Cytotoxicity of Labruscol, a New Resveratrol Dimer Produced by Grapevine Cell Suspensions, on Human Skin Melanoma Cancer Cell Line HT-144
Abstract
:1. Introduction
2. Results and Discussion
2.1. CPC Purification and Chemical Characterization of Labruscol (1)
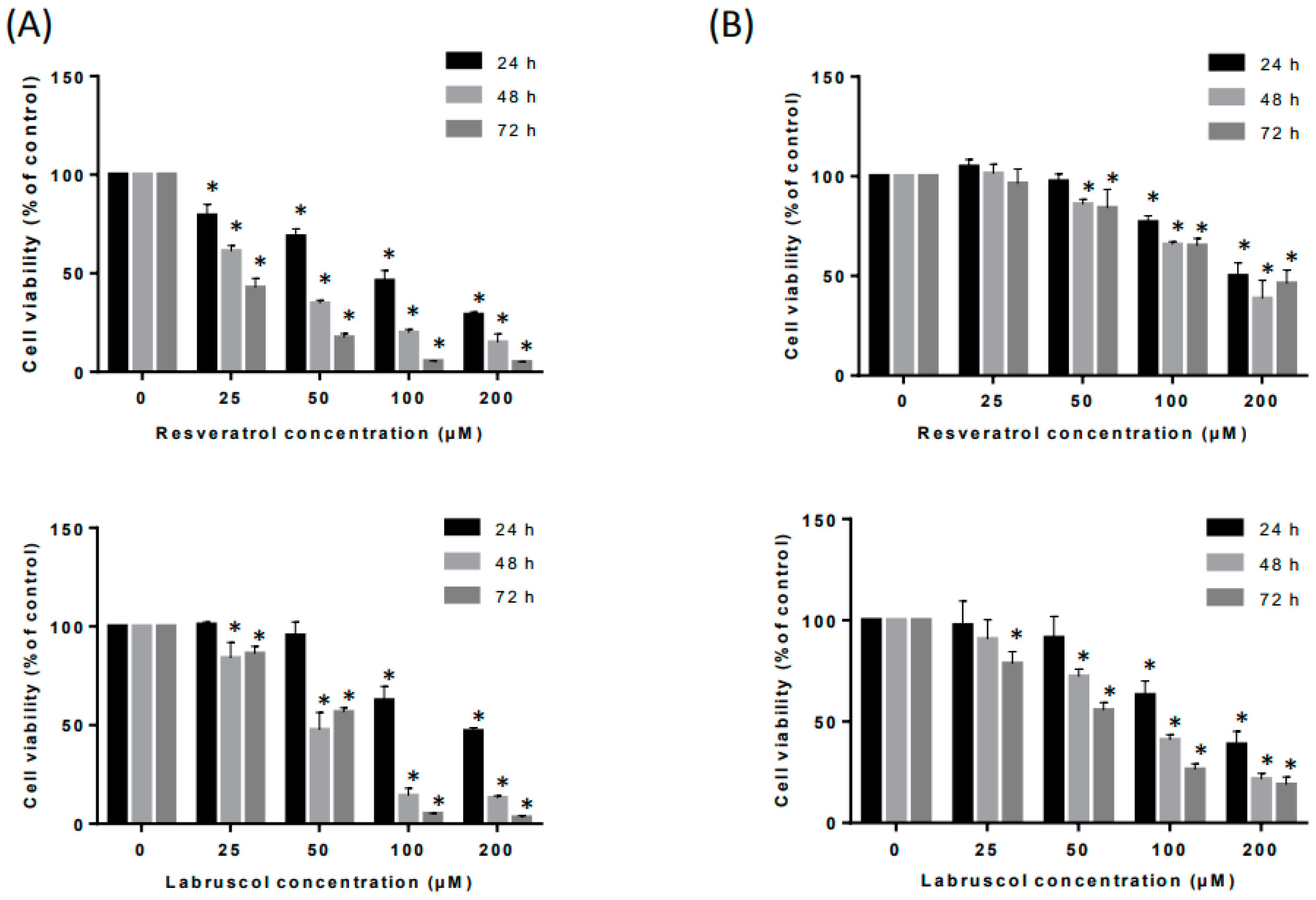
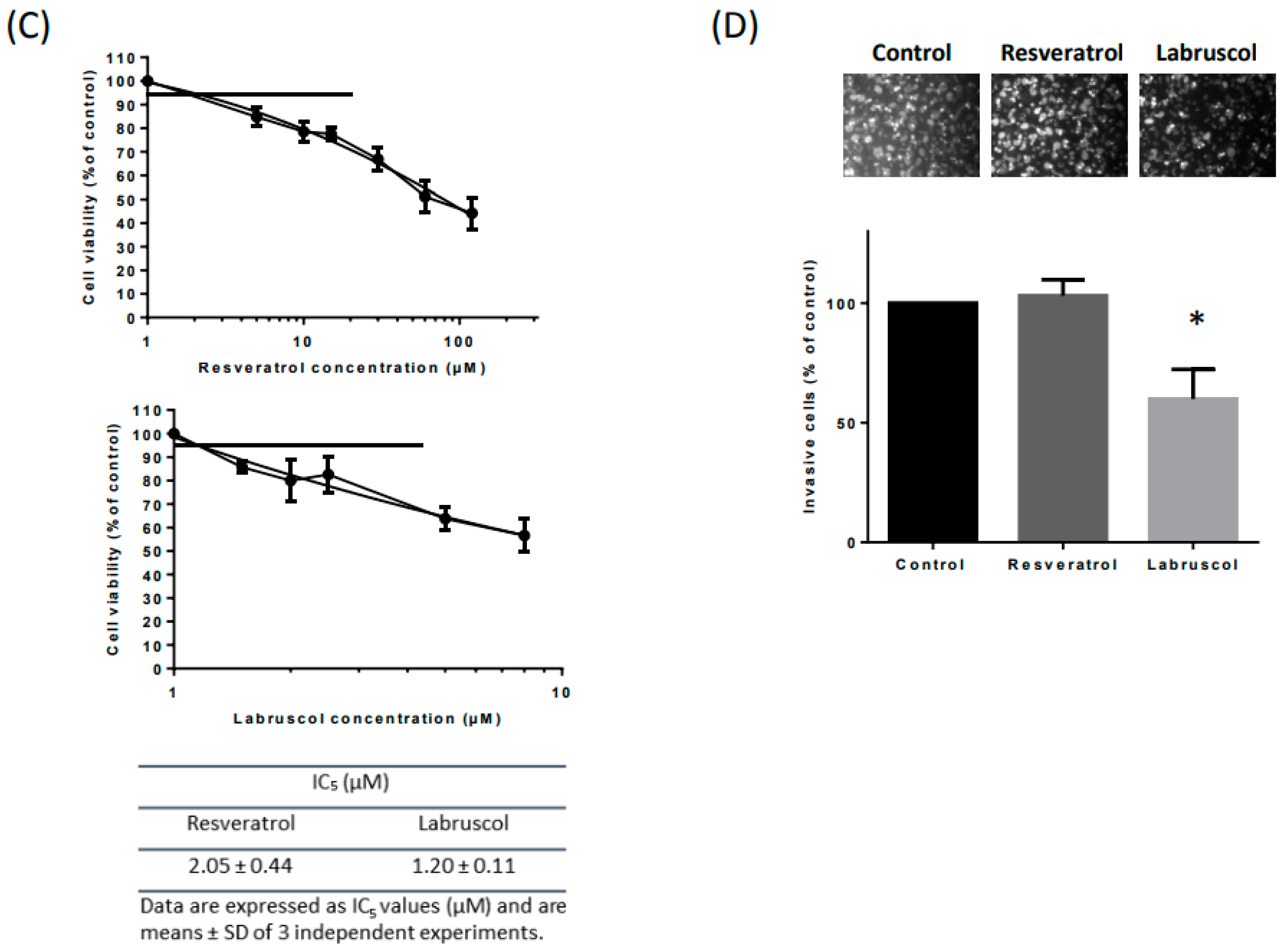
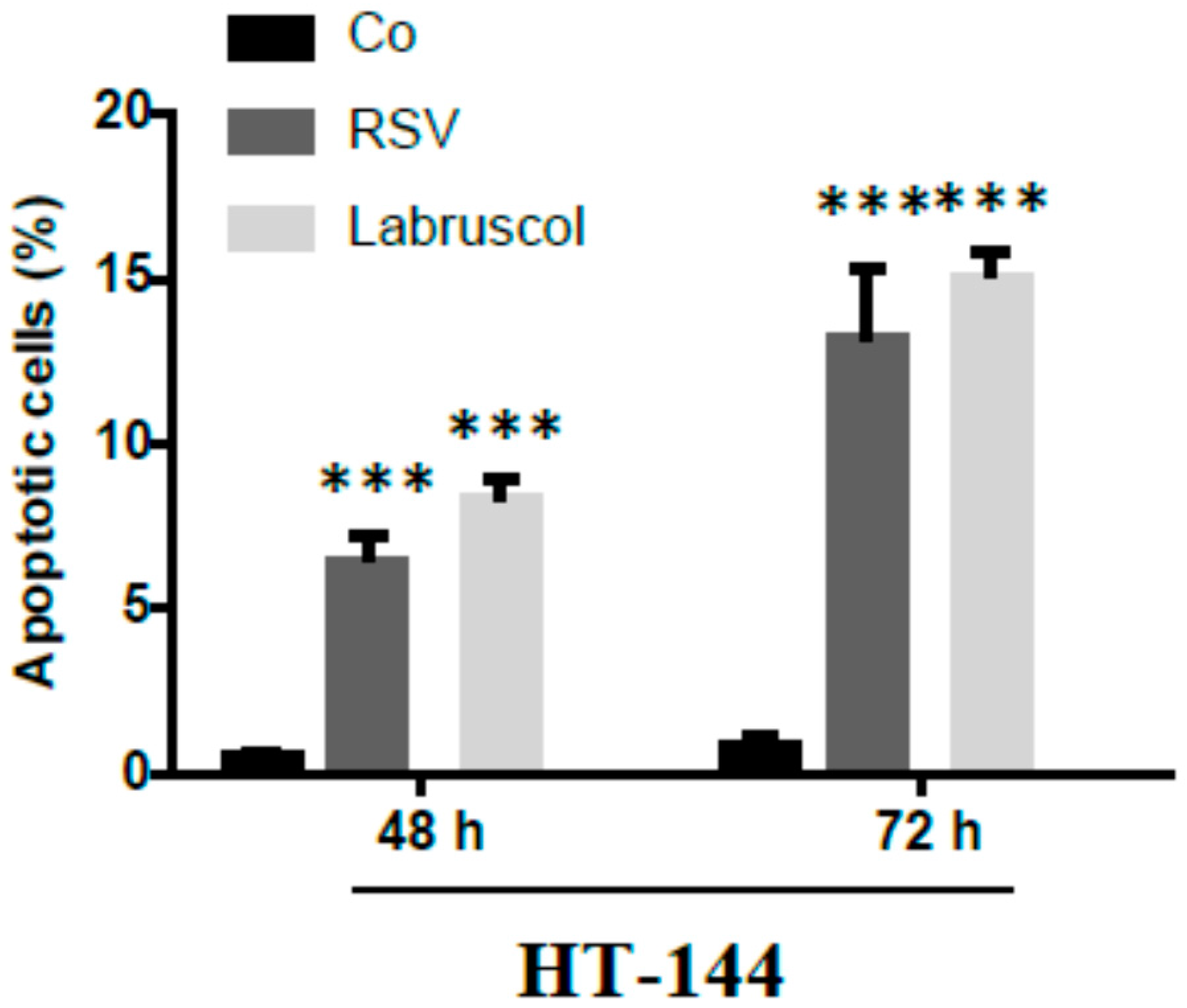
2.2. Biological Activity of Resveratrol and Labruscol
3. Materials and Methods
3.1. Chemicals, Reagents and Materials
3.2. Cultures in Bioreactor and Elicitation of Stilbene Production
3.3. Extraction of Total Stilbenes from the Culture Medium and CPC Purification of Labruscol
3.4. Structural Elucidation of Labruscol
3.5. Biological Tests
3.5.1. Cell Cultures
3.5.2. Experimental Treatments
3.5.3. Cell Viability Assay
3.5.4. In Vitro Wound Closure
3.5.5. In Vitro Invasion Assays
3.5.6. Apoptosis Identification
3.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rivière, C.; Pawlus, A.D.; Mérillon, J.-M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Privat, C.; Telo, J.P.; Bernades-Genisson, V.; Vieira, A.; Souchard, J.-P.; Nepveu, F. Antioxidant properties of trans-ε-viniferin as compared to stilbene derivatives in aqueous and non aqueous media. J. Agric. Food Chem. 2002, 50, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Nassra, M.; Krisa, S.; Papastamoulis, Y.; Kapche, G.D.; Bisson, J.; André, C.; Konsman, J.-P.; Schmitter, J.-M.; Mérillon, J.-M.; Waffo-Téguo, P. Inhibitory activity of plant stilbenoids against nitric oxide production by lipopolysaccharide-activated microglia. Planta Med. 2013, 79, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Billard, C.; Izard, J.-C.; Roman, V.; Kern, C.; Mathiot, C.; Mentz, F.; Kolb, J.-P. Comparative antiproliferative and apoptotic effects of resveratrol, ε -viniferin and vine-shots derived polyphenols (vineatrols) on chronic B lymphatic leukemia cells and normal human lymphocytes. Leuk. Lymphoma 2002, 43, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.; Gimazane, A.; Lizard, G.; Izard, J.