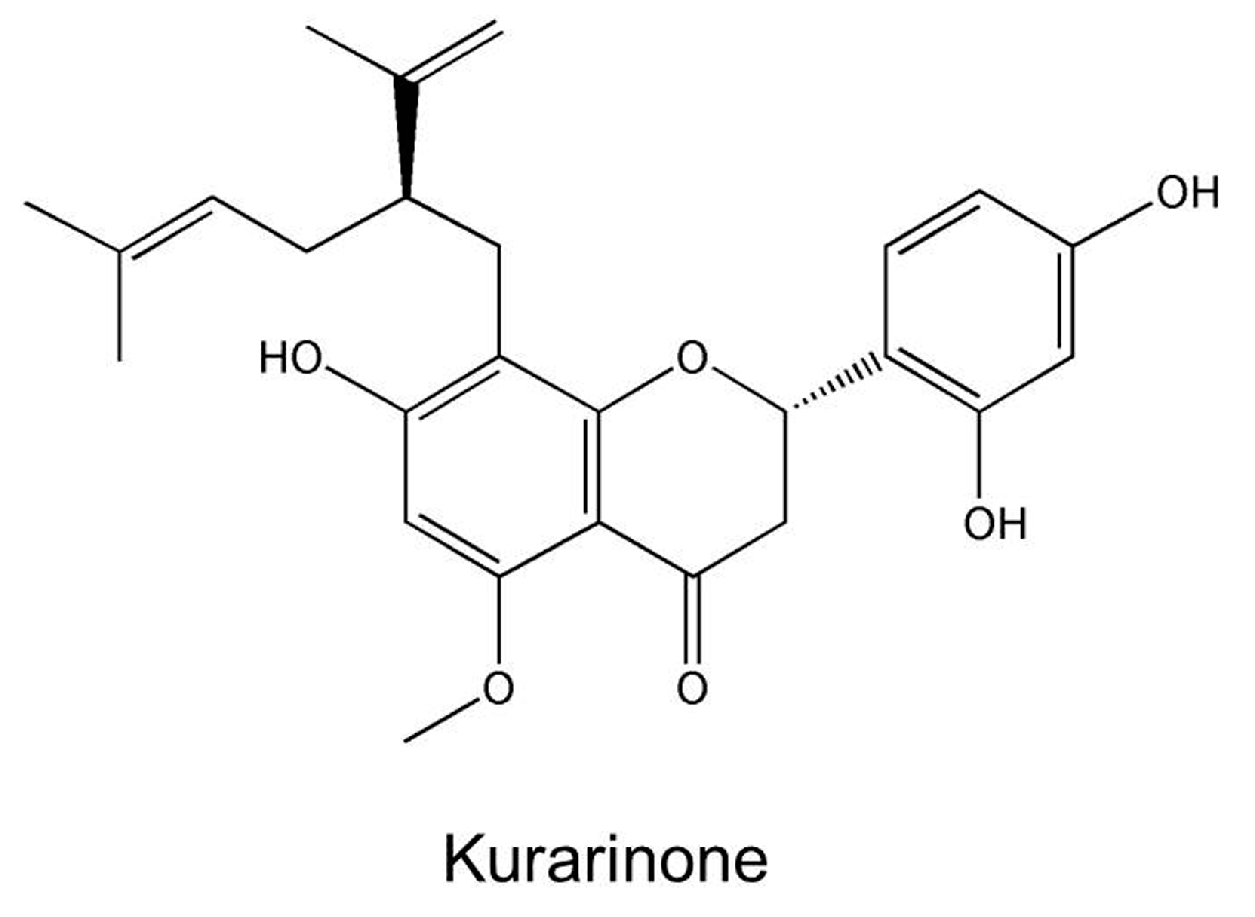
Hepatotoxicity Induced by Sophora flavescens and Hepatic Accumulation of Kurarinone, a Major Hepatotoxic Constituent of Sophora flavescens in Rats
Abstract
:1. Introduction
2. Results
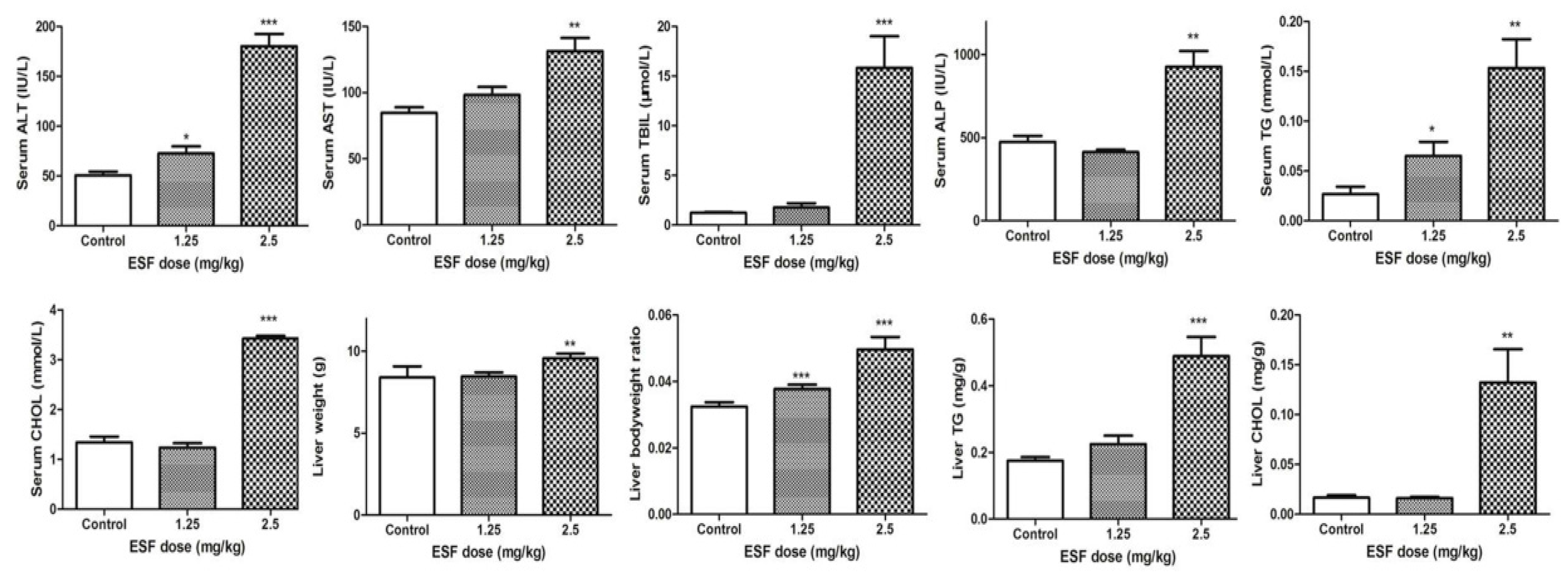
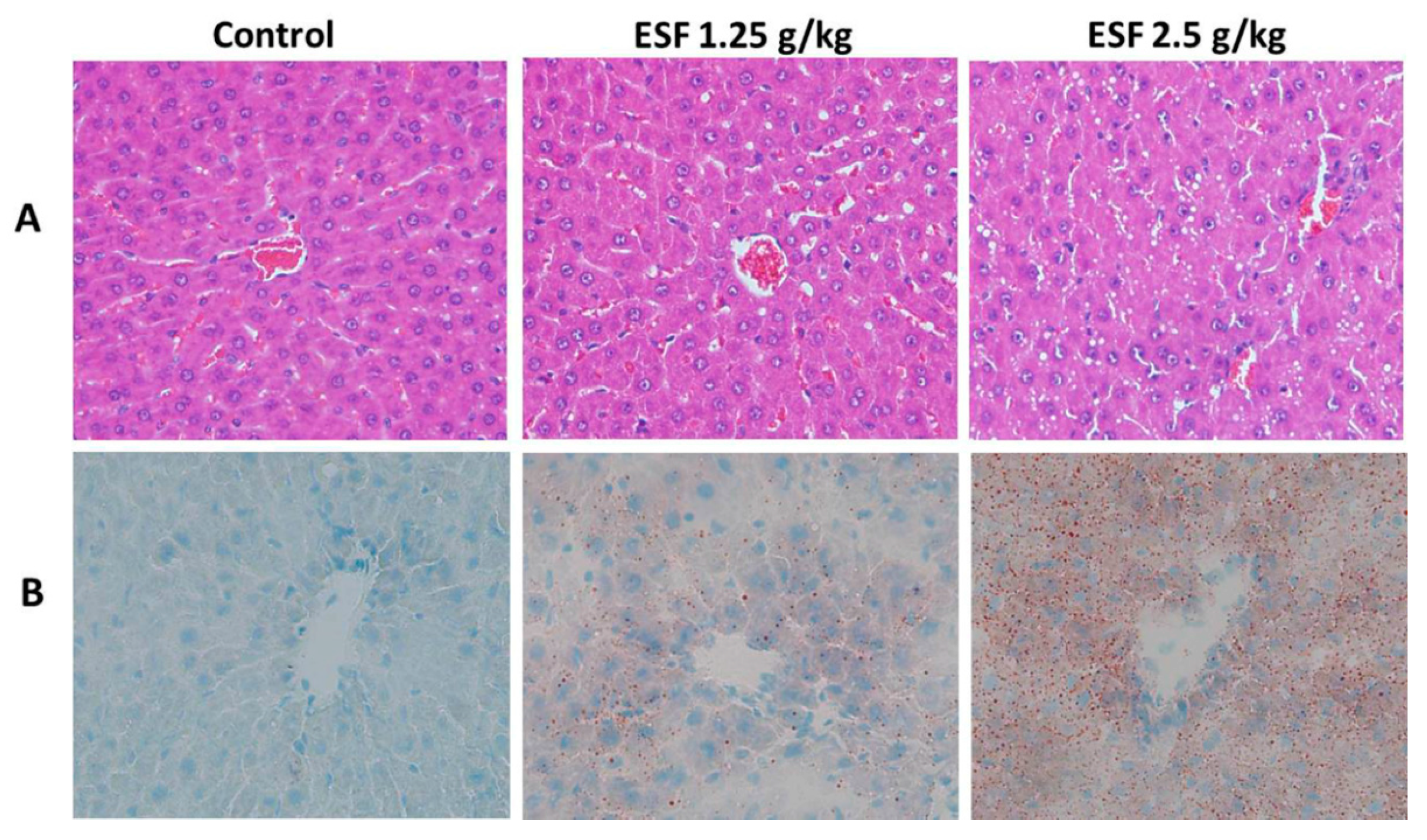
2.1. ESF Induces Hepatic Lipid Accumulation and Liver Injury
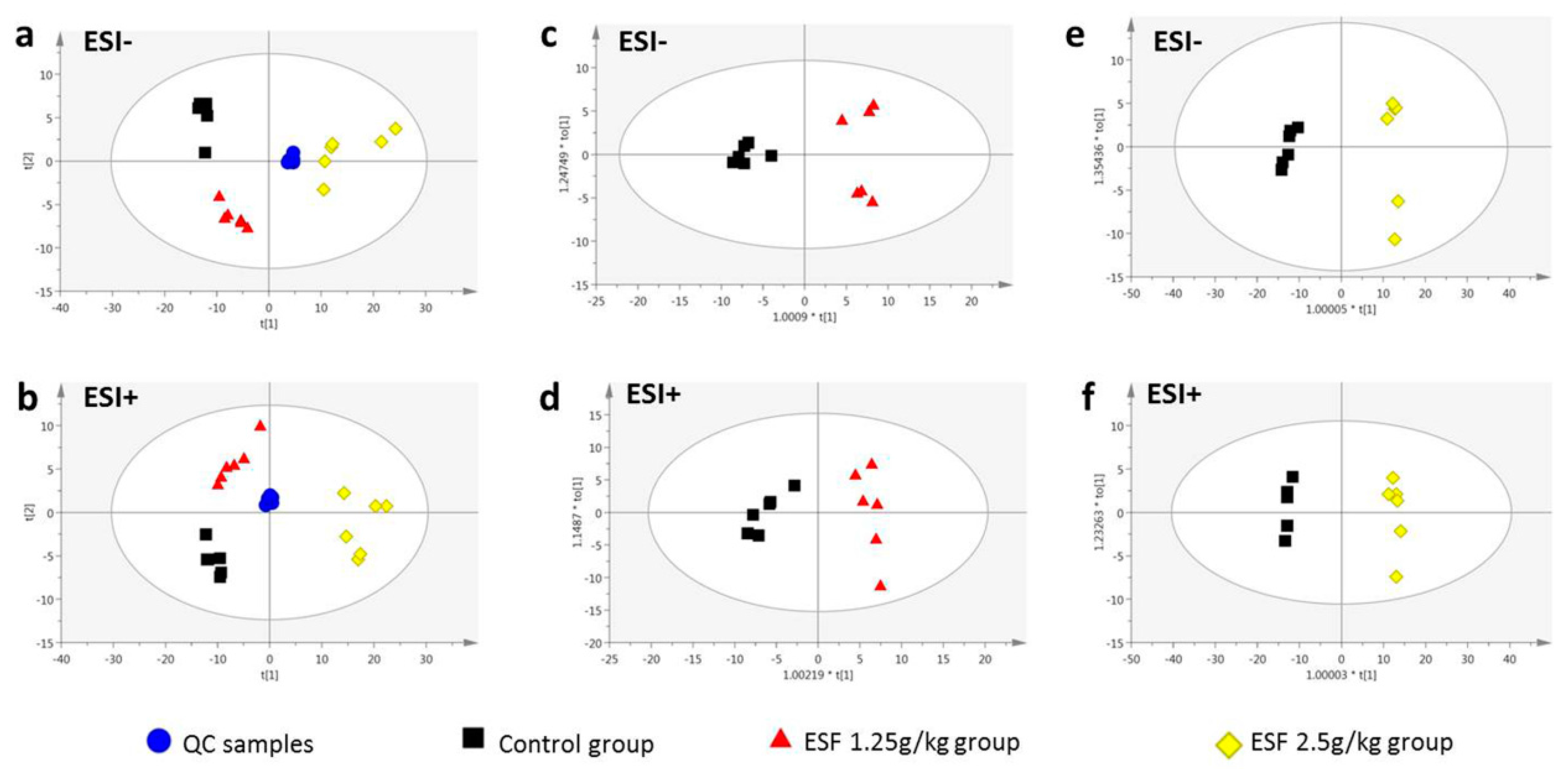
2.2. Metabolomics Study of the ESF Effects in Rats
2.2.1. Data Acquisition
2.2.2. Identification of Differential Metabolites and Analysis of Metabolic Pathways
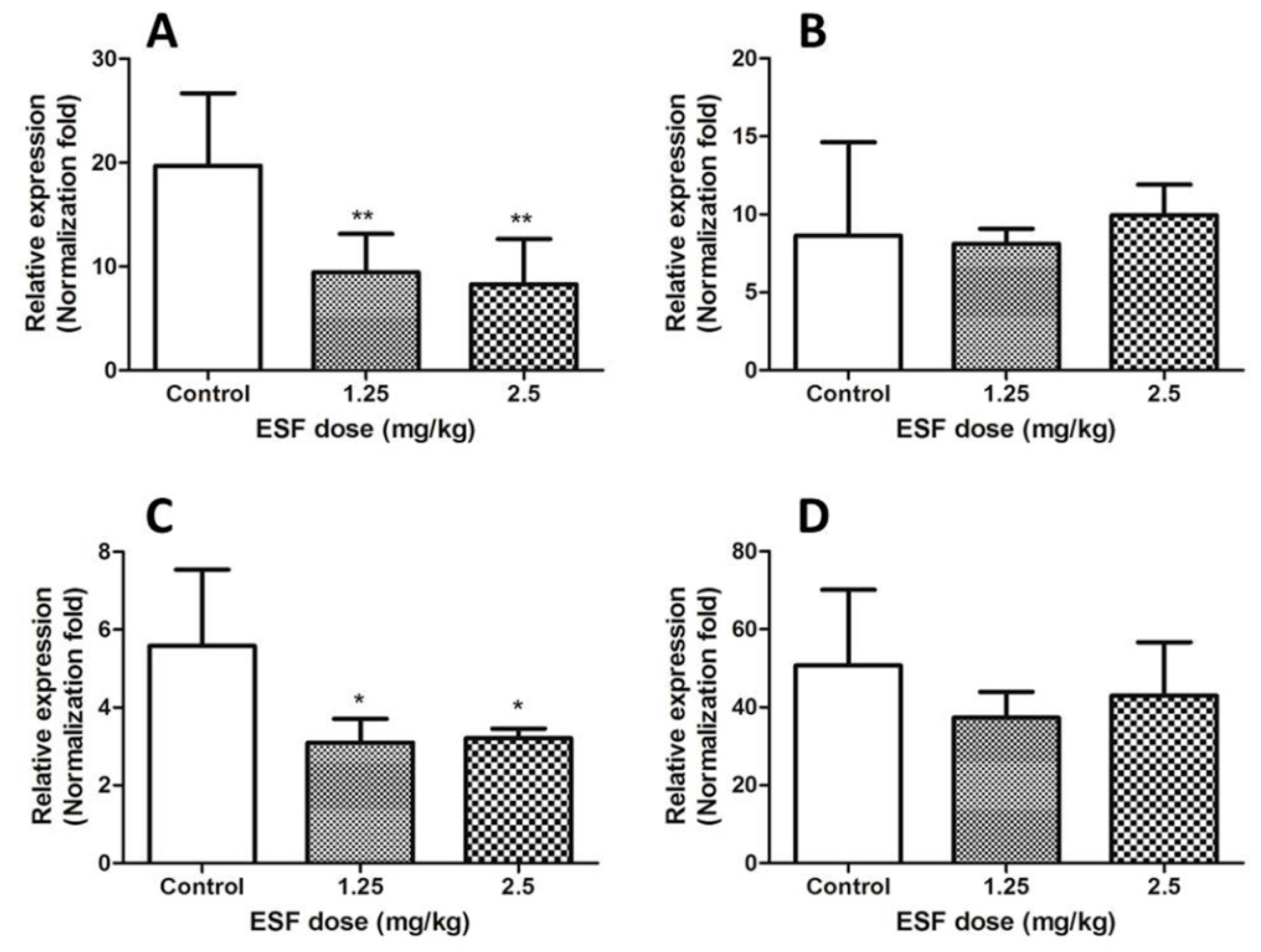
2.3. Quantitative Real-Time PCR
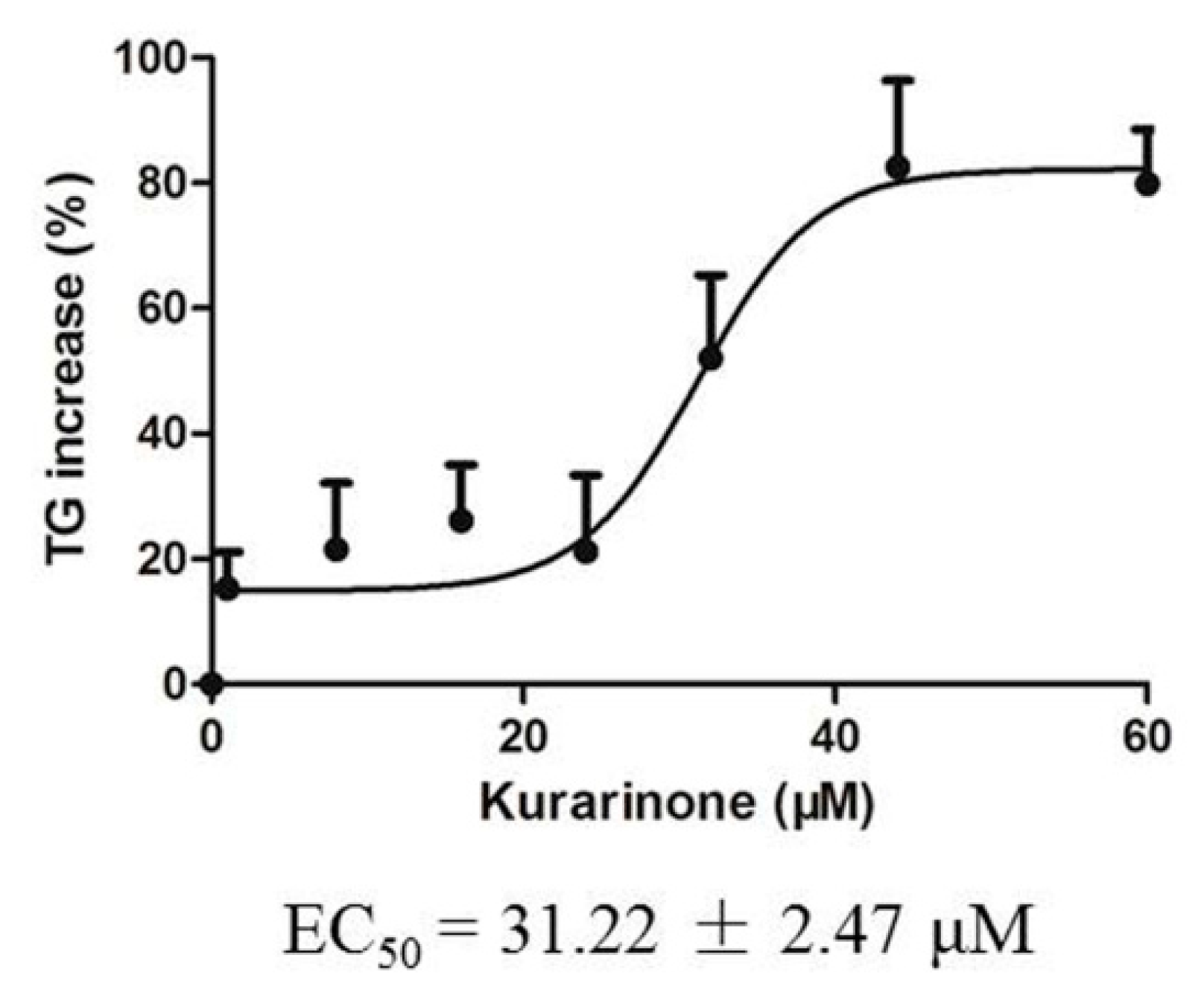
2.4. Effects of Kurarinone on Triglyceride Levels in HL 7702 Cells
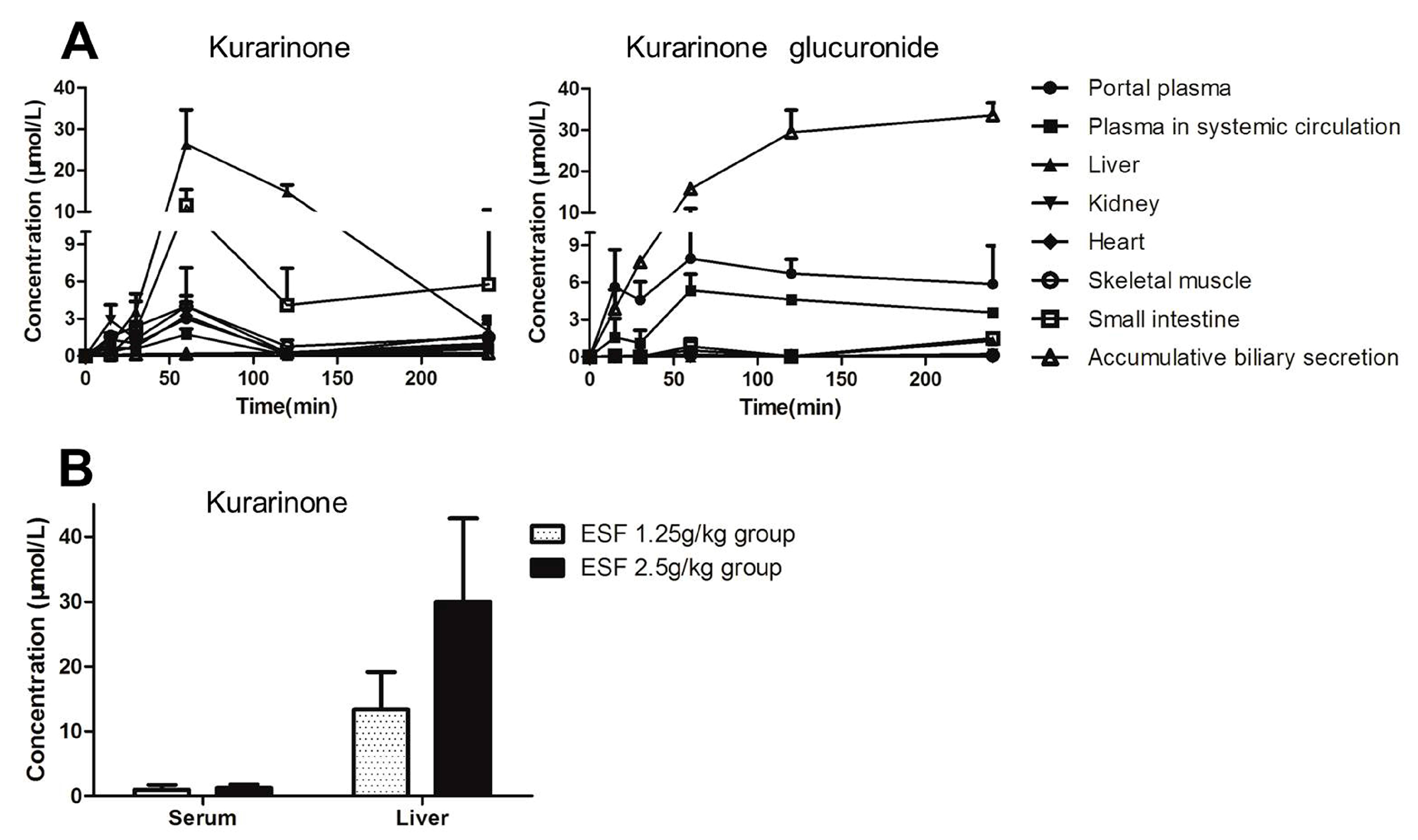
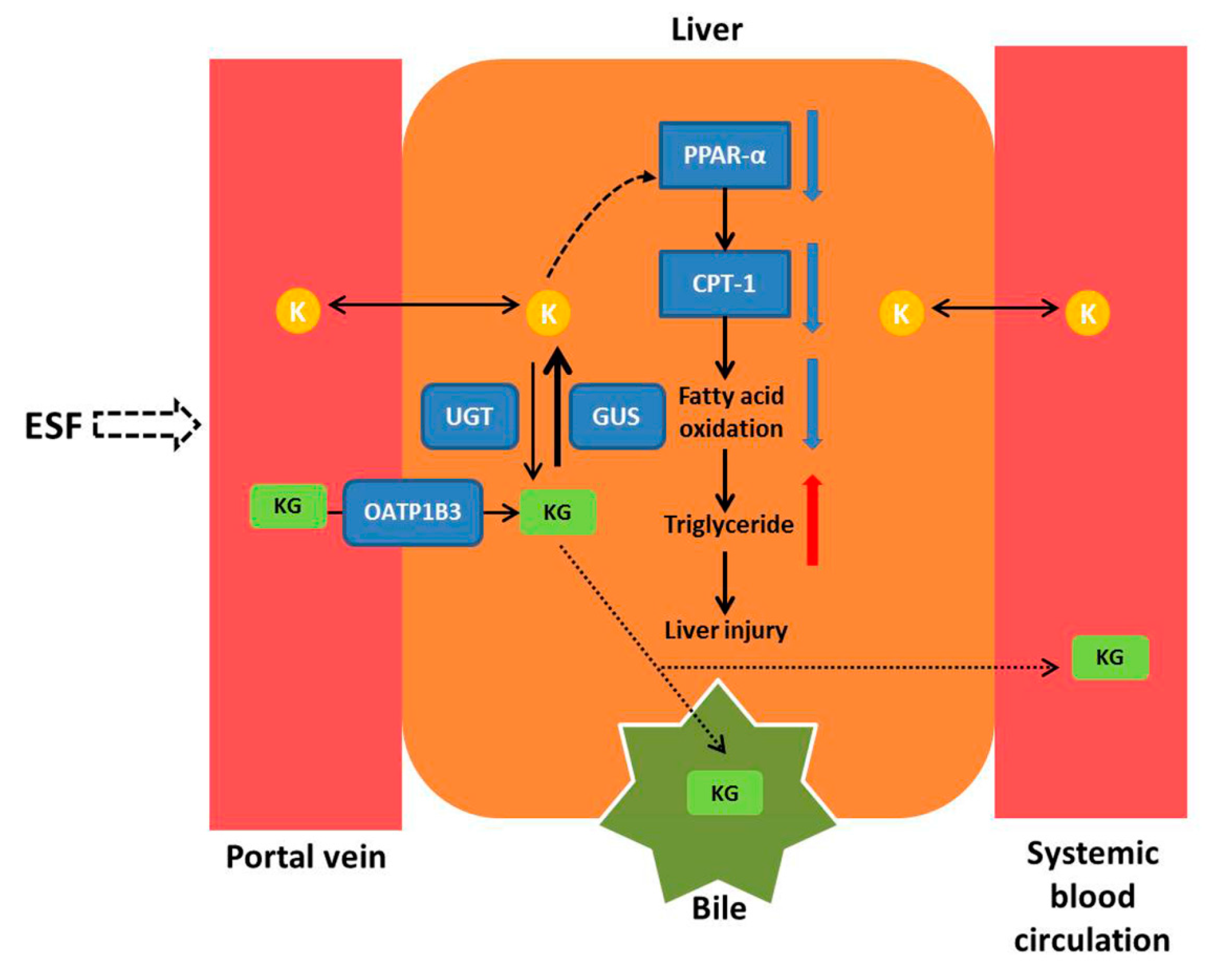
2.5. Tissue Distribution of Kurarinone and Kurarinone Glucuronides
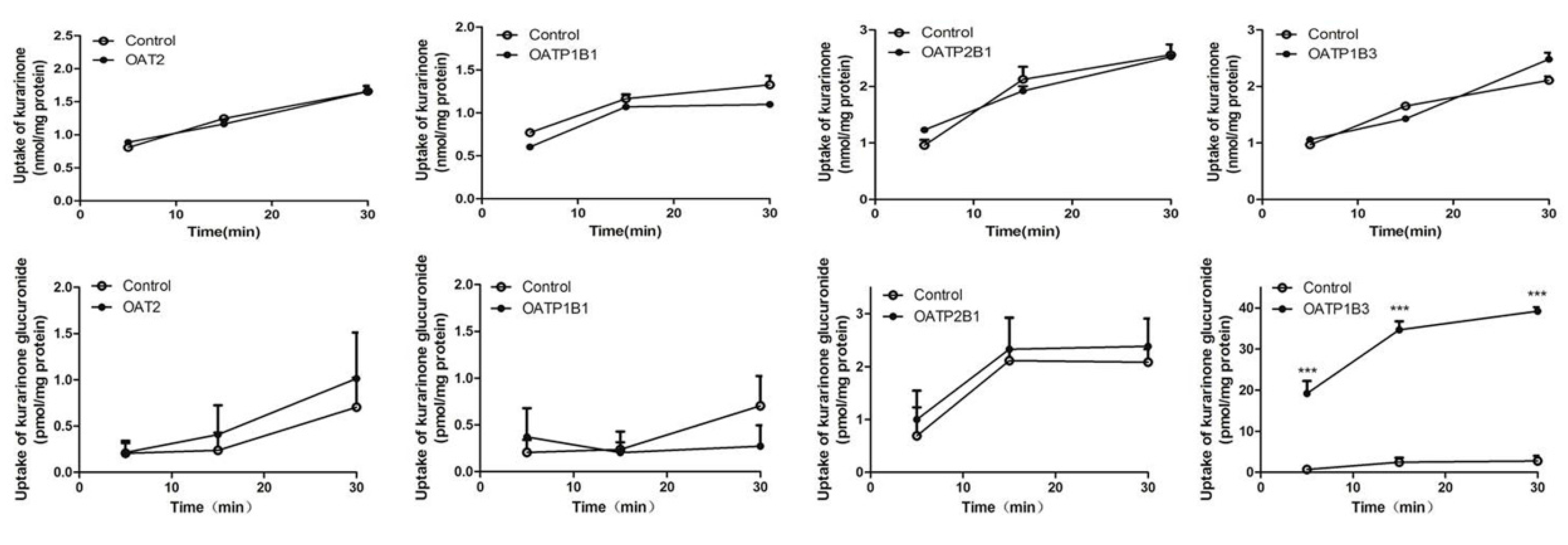
2.6. Transport Study of Kurarinone and Kurarinone Glucuronides
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Powdered ESF
4.3. Animals
4.4. ESF Hepatotoxicity Study in Rats
4.5. Serum Biochemistry Analysis and Histological Analysis
4.6. Metabolomics Study
4.6.1. Serum Sample Preparation
4.6.2. Instrumentation and Conditions
4.6.3. Data Processing and Statistical Analysis
4.7. Quantitative Real-Time PCR
4.8. Effect of Kurarinone on Triglyceride Levels in HL-7702 Cells
4.9. ESF Tissue Distribution Study in Rats
4.10. Uptake Transporter Study of Kurarinone and Kurarinone Glucuronides
