The killer of Socrates: Coniine and Related Alkaloids in the Plant Kingdom
Abstract
:1. Introduction
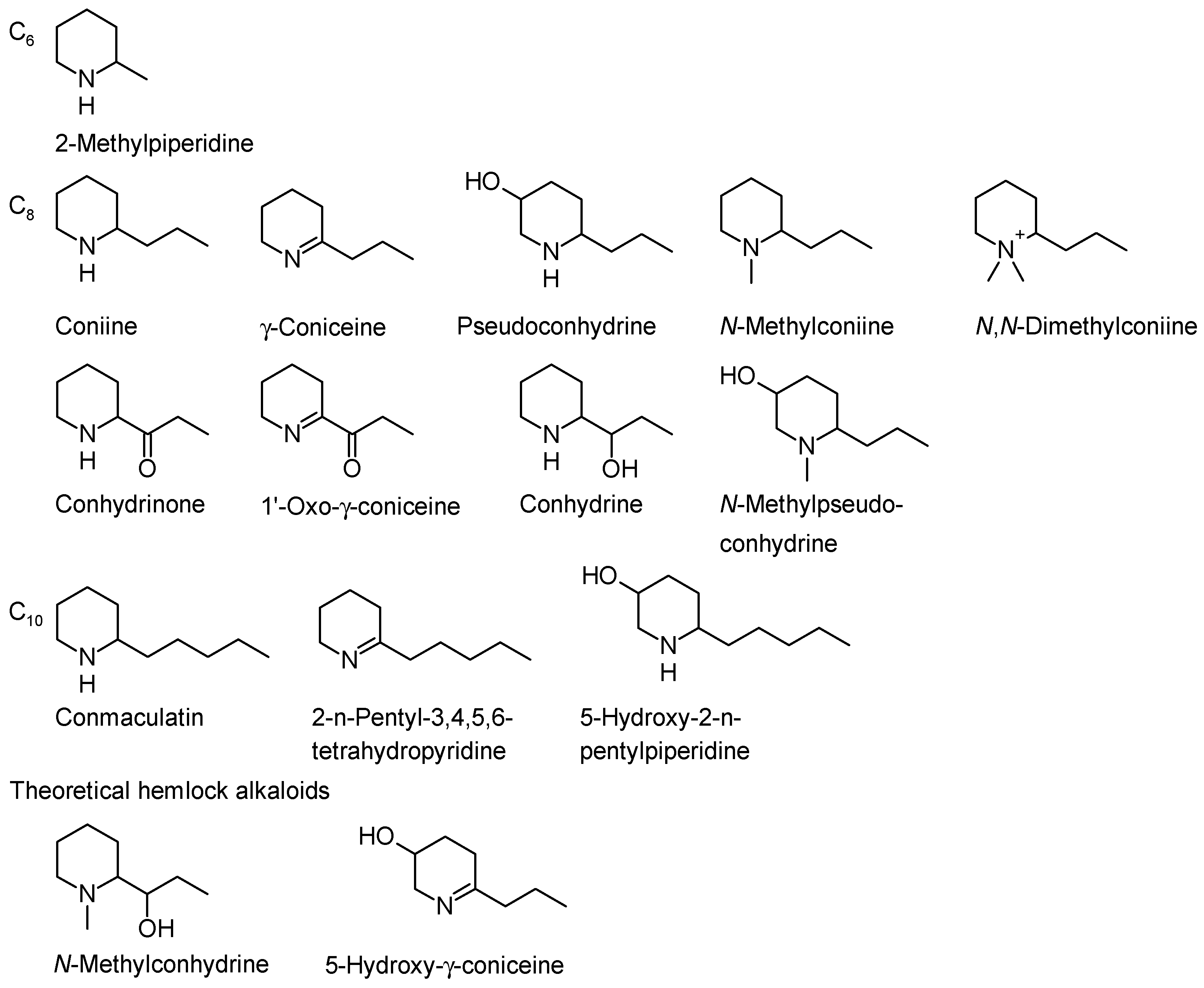
2. Hemlock Alkaloids and Their Chemistry
3. Plant Containing Hemlock Alkaloids
3.1. Conium sp.
3.2. Aloe sp.
3.3. Sarracenia sp.
3.4. Other Plants Possibly Containing Hemlock Alkaloids
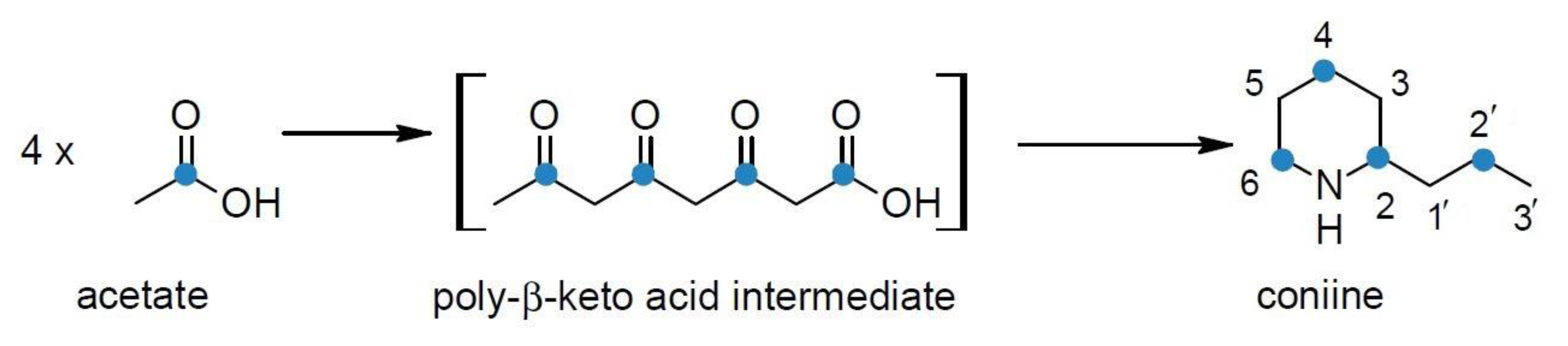
4. Biosynthesis of Hemlock Alkaloids
5. Biological Activity of Hemlock Alkaloids
5.1. Mode of Action of Hemlock Alkaloids in Organisms
5.2. Pharmacology of Poison Hemlock and Hemlock Alkaloids
5.3. Toxicity to Animals
5.4. Teratogenicity to Animals
5.5. Toxicity to Humans
5.6. Socrates
6. Ecological Role of Hemlock Alkaloids
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dring, J.V.; Nash, R.J.; Roberts, M.F.; Reynolds, T. Hemlock alkaloids in Aloes. Occurrence and distribution of γ-coniceine. Planta Med. 1984, 50, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Nash, R.J.; Beaumont, J.; Veitch, N.C.; Reynolds, T.; Benner, J.; Hughes, C.N.G.; Dring, J.V.; Bennett, R.N.; Dellar, J.E. Phenylethylamine and piperidine alkaloids in Aloe species. Planta Med. 1992, 58, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Blitzke, T.; Porzel, A.; Masoud, M.; Schmidt, J. A chlorinated amide and piperidine alkaloids from Aloe sabaea. Phytochemistry 2000, 55, 979–982. [Google Scholar] [CrossRef]
- Hotti, H.; Häkkinen, S.T.; Seppänen-Laakso, T.; Rischer, H. Polyketide-derived alkaloids and anthraquinones in Aloe plants and cell cultures. Unpublished work. 2017. [Google Scholar]
- Cromwell, B.T. The separation, micro-estimation and distribution of the alkaloids of hemlock (Conium maculatum L.). Biochem. J. 1956, 64, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Mody, N.V.; Henson, R.; Hedin, P.A.; Kokpol, U.; Miles, D.H. Isolation of insect paralysing agent coniine from Sarracenia flava. Experientia 1976, 32, 829–830. [Google Scholar] [CrossRef]
- Hotti, H.; Gopalacharyulu, P.; Seppänen-Laakso, T.; Rischer, H. Metabolite profiling of the carnivorous pitcher plants Darlingtonia and Sarracenia. PLoS ONE 2017, 12, e0171078. [Google Scholar] [CrossRef] [PubMed]
- López, T.A.; Cid, M.S.; Bianchini, M.L. Biochemistry of hemlock (Conium maculatum L.) alkaloids and their acute and chronic toxicity in livestock. A review. Toxicon 1999, 37, 841–865. [Google Scholar] [CrossRef]