-C.; Solary, E.; Latruffe, N.; Delmas, D. Effects of resveratrol analogs on cell cycle progression, cell cycle associated proteins and 5-fluoro-uracil sensitivity in human derived colon cancer cells. Int. J. Cancer 2009, 124, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.A.; Kishino, K.; Satoh, R.; Hashimoto, K.; Kikuchi, H.; Nishikawa, H.; Shirataki, Y.; Sakagami, H. Tumor-specificity and apoptosis-inducing activity of stilbenes and flavonoids. Anticancer Res. 2005, 25, 2055–2063. [Google Scholar] [PubMed]
- Muhtadi, H.E.H.; Juliawaty, L.D.; Syah, Y.M.; Achmad, S.A.; Latip, J.; Ghisalberti, E.L. Cytotoxic resveratrol oligomers from the tree bark of Dipterocarpus hasseltii. Fitoterapia 2006, 77, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Barjot, C.; Tournaire, M.; Castagnino, C.; Vigor, C.; Vercauteren, J.; Rossi, J.-F. Evaluation of antitumor effects of two vine stalk oligomers of resveratrol on a panel of lymphoid and myeloid cell lines: Comparison with resveratrol. Life Sci. 2007, 81, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Nivelle, L.; Hubert, J.; Courot, E.; Jeandet, P.; Aziz, A.; Nuzillard, J.-M.; Renault, J.-H.; Clément, C.; Martiny, L.; Delmas, D.; Tarpin, M. Anti-cancer activity of resveratrol and derivatives produced by grapevine cell suspensions in a 14 L stirred bioreactor. Molecules 2017, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- Zghonda, N.; Yoshida, S.; Araki, M.; Kusunoki, M.; Mliki, A.; Ghorbel, A.; Miyazaki, H. Greater effectiveness of ε-viniferin in red wine than its monomer resveratrol for inhibiting vascular smooth muscle cell proliferation and migration. Biosci. Biotechnol. Biochem. 2011, 75, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Bru, R.; García-Carmona, F.; Ros Barceló, A.; Pedreño, M.A. Effect of dimethyl-β-cyclodextrins on resveratrol metabolism in Gamay grapevine cell cultures before and after inoculation with shape Xylophilus ampelinus. Plant Cell Tissue Org. Cult. 1998, 53, 179–187. [Google Scholar] [CrossRef]
