khalifeh, R. Eco-Friendly Synthesis of Novel Lariat Ethers via Mannich Reaction under Solventless Conditions. Heterocycles 2007, 71, 1601–1614. [Google Scholar] [CrossRef]
- Dhanak, D.; Reese, C.R. Studies in the protection of pyrrole and indole derivatives. J. Chem. Soc. Perkin Trans 1 1986, 2181–2186. [Google Scholar] [CrossRef]
- Deguest, G.; Bischoff, L.; Fruit, C.; Marsais, F. Anionic, in situ generation of formaldehyde: A very useful and versatile tool in synthesis. Org. Lett. 2007, 9, 1165–1167. [Google Scholar] [CrossRef] [PubMed]
- Nasreen, A.; Varala, R.; Adapa, S.R. Copper nitrate trihydrate catalyzed efficient synthesis of bis (indolyl) methanes in acetonitrile at room temperature. J. Heterocycl. Chem. 2007, 44, 983–987. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, Y.-M. An efficient and practical process for the synthesis of bis (indolyl) methanes catalyzed by zirconium tetrachloride. Synthesis 2005, 12, 1949–1954. [Google Scholar] [CrossRef]
- Khalafi-Nezhad, A.; Pharami, A.; Zare, A.; Moosavi Zare, A.R.; Hasaninejad, A.; Panahi, F. Trityl chloride as a novel and efficient organic catalyst for room temperature preparation of bis (indolyl) methanes under solvent-free conditions in neutral media. Synthesis 2008, 4, 617–621. [Google Scholar] [CrossRef]
- Deb, M.L.; Bhuyan, P.J. An efficient and clean synthesis of bis (indolyl) methanes in a protic solvent at room temperature. Tetrahedron Lett. 2006, 47, 1441–1443. [Google Scholar] [CrossRef]
- Barcock, R.A.; Moorcroft, N.A.; Storr, R.C. 1-and 2-azafulvenes. Tetrahedron Lett. 1993, 34, 1187–1190. [Google Scholar] [CrossRef]
- Ballini, R.; Palmieri, A.; Petrini, M.; Shaikh, R. Reaction of 3-(1-Arylsulfonylalkyl)-indoles with Easily Enolisable Derivatives Promoted by Potassium Fluoride on Basic Alumina. Adv. Synth. Catal. 2008, 350, 129–134. [Google Scholar] [CrossRef]
- Liu, Y.; She, W.; Wang, F.; Li, J.; Wang, J.; Jiang, W. 3,3′-Diindolylmethane alleviates steatosis and the progression of NASH partly through shifting the imbalance of Treg/Th17 cells to Treg dominance. Int. Immunopharmacol. 2014, 23, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Firestone, G.L.; Bjeldanes, L.F. 3,3′-Diindolylmethane is a novel topoisomerase IIα catalytic inhibitor that induces S-phase retardation and mitotic delay in human hepatoma HepG2 cells. Mol. Pharmacol. 2006, 69, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, H.; Lu, Y.; Ren, W.; Teng, K.; Chiang, C.; Yang, Z.; Yu, B.; Hsu, S.; Jacob, S.T.; et al. Indole-3-carbinol inhibits tumorigenicity of hepatocellular carcinoma cells via suppression of microRNA-21 and upregulation of phosphatase and tensin homolog. BBA Mol. Cell Res. 2015, 1853, 244–253. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Entry | Base | Base/(1) | Time (h) | Yields (%) | |
|---|---|---|---|---|---|
| 3a | 4a | ||||