- Vetter, J. Poison hemlock (Conium maculatum L.). Food Chem. Toxicol. 2004, 42, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T. Hemlock alkaloids from Socrates to poison aloes. Phytochemistry 2005, 66, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Veerporte, R.; van der Heijden, R.; van Gulik, W.M.; ten Hoopen, H.J.G. Plant biotechnology for the production of alkaloids: Present status and prospects. In The Alkaloids; Brossi, A., Ed.; Academic Press: New York, NY, USA, 1991; Volume 40, pp. 1–188. ISBN 978-0-12-469540-5. [Google Scholar]
- Roberts, M.F. Alkaloid production in Conium fruit. J. Pharm. Pharmacol. 1985, 37, 141. [Google Scholar] [CrossRef]
- Leete, E.; Adityachaudhury, N. Biosynthesis of the hemlock alkaloids—II. The conversion of γ-coniceine to coniine and Ψ-conhydrine. Phytochemistry 1967, 6, 219–223. [Google Scholar] [CrossRef]
- Hotti, H. The killer of Socrates exposed—Coniine in the plant kingdom. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2016. [Google Scholar]
- Büchel, K.H.; Korte, F. Acyl-lactone rearrangement. XXIV. Synthesis of hemlock alkaloids by the reaction principle of the acyl-lactone rearrangement. Chem. Ber. 1962, 95, 2460–2464. [Google Scholar] [CrossRef]
- Jean-Claude Bradley Double Plus Good (Highly Curated and Validated) Melting Point Dataset. Available online: https://dx.doi.org/10.6084/m9.figshare.1031638.v1 (accessed on 16 February 2016).
- Holstege, D.M.; Galey, F.D.; Johnson, B.; Seiber, J.N. Determination of alkaloid exposure in a model ruminant (goat) using a multiresidue screening method. J. Agric. Food Chem. 1996, 44, 2310–2315. [Google Scholar] [CrossRef]
- Giseke, A.L. Über das wirksame Princip des Schierlings Conium maculatum. Archiv des Apotheker-Vereins im nördlich Deutschland 1826, 20, 97–111. [Google Scholar]
- Hofmann, A.W. Einwirkung der Wärmeauf die Ammoniumbasen. Berichte der Deutschen Chemischen Gesellschaft 1881, 14, 659–669. [Google Scholar] [CrossRef]
- Ladenburg, A. Versuche zur Synthese des Coniin. Berichte der Deutschen Chemischen Gesellschaft 1886, 19, 439–441. [Google Scholar] [CrossRef]
- Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11; United States Environmental Protection Agency: Washington, DC, USA, 2016.