- Bru, R.; Sellés, S.; Casado-Vela, J.; Belchí-Navarro, S.; Pedreño, M.A. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J. Agric. Food Chem. 2006, 54, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Donnez, E.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol. 2009, 27, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, C.; Courot, E. Resveratrol production at large scale using plant cell suspensions. Eng. Life Sci. 2014, 14, 622–632. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Tisserant, L.-P.; Crouzet, J.; Courot, E. Use of grapevine cell cultures for the production of phytostilbenes of cosmetic interest. C. R. Chim. 2016, 19, 1062–1070. [Google Scholar] [CrossRef]
- Friesen, J.-B.; McAlpine, J.-B.; Chen, S.-N.; Pauli, G.-F. Countercurrent separation of natural products: An update. J. Nat. Prod. 2015, 78, 1765–1796. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Nuzillard, J.-M.; Purson, S.; Hamzaoui, M.; Borie, N.; Reynaud, R.; Renault, J.-H. Identification of natural metabolites in mixture: A pattern recognition strategy based on 13C-NMR. Anal. Chem. 2014, 86, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Tisserant, L.-P.; Hubert, J.; Lequart, M.; Borie, N.; Maurin, N.; Pilard, S.; Jeandet, P.; Aziz, A.; Renault, J.-H.; Nuzillard, J.-M.; et al. 13C-NMR and LC-MS profiling of stilbenes from elicited grapevine hairy root cultures. J. Nat. Prod. 2016, 79, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.M. Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol. Biol. 1998, 79, 179–183. [Google Scholar] [PubMed]
- Aziz, M.H.; Nihal, M.; Fu, V.X.; Jarrard, D.F.; Ahmad, N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol. Cancer Ther. 2006, 5, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.; Limagne, E.; Jeanningros, S.; Jacquel, A.; Lizard, G.; Athias, A.; Gambert, P.; Hichami, A.; Latruffe, N.; Solary, E.; et al. Endocytosis of resveratrol via lipid rafts and activation of downstream signaling pathways in cancer cells. Cancer Prev. Res. 2011, 4, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-B.; Hsieh, M.