| 1 | - | - | 6 | N.I. a | N.I. a |
| 2 | CaO | 0.1/1 | 6 | N.I. a | N.I. a |
| 3 | CaO | 1/1 | 6 | 50 | 8 |
| 4 | CaO | 17/1 | 3 | 65 | 25 |
| 5 | CaO b | 17/1 | 3 | N.I. a | 62 |
| 6 | KOH | 1/1 | 1.5 | 61 | 37 |
| Entry | Substrate | Base | Time (h) | Products | Yields (%) [ref.] | ||
|---|---|---|---|---|---|---|---|
| 3 | 4 | 3 | 4 | ||||
| 1 | CH3CH2CHO 2b | CaO | 5 | 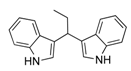 | 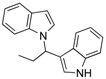 | 5 | N.I. b |
| 2 | CH3CH2CHO 2b | - | 3.5 | 57 [32] | 38 | ||
| 3 | CH3(CH2)4CHO 2c | - | 3.5 |  |  | 43 [32] | 34 |
| 4 | CH3(CH2)5CHO 2d | - | 3.5 | 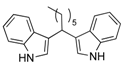 |  | 45 [33] | 22 |
| 5 | CH3(CH2)10CHO 2e | - | 3.5 |  | 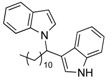 | 10 | 5 |
| 6 | 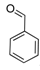 2f 2f | - | 3.5 | 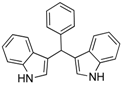 | - | 70 [32] | - |
| 7 |  2g 2g | - | 3.5 |  | - | 50 [9] | - |
| 8 | 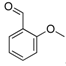 2g 2g | CaO | 5 | - | N.I. b | - | |
| 9 |  2h 2h | - | 3.5 | 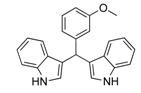 | - | 10 [13] | - |
| 10 | 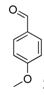 2j 2j | - | 3.5 | 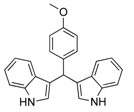 | - | 5 [32] | - |
| 11 |  2k 2k | - | 3.5 |  | - | 70 [34] | - |
| 12 | 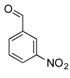 2l 2l | - | 3.5 | 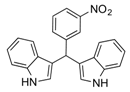 | - | N.I. b [34] | - |
| 13 | 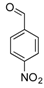 2m 2m | - | 3.5 | 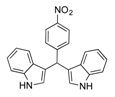 | - | N.I.b [32] | - |
| 14 | 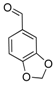 2n 2n | - | 3.5 | 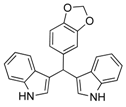 | - | 5 [8] | - |
| 15 | 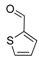 2o 2o | - | 3.5 |  | - | 73 [34] | - |
| 16 | 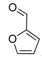 2p 2p | - | 3.5 |  | - | 88 [32] | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tocco, G.; Zedda, G.; Casu, M.; Simbula, G.; Begala, M. Solvent-Free Addition of Indole to Aldehydes: Unexpected Synthesis of Novel 1-[1-(1H-Indol-3-yl) Alkyl]-1H-Indoles and Preliminary Evaluation of Their Cytotoxicity in Hepatocarcinoma Cells. Molecules 2017, 22, 1747. https://doi.org/10.3390/molecules22101747
Tocco G, Zedda G, Casu M, Simbula G, Begala M. Solvent-Free Addition of Indole to Aldehydes: Unexpected Synthesis of Novel 1-[1-(1H-Indol-3-yl) Alkyl]-1H-Indoles and Preliminary Evaluation of Their Cytotoxicity in Hepatocarcinoma Cells. Molecules. 2017; 22(10):1747. https://doi.org/10.3390/molecules22101747
Chicago/Turabian StyleTocco, Graziella, Gloria Zedda, Mariano Casu, Gabriella Simbula, and Michela Begala. 2017. "Solvent-Free Addition of Indole to Aldehydes: Unexpected Synthesis of Novel 1-[1-(1H-Indol-3-yl) Alkyl]-1H-Indoles and Preliminary Evaluation of Their Cytotoxicity in Hepatocarcinoma Cells" Molecules 22, no. 10: 1747. https://doi.org/10.3390/molecules22101747