- Wolffenstein, R. Über Conium-alkaloïde. Berichte der Deutschen Chemischen Gesellschaft 1895, 28, 302–305. [Google Scholar] [CrossRef]
- Beyerman, H.C.; van Leeuwen, M.; Smidt, J.; van Veen, A. γ-Coniceine of Conium maculatum L., a revision of the generally accepted structure. Recueil des Travaux Chimiques des Pays-Bas 1961, 80, 513–525. [Google Scholar] [CrossRef]
- Gabriel, S. Synthese des γ-Coniceins. Berichte der Deutschen Chemischen Gesellschaft 1909, 42, 4059–4062. [Google Scholar] [CrossRef]
- Merck, E. Reported as “pseudoconhydrine”. Pharm. J. 1891, 21, 662. [Google Scholar]
- Yanai, H.S.; Lipscomb, W.N. The structure of Ψ-conhydrine. Tetrahedron 1959, 6, 103–108. [Google Scholar] [CrossRef]
- Von Planta, A.; Kekulé, A. Beiträge zur Kenntnis einiger flüchtiger Basen. Ann. Chem. Pharm. 1854, 89, 129–155. [Google Scholar] [CrossRef]
- Wolffenstein, R. Über Conium-Alkaloïde. Berichte der Deutschen Chemischen Gesellschaft 1894, 27, 2611–2615. [Google Scholar] [CrossRef]
- Hess, K.; Eichel, A. Alkaloids of the pomegranate tree. IV. A scheme for the isolation of the pure pelletierine alkaloids. Determination of the constitution of methylisopelletierine (“methylpelletierine,” “isomethylpelletierine”). Transformation of conhydrine into methylisopelletierine. Constitution of conhydrine. J. Chem. Soc. Abstr. 1918, 114, 34–35. [Google Scholar] [CrossRef]
- Leete, E.; Olson, J.O. Biosynthesis and metabolism of the hemlock alkaloids. J. Am. Chem. Soc. 1972, 94, 5472–5477. [Google Scholar] [CrossRef]
- Wertheim, T. Über ein neues Alkaloïd in Conium maculatum. Liebigs Ann. 1856, 100, 328–339. [Google Scholar] [CrossRef]
- Roberts, M.F.; Brown, R.T. A new alkaloid from South African Conium species. Phytochemistry 1981, 20, 447–449. [Google Scholar] [CrossRef]
- Wegler, R.; Pieper, G. The addition of alkylpyridines to butadiene and styrene in the presence of alkali metal. Chem. Ber. 1950, 83, 6–10. [Google Scholar] [CrossRef]
- Radulović, N.; Đorđević, N.; Denić, M.; Pinheiro, M.M.G.; Fernandes, P.D.; Boylan, F. A novel alkaloid from poison hemlock (Conium maculatum L., Apiaceae): Identification, synthesis and antinociceptive activity. Food Chem. Toxicol. 2012, 50, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.G.; Smith, R.A. Two new alkaloids of Conium maculatum, and evidence for a tautomeric form for “γ”-coniceine. In Toxic Plants and Other Natural Toxicants; Garland, T., Barr, A.C., Eds.; CAB International: Oxford, UK, 1997; pp. 419–422. [Google Scholar]
- Jones, T.H.; Blum, M.S.; Fales, H.M. Ant venom alkaloids from Solenopsis and Monorium species: Recent developments. Tetrahedron 1982, 38, 1949–1958. [Google Scholar] [CrossRef]
- Fairbairn, J.W.; Suwal, P.N. The alkaloids of hemlock (Conium maculatum L.)—II Evidence for a rapid turnover of the major alkaloids. Phytochemistry 1961, 1, 38–46. [Google Scholar] [CrossRef]
- Castells, E.; Berhow, M.A.; Vaughn, S.F.; Berenbaum, M.R. Geographic variation in alkaloid production in Conium maculatum populations experiencing differential herbivory by Agonopterix alstroemeriana. J. Chem. Ecol. 2005, 31, 1693–1709. [Google Scholar] [CrossRef] [PubMed]
- Hande, S.M.; Kawai, N.; Uenishi, J. An efficient synthesis of 2- and 2,6-substituted piperidines using PdII-catalyzed 1,3-chirality transfer reaction. J. Org. Chem. 2008, 74, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Denić, M.; Blagovejić, P.; Radulović, N. Synthetic approaches to coniine and other 2-alkyl piperidines. FU Phys. Chem. Technol. 2013, 11, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Panter, K.E.; Bunch, T.D.; Keeler, R.F.; Sisson, D.V. Radio ultrasound observation of the fetotoxic effects in sheep from ingestion of Conium maculatum (poison-hemlock). J. Toxicol. Clin. Toxicol. 1988, 26, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Magee, A.R.; Clark, V.R. Mzansi’s mountain hemlocks: The identities of Hilliard and Burtt’s Conium species 3 and 4 (Apiaceae) and a revised key for the genus in sub-Saharan Africa. S. Afr. J. Bot. 2017, 108, 243–247. [Google Scholar] [CrossRef]
- Angiosperm Phylogeny Website, version 13. September 2013. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 18 April 2016).
- Holm, L.; Doll, J.; Holm, E.; Pancho, J.; Herberger, J. Chapter 27. Conium maculatum L. In World Weeds—Natural Histories and Distribution; John Wiley & Sons, Inc.: New York, NY, USA; Chichester, UK; Weinheim, Germany; Brisbane, Australia; Singapore; Toronto, ON, Canada, 1997; pp. 221–225. ISBN 978-0-471-04701-8. [Google Scholar]
- De Landoni, J.H. Conium maculatum L. (PIM 144). Available online: http://www.inchem.org/documents/pims/plant/conium.htm (accessed on 9 May 2015).
- Drummer, O.H.; Roberts, A.N.; Bedford, P.J.; Crump, K.L.; Phelan, M.H. Case report—Three deaths from hemlock poisoning. The weed that killed Socrates is common in many parts of Australia. Med. J. Aust. 1995, 162, 592–593. [Google Scholar] [PubMed]
- Mitich, L.W. Poison-hemlock (Conium maculatum L.). Weed Technol. 1998, 12, 194–197. [Google Scholar] [CrossRef]
- Field Guide for Managing Poison Hemlock in the Southwest. TP-R3-16-18. Available online: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5410121.pdf (accessed on 20 October 2017).