-J.; Lin, C.-W.; Chiou, H.-L.; Lin, P.-Y.; Chen, T.-Y.; Yang, S.-F. The antimetastatic effects of resveratrol on hepatocellular carcinoma through the downregulation of a metastasis-associated protease by SP-1 modulation. PLoS ONE 2013, 8, e56661. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.-Y.; Su, Y.-C.; Chen, N.-C.; Hsieh, H.-S.; Chen, K.-S. Resveratrol inhibits migration and invasion of human breast-cancer cells. Mol. Nutr. Food Res. 2008, 52, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-J.; Wu, C.-F.; Huang, H.-W.; Wu, C.-H.; Ho, C.-T.; Yen, G.-C. Evaluation of anti-invasion effect of resveratrol and related methoxy analogues on human hepatocarcinoma cells. J. Agric. Food Chem. 2010, 58, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Tang, L.; Chen, H.; Wu, C.; Zhao, M.; Yang, Y.; Chen, X.; Liu, G. Resveratrol inhibits TGF-β1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology 2013, 303, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Liu, X.; Fu, X.; Zhang, L.; Sui, H.; Zhou, L.; Sun, J.; Cai, J.; Qin, J.; Ren, J.; et al. Resveratrol Inhibits Invasion and Metastasis of Colorectal Cancer Cells via MALAT1 Mediated Wnt/β-Catenin Signal Pathway. PLoS ONE 2013, 8, e78700. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Gromek, S.; Niesen, D.; Seeram, N.P.; Henry, G.E. Resveratrol oligomers isolated from Carex species inhibit growth of human colon tumorigenic cells mediated by cell cycle arrest. J. Agric. Food Chem. 2011, 59, 8632–8638. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; He, C.; Ran, R.; Zhang, D.; Li, D.; Xiao, P.-G.; Altman, E. The resveratrol oligomers, cis- and trans-gnetin H, from Paeonia suffruticosa seeds inhibit the growth of several human cancer cell lines. J. Ethnopharmacol. 