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, G.J.; Mann, S.P.; Ngu, M.C. Acute hepatitis induced by Shou-Wu-Pian, a herbal product derived from Polygonum multiflorum. J. Gastroenterol. Hepatol. 2001, 16, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ji, L.; Liu, H.; Wang, Z. Study of the hepatotoxicity induced by Dioscorea bulbifera L. rhizome in mice. Biosci. Trends 2010, 4, 79–85. [Google Scholar] [PubMed]
- Wang, J.; Jiang, Z.; Ji, J.; Li, Y.; Chen, M.; Wang, Y.; Zhang, Y.; Tai, T.; Wang, T.; Zhang, L. Evaluation of hepatotoxicity and cholestasis in rats treated with EtOH extract of Fructus Psoraleae. J. Ethnopharmacol. 2012, 144, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Slain, D.; Cheng, J.; Ma, W.; Liang, W. Eighteen cases of liver injury following ingestion of Polygonum multiflorum. Complement. Ther. Med. 2014, 22, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.A.; Min, H.J.; Yoo, S.S.; Kim, H.J.; Choi, S.N.; Ha, C.Y.; Kim, T.H.; Jung, W.T.; Lee, O.J.; Lee, J.S.; et al. Drug-Induced Liver Injury: Twenty Five Cases of Acute Hepatitis Following Ingestion of Polygonum multiflorum Thunb. Gut Liver 2011, 5, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, F.; Lu, J.; Li, G.; Li, D.; Zhong, X.B.; Guo, G.L.; Ma, X. The Chinese herbal medicine Sophora flavescens activates pregnane X receptor. Drug Metab. Dispos. 2010, 38, 2226–2231. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.P.; Yue, H.; Zhai, H.Z. Zhixue capsule caused seven cases of drug induced hepatitis. Hepatogastroenterology 2010, 57, 571–572. [Google Scholar] [PubMed]