- Fröberg, L. Conium maculatum . In Flora Nordica 6; Jonsell, B., Karlsson, T., Eds.; The Swedish Museum of Natural History: Stockholm, Sweden, 2010; pp. 210–211. ISBN 978-91-86510-61-9. [Google Scholar]
- Corsi, G.; Biasci, D. Secretory structures and localization of alkaloids in Conium maculatum L. (Apiaceae). Ann. Bot. 1998, 81, 157–162. [Google Scholar] [CrossRef]
- Roberts, M.F. Enzymology of alkaloid biosynthesis. In Alkaloids: Biochemistry, Ecology, and Medicinal Applications; Roberts, M.F., Wink, M., Eds.; Springer: New York, NY, USA, 1998; pp. 109–146. ISBN 978-1-4757-2905-4. [Google Scholar]
- Fairbairn, J.W.; Challen, S.B. The alkaloids of hemlock (Conium maculatum L.). Distribution in relation to the development of the fruit. Biochem. J. 1959, 72, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Gulezian, P.Z.; Ison, J.L.; Granberg, K.J. Establishment of an invasive species (Conium maculatum) in contaminated roadside soil in Cook County, Illinois. Midl. Nat. 2012, 168, 375–395. [Google Scholar] [CrossRef]
- Panter, K.E.; Keeler, R.F.; Baker, D.C. Toxicosis in livestock from the hemlocks (Conium and Cicuta spp.). J. Anim. Sci. 1988, 66, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C. Seed germination ecology of poison hemlock, Conium maculatum. Can. J. Bot. 1990, 68, 2018–2024. [Google Scholar] [CrossRef]
- Pokorny, M.; Sheley, R. Poison hemlock Conium maculatum. MT200013AG, MontGuide. Montana State University Extension: Bozeman, MT, USA. Available online: http://store.msuextension.org/publications/AgandNaturalResources/MT200013AG.pdf (accessed on 9 May 2015).
- Smith, L.J.; Thill, D.C.; Callihan, R.H.; Lish, J.M. Poison Hemlock. A Threat to Man and Livestock. Current Information Series No. 632; Cooperative Extension Service Agricultural Experiment Station, College of Agriculture, University of Idaho: Moscow, ID, USA, 1984; pp. 1–2. [Google Scholar]
- DiTomaso, J.M. Invasive weeds in rangelands: Species, impacts, and management. Weed Sci. 2000, 48, 255–265. [Google Scholar] [CrossRef]
- Pimental, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Krezelok, E.P.; Jaconsen, T.D.; Aronis, J.M. Hemlock ingestions: The most deadly plant exposures. J. Toxicol. Clin. Toxicol. 1996, 34, 601–602. [Google Scholar]
- Jeffery, L.S.; Robinson, L.R. Poison-hemlock (Conium maculatum) control in alfalfa (Medicago sativa). Weed Technol. 1990, 4, 585–587. [Google Scholar] [CrossRef]
- Woodard, C.A. Poison Hemlock (Conium maculatum L.): Biology, Implications for Pastures and Response to Herbicides. Master’s Thesis, University of Missouri, Columbia, MO, USA, May 2008. [Google Scholar]
- The Angiosperm Phylogeny Group. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 2003, 141, 399–436. [Google Scholar] [CrossRef]
- Viljoen, A.M.; van Wyk, B.-E.; van Heerden, F.R. Distribution and chemotaxonomic significance of flavonoids in Aloe (Asphodelaceae). Plant. Syst. Evol. 1998, 211, 31–42. [Google Scholar] [CrossRef]
- Carter, S.; Lavranos, J.J.; Newton, L.E.; Walker, C.C. Aloe—The Definitive Guide, 1st ed.; Kew Publishing: Kew, UK, 2011; 720p, ISBN 978-1842464397. [Google Scholar]
- Rowley, G.D. A History of Succulent Plants; Strawberry Press: Mill Valley, CA, USA, 1997; p. 409. ISBN 0912647160. [Google Scholar]
- Dagne, E.; Bisrat, D.; Viljoen, A.; van Wyk, B.-E. Chemistry of Aloe species. Curr. Org. Chem. 2000, 4, 1055–1078. [Google Scholar] [CrossRef]
- Loots, D.T.; van der Westhuizen, F.H.; Botes, L. Aloe ferox leaf gel phytochemical content, antioxidant capacity, and possible health benefits. J. Agric. Food Chem. 2007, 55, 6891–6896. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.B.; Gelfand, M.; Mavi, S. Medicinal and other uses of succulents by the Rhodesian African. Excelsa 1975, 5, 51–56. [Google Scholar]
- Parry, O.; Matambo, C. Some pharmacological actions of aloe extracts and Cassia abbreviata on rats and mice. Cent. Afr. J. Med. 1992, 38, 409–414. [Google Scholar] [PubMed]
- McPherson, S. Pitcher Plants of the North America; The McDonald & Woodward Publishing Company: Blacksburg, VA, USA, 2006; p. 320. ISBN 978-1604691085. [Google Scholar]
- Ellison, A.M.; Butler, E.D.; Hicks, E.J.; Naczi, R.F.C.; Calie, P.J.; Bell, C.D.; Davis, C.C. Phylogeny and biogeography of the carnivorous plant family Sarraceniaceae. PLoS ONE 2012, 7, e39291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harborne, J.B. Introduction to Ecological Biochemistry, 2nd ed.; Academic Press: London, UK, 1982; p. 278. ISBN 0123246806. [Google Scholar]