2015, 169, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol. Nutr. Food Res. 2013, 57, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.; Limagne, E.; Ragot, K.; Lizard, G.; Ghiringhelli, F.; Solary, E.; Chauffert, B.; Latruffe, N.; Delmas, D. Resveratrol transiently induces cell death and senescence in colon cancer cells. Cell Death Dis. 2014, 5, e1533. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.; Lancon, A.; Delmas, D.; Lizard, G.; Abrossinow, J.; Kahn, E.; Jannin, B.; Latruffe, N. Antiproliferative activities of resveratrol and related compounds in human hepatocyte derived HepG2 cells are associated with biochemical cell disturbance revealed by fluorescence analyses. Biochimie 2008, 90, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, L.; Innocenti, M.; Santamaria, A.R.; Bigagli, E.; Pasqua, G.; Mulinacci, N. Antitumoural activity of viniferin-enriched extracts from Vitis vinifera L. cell cultures. Nat. Prod. Res. 2014, 28, 2006–2016. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nivelle, L.; Hubert, J.; Courot, E.; Borie, N.; Renault, J.-H.; Nuzillard, J.-M.; Harakat, D.; Clément, C.; Martiny, L.; Delmas, D.; et al. Cytotoxicity of Labruscol, a New Resveratrol Dimer Produced by Grapevine Cell Suspensions, on Human Skin Melanoma Cancer Cell Line HT-144. Molecules 2017, 22, 1940. https://doi.org/10.3390/molecules22111940
Nivelle L, Hubert J, Courot E, Borie N, Renault J-H, Nuzillard J-M, Harakat D, Clément C, Martiny L, Delmas D, et al. Cytotoxicity of Labruscol, a New Resveratrol Dimer Produced by Grapevine Cell Suspensions, on Human Skin Melanoma Cancer Cell Line HT-144. Molecules. 2017; 22(11):1940. https://doi.org/10.3390/molecules22111940
Chicago/Turabian StyleNivelle, Laetitia, Jane Hubert, Eric Courot, Nicolas Borie, Jean-Hugues Renault, Jean-Marc Nuzillard, Dominique Harakat, Christophe Clément, Laurent Martiny, Dominique Delmas, and et al. 2017. "Cytotoxicity of Labruscol, a New Resveratrol Dimer Produced by Grapevine Cell Suspensions, on Human Skin Melanoma Cancer Cell Line HT-144" Molecules 22, no. 11: 1940. https://doi.org/10.3390/molecules22111940