- Yu, Q.; Cheng, N.; Ni, X. Identifying 2 prenylflavanones as potential hepatotoxic compounds in the ethanol extract of Sophora flavescens. J. Food Sci. 2013, 78, T1830–T1834. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Zhao, H.P.; Zhao, Y.L.; Jin, C.; Liu, D.J.; Kong, W.J.; Fang, F.; Zhang, L.; Wang, H.J.; Xiao, X.H. Hepatotoxicity or hepatoprotection? Pattern recognition for the paradoxical effect of the Chinese herb Rheum palmatum L. in treating rat liver injury. PLoS ONE 2011, 6, e24498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, Z.; Sun, W.; Wang, P.; Wang, X. Metabolomics: Towards understanding traditional Chinese medicine. Planta Med. 2010, 76, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Ma, C.; Zhang, L.; Yang, G.; Cui, Y.; Su, D.; Zhao, X.; Liu, Z.; Bi, K.; Chen, X. Metabonomic study of genkwa flos-induced hepatotoxicity and effect of herb-processing procedure on toxicity. Phytother. Res. 2013, 27, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Korzekwa, K.; Elsby, R.; Fenner, K.; Galetin, A.; Lai, Y.; Matsson, P.; Moss, A.; Nagar, S.; Rosania, G.R.; et al. Intracellular drug concentrations and transporters: Measurement, modeling, and implications for the liver. Clin. Pharmacol. Ther. 2013, 94, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tian, Y.; Li, G.; Li, Y.; Yin, X.; Peng, C.; Xu, F.; Zhang, Z. Discovery of safety biomarkers for realgar in rat urine using UFLC-IT-TOF/MS and 1H NMR based metabolomics. Anal. Bioanal. Chem. 2013, 405, 4811–4822. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Massart, J.; Robin, M.A.; Borgne-Sanchez, A.; Fromenty, B. Drug-induced toxicity on mitochondria and lipid metabolism: Mechanistic diversity and deleterious consequences for the liver. J. Hepatol. 2011, 54, 773–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.J.; Chen, C.W.; Fu, Q.C.; Zhang, J.H. Drug-induced liver injury related with Zhixue Capsules: Analysis of 30 gregarious patients. Chin. Hepatol. 2009, 14, 287–290. [Google Scholar]
- Konig, B.; Koch, A.; Spielmann, J.; Hilgenfeld, C.; Hirche, F.; Stangl, G.I.; Eder, K. Activation of PPARalpha and PPARgamma reduces triacylglycerol synthesis in rat hepatoma cells by reduction of nuclear SREBP-1. Eur. J. Pharmacol. 2009, 605, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Yoo, S.H.; Henderson, L.E.; Gonzalez, F.J.; Woodcroft, K.J.; Song, B.J. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J. Nutr. 2011, 141, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Park, S.Y.; Shinzawa, K.; Kim, S.; Chung, K.W.; Lee, J.H.; Kwon, C.H.; Lee, K.W.; Park, C.K.; Chung, W.J.; et al. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J. Lipid Res. 2008, 49, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Kakisaka, K.; Cazanave, S.C.; Fingas, C.D.; Guicciardi, M.E.; Bronk, S.F.; Werneburg, N.W.; Mott, J.L.; Gores, G.J. Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G77–G84. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.Y.; Huskey, S.E.; Braun, M.P.; Sarkadi, B.; Evans, D.C.; Evers, R. Transport of ethinylestradiol glucuronide and ethinylestradiol sulfate by the multidrug resistance proteins MRP1, MRP2, and MRP3. J. Pharmacol. Exp. Ther. 2004, 309, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Wittgen, H.G.; Van Den Heuvel, J.J.; Van Den Broek, P.H.; Siissalo, S.; Groothuis, G.M.; de Graaf, I.A.; Koenderink, J.B.; Russel, F.G. Transport of the coumarin metabolite 7-hydroxycoumarin glucuronide is mediated via multidrug resistance-associated proteins 3 and 4. Drug Metab. Dispos. 2012, 40, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, B.; Wu, B.; Yu, R.; Hu, M. UDP-glucuronosyltransferase (UGT) 1A9-overexpressing HeLa cells is an appropriate tool to delineate the kinetic interplay between breast cancer resistance protein (BRCP) and UGT and to rapidly identify the glucuronide substrates of BCRP. Drug Metab. Dispos. 2012, 40, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Usui, I.; Kato, I.; Oya, T.; Kanatani, Y.; Yamazaki, Y.; Fujisaka, S.; Senda, S.; Ishii, Y.; Urakaze, M.; et al. Deletion of platelet-derived growth factor receptor-beta improves diabetic nephropathy in Ca(2)(+)/calmodulin-dependent protein kinase IIalpha (Thr286Asp) transgenic mice. Diabetologia 2011, 54, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.M.; Li, R.F.; Qiu, J.F.; Zhang, Z.Y.; Wang, H.B.; Bian, L.; Lei, J.H. Determination of kurarinone in rat plasma by UPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 986–987, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, P.; Chen, P.; Cheng, N. Metabolism of kurarinone by human liver microsomes and its effect on cytotoxicity. Pharm. Biol. 2016, 54, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, H.; Guo, Z.; You, T.; Chen, X.; Zhong, D. Mechanistic studies on the absorption and disposition of scutellarin in humans: Selective OATP2B1-mediated hepatic uptake is a likely key determinant for its unique pharmacokinetic characteristics. Drug Metab. Dispos. 2012, 40, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.Q.; Yan, R. Regioselective glucuronidation of the isoflavone calycosin by human liver microsomes and recombinant human UDP-glucuronosyltransferases. Chem. Biol. Interact. 2014, 220, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Welsch, T.; Humpf, H.U. HT-2 toxin 4-glucuronide as new T-2 toxin metabolite: Enzymatic synthesis, analysis, and species specific formation of T-2 and HT-2 toxin glucuronides by rat, mouse, pig, and human liver microsomes. J. Agric. Food Chem. 2012, 60, 10170–10178. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
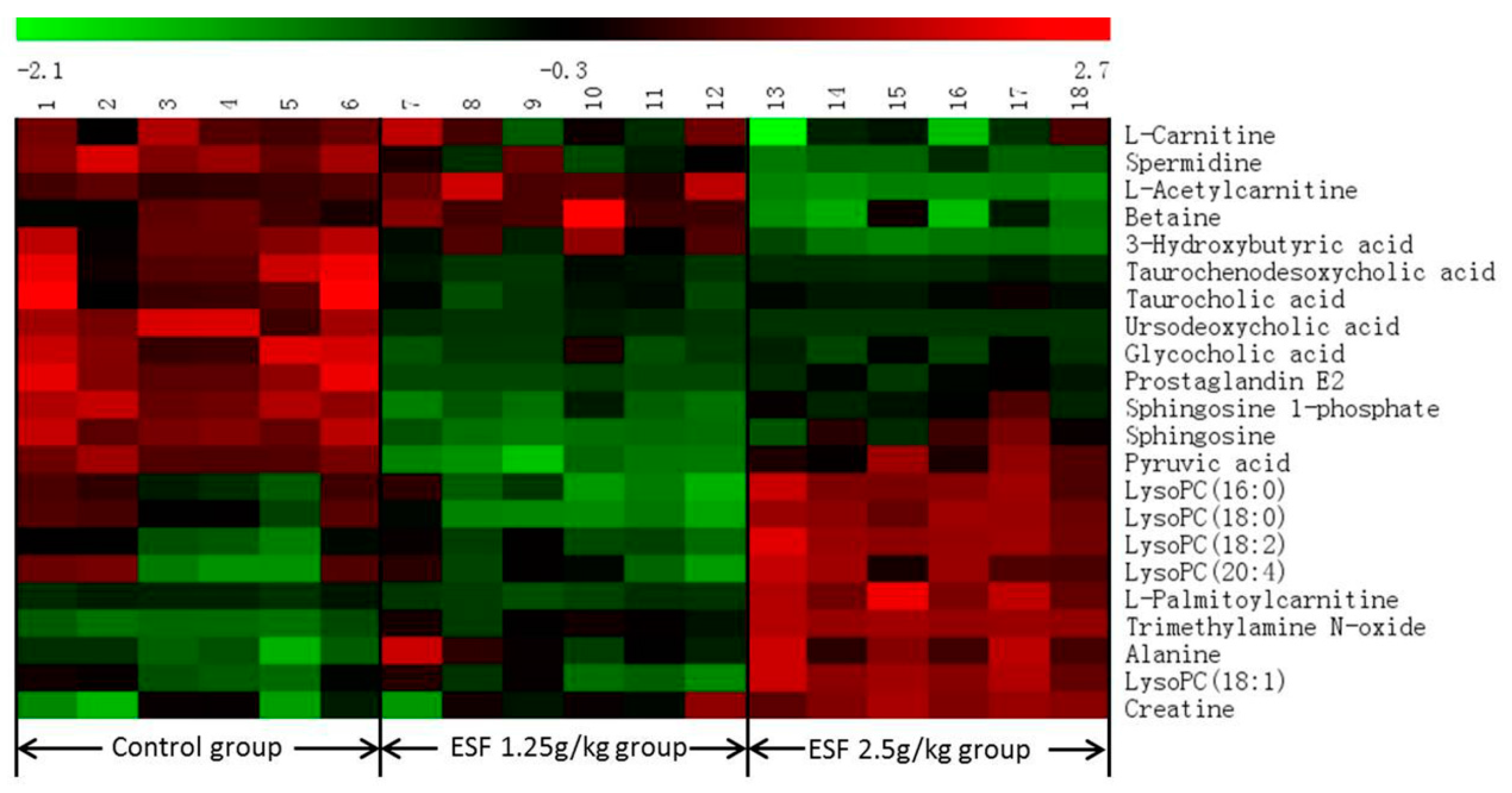
| No. | tR (min) | Detected m/z | Metabolites a | VIP d | Trend e | Related Pathway f | p-Value g | ESI Mode |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.90 | 146.1647 | Spermidine | 1.02 | ↓ | Glutathione metabolism | 0.00 | + |
| 2 | 1.00 | 87.0084 | Pyruvic acid b | 1.32 | ↓ | Pyruvate metabolism | 0.01 | − |
| 3 | 1.02 | 162.1115 | l-Carnitine | 1.96 | ↓ | Fatty acid metabolism | 0.00 | + |
| 4 | 1.03 | 118.0854 | Betaine | 1.66 | ↓ | Betaine metabolism | 0.03 | + |
| 5 | 1.04 | 76.0751 | Trimethylamine N-oxide | 1.01 | ↑ | Choline metabolism | 0.00 | + |