- Li, S.-L.; Lin, G.; Chan, S.-W.; Li, P. Determination of the major isosteroidal alkaloids in bulbs of Fritillaria by high-performance liquid chromatography coupled with evaporative light scattering detection. J. Chromatogr. A 2001, 909, 207–214. [Google Scholar] [CrossRef]
- Hébert, A.; Heim, F. Recherches sur les principes actifs de quelques aroïdées. Bull. Soc. Chim. Fr. 1898, 17, 664–669. [Google Scholar]
- Raffauf, R.F. A Handbook of Alkaloids and Alkaloid-Containing Plants; Wiley: New York, NY, USA; London, UK; Sydney, Australia; Toronto, ON, Canada, 1970; p. 1275. ISBN 978-0471704782. [Google Scholar]
- Power, F.D.; Tutin, F. Chemical examination of Aethusa cynapium. J. Am. Chem. Soc. 1905, 27, 1461–1476. [Google Scholar] [CrossRef]
- Teuscher, E.; Greger, H.; Adrian, V. Investigation of the toxicity of Aethusa cynapium L. (Hundspetersilie). Pharmazie 1990, 45, 537–538. [Google Scholar] [PubMed]
- Naef, R.; Velluz, A.; Mayenzet, F.; Starkenmann, C.; Sun, H.-D. Volatile constituents of Semnostachya menglaensis Tsui. J. Agric. Food Chem. 2005, 53, 9161–9164. [Google Scholar] [CrossRef] [PubMed]
- Jananie, R.K.; Priya, V.; Vijayalakshmi, K. Secondary metabolites of Cynodon dactylon as an antagonist to angiotensin II type1 receptor: Novel in silico drug targeting approach for diabetic retinopathy. J. Pharmacol. Pharmacother. 2012, 3, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.P.; Boligon, A.A.; Appel, A.S.; Fachinetto, R.; Ceron, C.S.; Tanus-Santos, J.E.; Athayde, M.L.; Rocha, J.B.T. Chemical composition, antioxidant and anticholinesterase activity of Melissa officinalis. Ind. Crops Prod. 2014, 53, 34–45. [Google Scholar] [CrossRef]
- Ashraf, M.; Ahmad, R.; Asghar, B.; Bhatty, M.K. Studies on the essential oils of the Pakistani species of the family Umbelliferae. Part XX. Pimpinella acuminate (Edgew) Clarke (jungle anise) seed oil. Pak. J. Sci. Ind. Res. 1979, 23, 79–81. [Google Scholar]
- Robinson, R. LXXV.—A theory of the mechanism of the phytochemical synthesis of certain alkaloids. J. Chem. Soc. Trans. 1917, 111, 876–899. [Google Scholar] [CrossRef]
- Leete, E. Biosynthesis of the hemlock and related piperidine alkaloids. Acc. Chem. Res. 1971, 4, 100–107. [Google Scholar] [CrossRef]
- Aniszewski, T. Alkaloids: Chemistry, Biology, Ecology, and Applications, 2nd ed.; Elsevier Science: Oxford, UK, 2015; p. 475. ISBN 978-0444594334. [Google Scholar]
- Panter, K.E.; Keeler, R.F. Chapter 5—Piperidine alkaloids of poison hemlock (Conium maculatum). In Toxicants of Plant Origin; Cheeke, P.R., Ed.; CRC Press: Boca Raton, FL, USA, 1989; Volume I, pp. 109–132. ISBN 9780849369902. [Google Scholar]
- Leete, E. The Biosynthesis of coniine four acetate units. J. Am. Chem. Soc. 1963, 85, 3523–3524. [Google Scholar] [CrossRef]
- Cromwell, B.T.; Roberts, M.F. The biogenesis of γ-coniceine in hemlock (Conium maculatum L.). Phytochemistry 1964, 3, 369–375. [Google Scholar] [CrossRef]
- Leete, E. Biosynthesis of the hemlock alkaloids. The incorporation of the acetate-1-C14 into coniine and conhydrine. J. Am. Chem. Soc. 1964, 86, 2509–2513. [Google Scholar] [CrossRef]
- Leete, E. Biosynthesis of coniine from octanoic acid in hemlock plants (Conium maculatum). J. Am. Chem. Soc. 1970, 92, 3835. [Google Scholar] [CrossRef] [PubMed]
- Leete, E.; Olson, J.O. 5-Oxo-octanoic acid and 5-oxo-octanal, precursors of coniine. J. Chem. Soc. D 1970, 23, 1651–1652. [Google Scholar] [CrossRef]
- Hotti, H.; Seppänen-Laakso, T.; Arvas, M.; Teeri, T.H.; Rischer, H. Polyketide synthases from poison hemlock (Conium maculatum L.). FEBS J. 2015, 282, 4141–4156. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Seshime, Y.; Juvvadi, P.R.; Kitamoto, K.; Ebizuka, Y.; Fujii, I. Identification of csypyrone B1 as the novel product of Aspergillus oryzae type III polyketide synthase CsyB. Bioorg. Med. Chem. 2010, 18, 4542–4546. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.F. The formation of γ-coniceine from 5-ketooctanal by a transaminase of Conium maculatum. Phytochemistry 1971, 10, 3057–3060. [Google Scholar] [CrossRef]
- Roberts, M.F. Purification and properties of l-alanine:5-ketooctanal aminotransferase from Conium maculatum. Phytochemistry 1977, 16, 1381–1386. [Google Scholar] [CrossRef]
- Roberts, M.F. Separation of the formation of γ-coniceine and aliphatic amines from GOT activity in Conium maculatum. Phytochemistry 1978, 17, 107–112. [Google Scholar] [CrossRef]
- Roberts, M.F. Enzymatic synthesis of γ-coniceine in Conium maculatum chloroplasts and mitochondria. Plant Cell Rep. 1981, 1, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Unger, W. Enzymatische In-vitro-synthase von γ-Conicein durch eine Aldehyd-aminosäure-transaminase aus Spinatblättern. Planta Med. 1977, 31, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, S.M.C.; Martin, R.O. Biosynthesis of Conium alkaloids using carbon-14 dioxide. Interrelation of γ-coniceine, coniine, and N-methylconiine. J. Am. Chem. Soc. 1968, 90, 1921–1923. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, S.M.C.; Martin, R.O. The biosynthesis of Conium alkaloids using carbon-14 dioxide. The kinetics of 14C incorporation into the known alkaloids and some new alkaloids. Biochemistry 1969, 8, 4163–4172. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, J.W.; Ali, A.A.E.R. The alkaloids of hemlock (Conium maculatum L.)—III. The presence of bound forms in the plant. Phytochemistry 1968, 7, 1593–1597. [Google Scholar] [CrossRef]
- Fairbairn, J.W.; Ali, A.A.E.R. The alkaloids of hemlock (Conium maculatum L.)—IV. Isotopic studies of the bound forms of alkaloids in the plant. Phytochemistry 1968, 7, 1599–1603. [Google Scholar] [CrossRef]
- Roberts, M.F. An S-adenosyl-L-methionine; coniine methyltransferase from Conium maculatum. Phytochemistry 1974, 13, 1847–1851. [Google Scholar] [CrossRef]
- Roberts, M.F. γ-Coniceine reductase in Conium maculatum. Phytochemistry 1975, 14, 2393–2397. [Google Scholar] [CrossRef]
- Roberts, M.F. Origin of the methyl carbon of methyl coniine in Conium maculatum. Phytochemistry 1974, 13, 1841–1845. [Google Scholar] [CrossRef]
- Nétien, G.; Combet, J. Etude comparative dans la composition chimique des cultures de tissus de Conium maculatum cultivées in vitro. I. Variation des substances azotées. Paris Soc. Biol. C. R. 1970, 165, 103–107. [Google Scholar]
- Meier, P.; Hotti, H.; Rischer, H. Elicitation of furanocoumarins in poison hemlock (Conium maculatum L.) cell culture. Plant Cell Tissue Organ Cult. 2015, 123, 443–453. [Google Scholar] [CrossRef]
- Castells, E.; Berenbaum, M.R. Host plant selection by monophagous herbivore is not mediated by quantitative changes in unique plant chemistry: Agonopterix alstroemeriana and Conium maculatum. Arthropod Plant Interact. 2008, 2, 43–51. [Google Scholar] [CrossRef]
- Green, B.T.; Lee, S.T.; Panter, K.E.; Welch, K.D.; Cook, D.; Pfister, J.A.; Kem, W.R. Actions of piperidine alkaloid teratogens at fetal nicotinic acetylcholine receptors. Neurotoxicol. Teratol. 2010, 32, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Bowman, W.C.; Sangvi, I.S. Pharmacological actions of hemlock (Conium maculatum L.) alkaloids. J. Pharm. Pharmacol. 1963, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.; Row, R. A comparison of the physiological actions and chemical constitution of piperidine, coniine and nicotine. J. Physiol. 1898, 22, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Sampson, S.R.; Esplin, D.W.; Zablocka, B. Effects of coniine on peripheral and central synaptic transmission. J. Pharmacol. Exp. Ther. 1966, 152, 313–324. [Google Scholar] [PubMed]
- Forsyth, C.S.; Speth, R.C.; Wecker, L.; Galey, F.D.; Frank, A.A. Comparison of nicotinic receptor binding and biotransformation of coniine in the rat and chick. Toxicol. Lett. 1996, 89, 175–183. [Google Scholar] [CrossRef]
- Wink, M.; Schmeller, T.; Latz-Brüning, B. Modes of action of allelochemical alkaloids: Interaction with neuroreceptors, DNA, and other molecular targets. J. Chem. Ecol. 1998, 24, 1881–1937. [Google Scholar] [CrossRef]
- Lee, S.T.; Green, B.T.; Welch, K.D.; Pfister, J.A.; Panter, K.E. Stereoselective potencies and relative toxicities of coniine enantiomers. Chem. Res. Toxicol. 2008, 21, 2061–2064. [Google Scholar] [CrossRef] [PubMed]
- Erkent, U.; Iskit, A.B.; Onur, R.; Ilhan, M. The effect of coniine on presynaptic nicotinic receptors. Z. Naturforsch. 2016, 71, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.C.; Gutbrod, O.; Witzermann, V.; Methfessel, C. Pharmacology of the nicotinic acetylcholine receptor from fetal rat muscle expressed in Xenopus oocytes. Eur. J. Pharmacol. 1996, 309, 287–298. [Google Scholar] [CrossRef]