| 6 | 1.05 | 103.0395 | 3-Hydroxybutyric acid | 2.13 | ↓ | Synthesis and degradation of ketone bodies | 0.00 | − |
| 7 | 1.06 | 132.0757 | Creatine b | 2.63 | ↑ | Glycine and serine metabolism | 0.00 | + |
| 8 | 1.18 | 90.0542 | Alanine | 6.68 | ↑ | Alanine metabolism | 0.00 | + |
| 9 | 1.52 | 204.1219 | l-Acetylcarnitine | 1.20 | ↓ | Beta oxidation of very long chain fatty acids | 0.00 | + |
| 10 | 3.87 | 514.2798 | Taurocholic acid b | 1.83 | ↓ | Primary bile acid biosynthesis | 0.01 | − |
| 11 | 5.39 | 464.2981 | Glycocholic acid b | 2.08 | ↓ | Primary bile acid biosynthesis | 0.00 | − |
| 12 | 5.48 | 498.2852 | Taurochenodesoxycholic acid b | 2.38 | ↓ | Primary bile acid biosynthesis | 0.00 | − |
| 13 | 5.68 | 353.2313 | Prostaglandin E2 b | 1.13 | ↓ | Arachidonic acid metabolism | 0.00 | + |
| 14 | 6.73 | 391.2817 | Ursodeoxycholic acid b | 3.68 | ↓ | Secondary bile acid biosynthesis | 0.00 | − |
| 15 | 8.41 | 378.2383 | Sphingosine 1-phosphate | 2.00 | ↓ | Phospholipid metabolism | 0.00 | − |
| 16 | 9.83 | 520.3358 | LysoPC (18:2) c | 2.53 | ↑ | Glycerophospholipid metabolism | 0.00 | + |
| 17 | 10.20 | 544.3350 | LysoPC (20:4) c | 2.67 | ↑ | Glycerophospholipid metabolism | 0.01 | + |
| 18 | 10.34 | 300.2883 | Sphingosine | 1.02 | ↓ | Phospholipid metabolism | 0.00 | + |
| 19 | 10.53 | 496.3361 | LysoPC (16:0) c | 6.56 | ↑ | Glycerophospholipid metabolism | 0.02 | + |
| 20 | 11.29 | 522.3516 | LysoPC (18:1) c | 2.59 | ↑ | Glycerophospholipid metabolism | 0.00 | + |
| 21 | 12.67 | 524.3668 | LysoPC (18:0) c | 7.41 | ↑ | Glycerophospholipid metabolism | 0.00 | + |