- Lee, S.T.; Green, B.T.; Welch, K.D.; Jordan, G.T.; Zhang, Q.; Panter, K.E.; Hughes, D.; Chang, C.-W.T.; Pfister, J.A.; Gardner, D.R. Stereoselective potencies and relative toxicities of γ-coniceine and N-methylconiine enantiomers. Chem. Res. Toxicol. 2013, 26, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Green, B.T.; Lee, S.T.; Welch, K.D.; Pfister, J.A.; Panter, K.E. Fetal muscle-type nicotinic acetylcholine receptor activation in TE-671 cells and inhibition of fetal movement in a day 40 pregnant goat model by optical isomers of the piperidine alkaloid coniine. J. Pharmacol. Exp. Ther. 2013, 344, 295–307. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J. The death of Socrates. A historical and experimental study on the actions of coniine and Conium maculatum. Arch. Int. Pharmacodyn. Ther. 1950, 83, 473–490. [Google Scholar] [PubMed]
- Arihan, O.; Boz, M.; Iskit, A.B.; Ilhan, M. Antinociceptive activity of coniine in mice. J. Ethnopharmacol. 2009, 125, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Madaan, R.; Kumar, S. Screening of alkaloidal fraction of Conium maculatum L. aerial parts for analgesic and anti-inflammatory activity. Indian J. Pharm. Sci. 2012, 74, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Castells, E.; Berenbaum, M.R. Resistance of the generalist moth Trichoplusia ni (Noctuidae) to a novel chemical defence in the invasive plant Conium maculatum. Chemoecology 2008, 18, 11–18. [Google Scholar] [CrossRef]
- Bhat, B.G.; Chandrasekhara, N. Studies on the metabolism of piperine: Absorption, tissue distribution and excretion urinary conjugates in rats. Toxicology 1986, 40, 83–92. [Google Scholar] [CrossRef]
- Bloch, E. Hemlock poisoning and the death of Socrates: Did Plato tell the truth? In The Trial and Execution of Socrates: Sources and Controversies; Brickhouse, T.C., Smith, N.D., Eds.; Oxford University Press: New York, NY, USA, 2001; pp. 255–278. ISBN 978-0195119800. [Google Scholar]
- Daugherty, D.G. The death of Socrates and the toxicology of hemlock. J. Med. Biogr. 1995, 3, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Linnilä, K.; Savikko, S.; Lempiäinen, T. (Eds.) Elias Lönnrotin Flora Fennica I-III; Kustannusosakeyhtiö Tammi: Hämeenlinna, Finland, 2003. [Google Scholar]
- Penny, R.H.C. Hemlock poisoning in cattle. Vet. Rec. 1953, 65, 669–670. [Google Scholar]
- Galey, F.D.; Holstege, D.M.; Fisher, E.G. Toxicosis of dairy cattle exposed to poison hemlock (Conium maculatum) in hay; isolation of Conium alkaloids in plants, hay, and urine. J. Vet. Diagn. Investig. 1992, 4, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Binev, R.; Mitev, J.; Miteva, T. Intoxication with poison hemlock (Conium maculatum L.) in calves. Trakia J. Sci. 2007, 5, 40–50. [Google Scholar]
- Swerczek, T.W.; Swerczek, S.J. Spotted hemlock poisoning in a herd of Angus cattle. J. Am. Vet. Med. Assoc. 2012, 240, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, J.L. Poisoning in a pig by hemlock (Conium maculatum). Vet. J. 1937, 92, 301–302. [Google Scholar] [CrossRef]
- Edmonds, L.D.; Selby, L.A.; Case, A.A. Poisoning and congenital malformations associated with consumption of poison hemlock by sows. J. Am. Vet. Med. Assoc. 1972, 160, 1319–1324. [Google Scholar] [PubMed]
- Dyson, D.A.; Wrathall, A.E. Congenital deformities in pigs possibly associated with exposure to hemlock (Conium maculatum). Vet. Rec. 1977, 100, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Panter, K.E.; Keeler, R.F.; Buck, W.B.; Shupe, J.L. Toxicity and teratogenicity of Conium maculatum in swine. Toxicon 1983, 21, 333–336. [Google Scholar] [CrossRef]
- Widmer, W.R. Poison hemlock toxicosis in swine. Vet. Med. 1984, 79, 405–408. [Google Scholar]
- Hannam, D.A.R. Hemlock (Conium maculatum) poisoning in the pig. Vet. Rec. 1985, 116, 322. [Google Scholar] [CrossRef] [PubMed]
- Markham, K. Hemlock poisoning in piglets. Vet. Rec. 1985, 116, 27. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, H. Hemlock poisoning in horses. Vet. Rec. 1937, 49, 1211–1212. [Google Scholar]
- Keeler, R.F.; Balls, L.D.; Shupe, J.L.; Crowe, M.W. Teratogenicity and toxicity of coniine in cows, ewes and mares. Cornell Vet. 1980, 70, 19–26. [Google Scholar] [PubMed]
- Nice, G.; Johnson, B.; Bauman, T.; Jordan, T. Poison Hemlock—The Toxic Parsnip; Purdue Extension—Weed Science, Purdue University: West Lafayette, IN, USA, 2005; Available online: http://www.carrotmuseum.co.uk/PHemlock03.pdf (accessed on 9 May 2015).
- Jessup, D.A.; Boermans, H.J.; Kock, N.D. Toxicosis in tule elk caused by ingestion of poison hemlock. J. Am. Vet. Med. Assoc. 1986, 189, 1173–1175. [Google Scholar] [PubMed]
- Copithorne, B. Suspected poisoning of goats by hemlock (Conium maculatum). Vet. Rec. 1937, 49, 1018–1019. [Google Scholar]
- Panter, K.E.; Bunch, T.D.; Keeler, R.F. Maternal and fetal toxicity of poison hemlock (Conium maculatum) in sheep. Am. J. Vet. Res. 1988, 49, 281–283. [Google Scholar] [PubMed]
- Short, S.B.; Edwards, W.C. Accidental Conium maculate poisoning in the rabbit. Vet. Hum. Toxicol. 1989, 31, 54–57. [Google Scholar] [PubMed]
- Frank, A.A.; Reed, W.M. Conium maculatum (poison hemlock) toxicosis in a flock of range turkeys. Avian Dis. 1986, 31, 386–388. [Google Scholar] [CrossRef]
- Keeler, R.F.; Balls, L.D. Teratogenic effects in cattle of Conium maculatum and conium alkaloids and analogs. Clin. Toxicol. 1978, 12, 49–64. [Google Scholar] [CrossRef] [PubMed]
- López, T.A.; de la Torre, M.L.; Cid, M.S. An efficient TLC method for analysis of γ-coniceine and coniine in Conium maculatum L. foliage. J. Planar Chromatogr. Mod. TLC 2004, 17, 218–223. [Google Scholar] [CrossRef]
- Bunch, T.D.; Panter, K.E.; James, L.F. Ultrasound studies of the effects of certain poisonous plants on uterine function and fetal development in livestock. J. Anim. Sci. 1992, 70, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Panter, K.E.; Bunch, T.D.; Keeler, R.F.; Sisson, D.V.; Callan, R.J. Multiple congenital contractures (MCC) and cleft palate induced in goats by ingestion of piperidine alkaloid-containing plants: Reduction in fetal movement as the probable cause. J. Toxicol. Clin. Toxicol. 1990, 28, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Reese, J.J. Text.-Book of Medical Jurisprudence and Toxicology; Forgotten Books: London, UK, 1884; pp. 386–387. ISBN 9781176328440. [Google Scholar]
- Rizzi, D.; Basile, C.; di Maggio, A.; Sebastio, A.; Introna, F., Jr.; Rizzi, R.; Scatizzi, A.; de Marco, S.; Smialek, J.E. Clinical spectrum of accidental hemlock poisoning: Neurotoxic manifestations, rhabdomyolosis and acute tubular necrosis. Nephrol. Dial. Transplant. 1991, 6, 393–943. [Google Scholar] [CrossRef]
- Biberci, E.; Altuntas, Y.; Çobanoglu, A.; Alpinar, A. Acute respiratory arrest following hemlock (Conium maculatum). J. Toxicol. Clin. Toxicol. 2002, 40, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Drummer, O.H.; Maurer, H.H. Analysis of toxic alkaloids in body samples. Forensic Sci. Int. 2009, 185, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Scutchfield, F.D.; Genovese, E.N. Terrible death of Socrates: Some medical and classical reflections. Pharos Alpha Omega Alpha Honor Med. Soc. 1997, 60, 30–33. [Google Scholar] [PubMed]
- Dayan, D.A. What killed Socrates? Toxicological considerations and questions. Postgrad. Med. J. 2009, 85, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J. A note on the death of Socrates. Class Q 2001, 51, 608–610. [Google Scholar] [CrossRef]
- Nitao, J.K. Test for toxicity of coniine to a polyphagous herbivore, Heliothis zea (Lepidoptera: Noctuidae). Environ. Entomol. 1987, 16, 656–659. [Google Scholar] [CrossRef]
- Castells, E.; Berenbaum, M.R. Laboratory rearing of Agonopterix alstroemeriana, the defoliating poison hemlock (Conium maculatum L.) moth, and effects of piperidine alkaloids on preference and performance. Ecol. Entomol. 2006, 35, 607–615. [Google Scholar] [CrossRef]
- Butler, J.L.; Ellision, A.M. Nitrogen cycling dynamics in the carnivorous northern pitcher plant, Sarracenia purpurea. Funct. Ecol. 2007, 21, 835–843. [Google Scholar] [CrossRef]





| Language | Name |
|---|---|
| Argentina | cicuta, denta |
| Belgium | dolle kervel, gevlekte scheerling |
| Brazil | cicuta, cicuta da europa, cigue, cuquta maior, funcho selvagem |
| Chile | cicuta, sarrac |
| Denmark | skarntyde |
| Finland | myrkkykatko |
| France | grande cique |
| Germany | Gefleckter Schierling |
| Italy | cicuta maggiore |
| Japan | doku-ninjin |
| Portugal | ansarina-malhada |
| Spain | perejillon cicuta |
| Sweden | odört |
| Turkey | tri baldiran |
| Aloe Species | Alkaloids |
|---|---|
| A. ballyii Reynolds | γ-coniceine, conhydrinone |
| A. deltoideodonta Baker | γ-coniceine, a trace of pseudoconhydrine |
| A. descoingsii Reynolds | coniine, conhydrine |
| A. gariepensis Pillans | γ-coniceine, conhydrine, a trace of coniine and N-methylconiine |
| A. globuligemma Pole Evans | γ-coniceine, coniine, conhydrine, N-methylconiine |
| A. gracilicaulis Reynolds and P.R.O. Bally | γ-coniceine |
| A. ibitiensis Perrier | γ-coniceine |
| A. krapholiana Marloth. | coniine, conhydrine |
| A. ortholopha Christian and Milne-Redh. | coniine, conhydrine |
| A. ruspoliana Baker | γ-coniceine |
| A. sabaea Schweinf. (syn. A. gillilandii Reynolds) | γ-coniceine, coniine, N,N-dimethylconiine |
| A. viguieri Perrier | coniine, γ-coniceine, N-methylconiine |
| EC50 of the Cell Line Expressing nAChR | ||
|---|---|---|
| Alkaloid | TE-671 | SH-SY5Y |
| (−)-coniine | 115 μM | 9.6 μM |
| (±)-coniine | 208 μM | 51.4 μM |
| (+)-coniine | 900 μM | 10.2 μM |
| γ-coniceine | 1.3 μM | - |
| (−)-N-methylconiine | 105 μM | - |
| (±)-N-methylconiine | 405 μM | - |
| (+)-N-methylconiine | 3000 μM | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotti, H.; Rischer, H. The killer of Socrates: Coniine and Related Alkaloids in the Plant Kingdom. Molecules 2017, 22, 1962. https://doi.org/10.3390/molecules22111962
Hotti H, Rischer H. The killer of Socrates: Coniine and Related Alkaloids in the Plant Kingdom. Molecules. 2017; 22(11):1962. https://doi.org/10.3390/molecules22111962
Chicago/Turabian StyleHotti, Hannu, and Heiko Rischer. 2017. "The killer of Socrates: Coniine and Related Alkaloids in the Plant Kingdom" Molecules 22, no. 11: 1962. https://doi.org/10.3390/molecules22111962
APA StyleHotti, H., & Rischer, H. (2017). The killer of Socrates: Coniine and Related Alkaloids in the Plant Kingdom. Molecules, 22(11), 1962. https://doi.org/10.3390/molecules22111962