| 22 | 12.78 | 400.3401 | l-Palmitoylcarnitine | 1.94 | ↑ | Fatty acid metabolism | 0.00 | + |
| AUC0–t (h·nmol/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Protal Plasma | Body Plasma | Intestine | Liver | Kidney | Heart | Skeletal Muscle | Biliary Secretion | |
| Kurarinone | 4.4 | 2.4 | 21.4 | 45.5 | 5.4 | 4.7 | 6.9 | 0.8 |
| Kurarinone glucuronide | 25.0 | 15.3 | 2.2 | 1.4 | 0.7 | 0.1 | 0.4 | 93.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, P.; Zhang, X.; Huang, Y.; Cheng, N.; Ma, Y. Hepatotoxicity Induced by Sophora flavescens and Hepatic Accumulation of Kurarinone, a Major Hepatotoxic Constituent of Sophora flavescens in Rats. Molecules 2017, 22, 1809. https://doi.org/10.3390/molecules22111809
Jiang P, Zhang X, Huang Y, Cheng N, Ma Y. Hepatotoxicity Induced by Sophora flavescens and Hepatic Accumulation of Kurarinone, a Major Hepatotoxic Constituent of Sophora flavescens in Rats. Molecules. 2017; 22(11):1809. https://doi.org/10.3390/molecules22111809
Chicago/Turabian StyleJiang, Peng, Xiuwen Zhang, Yutong Huang, Nengneng Cheng, and Yueming Ma. 2017. "Hepatotoxicity Induced by Sophora flavescens and Hepatic Accumulation of Kurarinone, a Major Hepatotoxic Constituent of Sophora flavescens in Rats" Molecules 22, no. 11: 1809. https://doi.org/10.3390/molecules22111809
APA StyleJiang, P., Zhang, X., Huang, Y., Cheng, N., & Ma, Y. (2017). Hepatotoxicity Induced by Sophora flavescens and Hepatic Accumulation of Kurarinone, a Major Hepatotoxic Constituent of Sophora flavescens in Rats. Molecules, 22(11), 1809. https://doi.org/10.3390/molecules22111809





