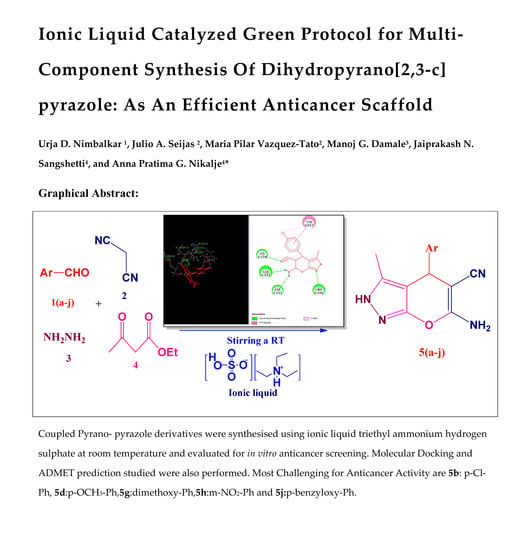
Ionic Liquid-Catalyzed Green Protocol for Multi-Component Synthesis of Dihydropyrano[2,3-c]pyrazoles as Potential Anticancer Scaffolds
Abstract
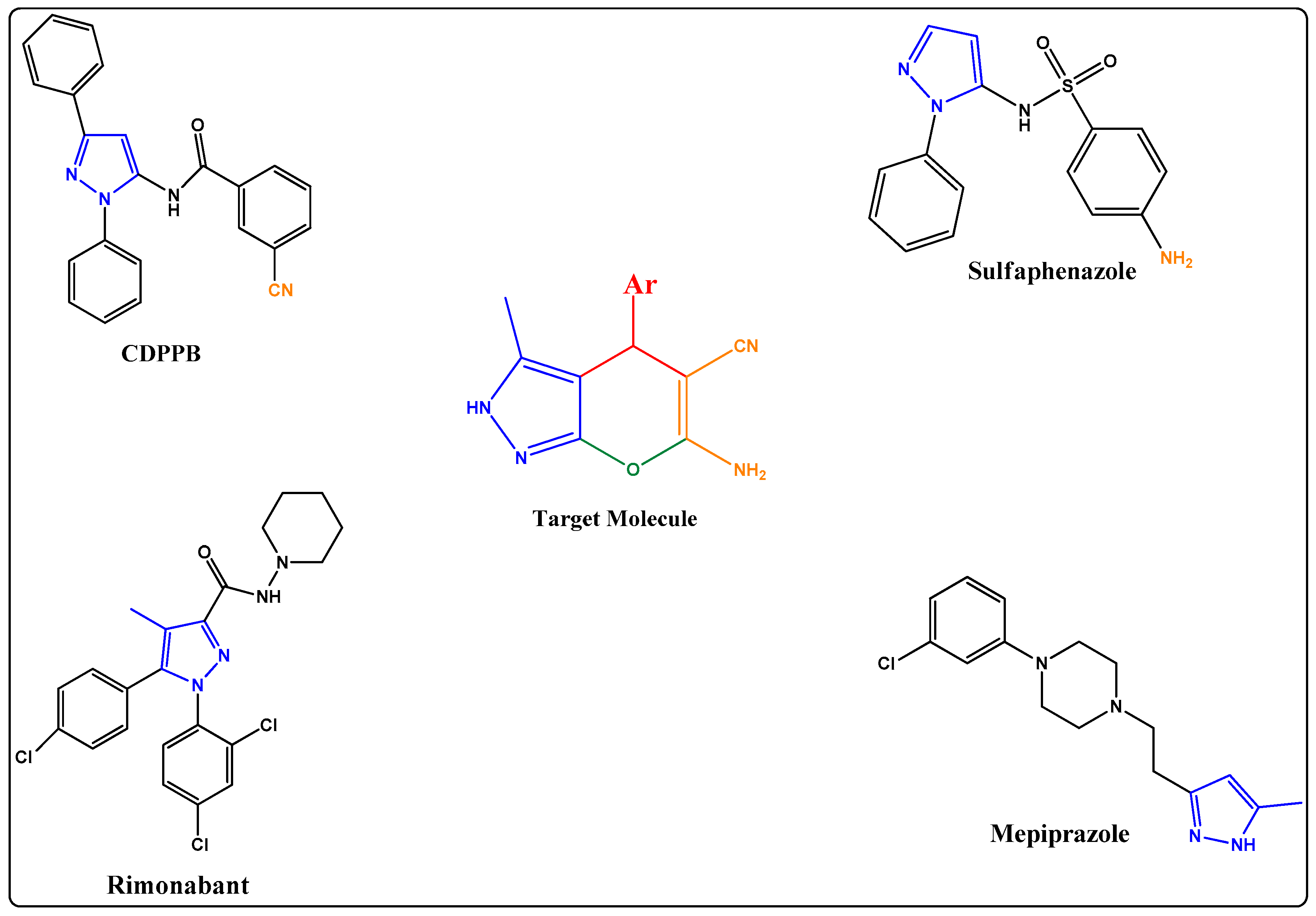
:1. Introduction
2. Results
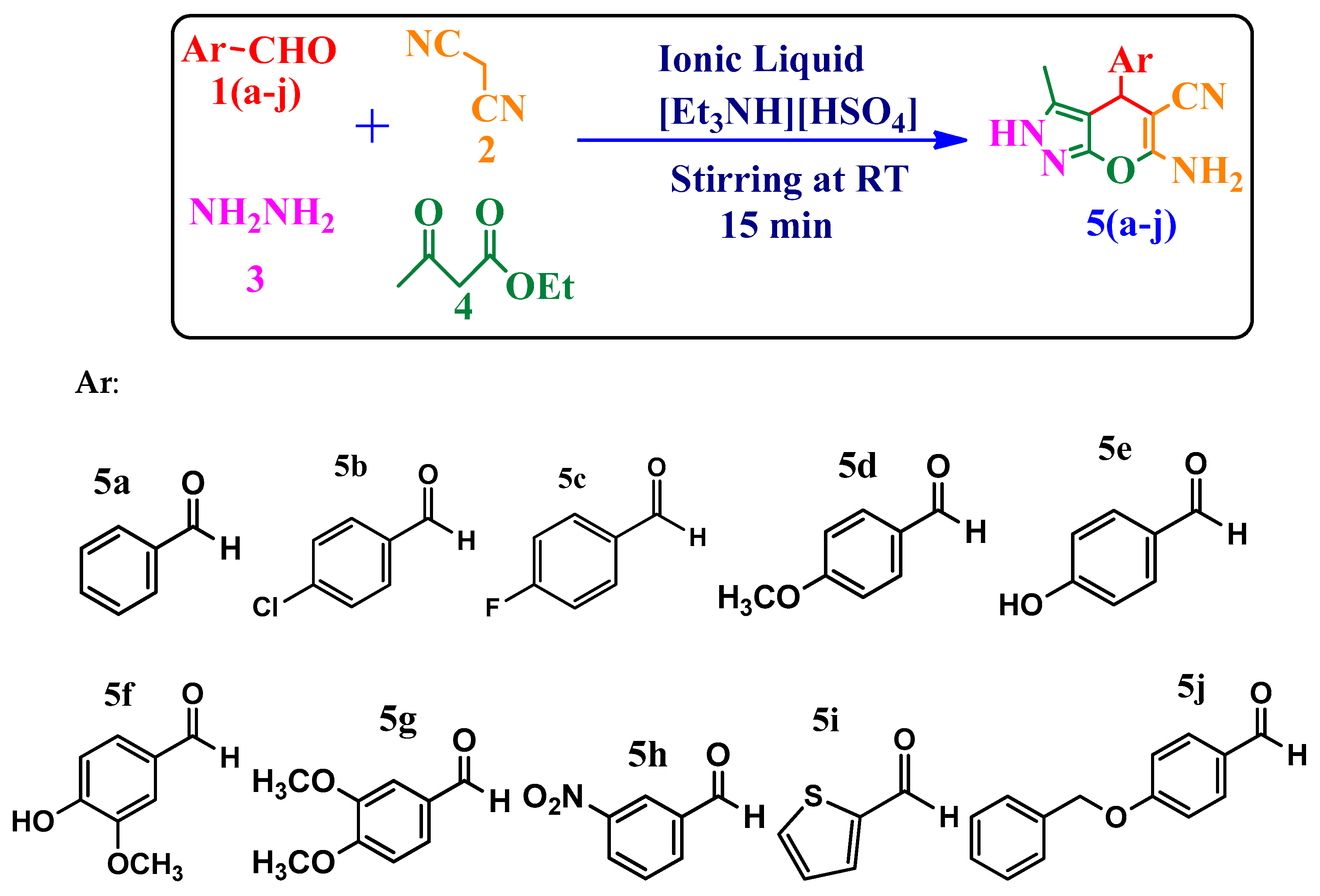
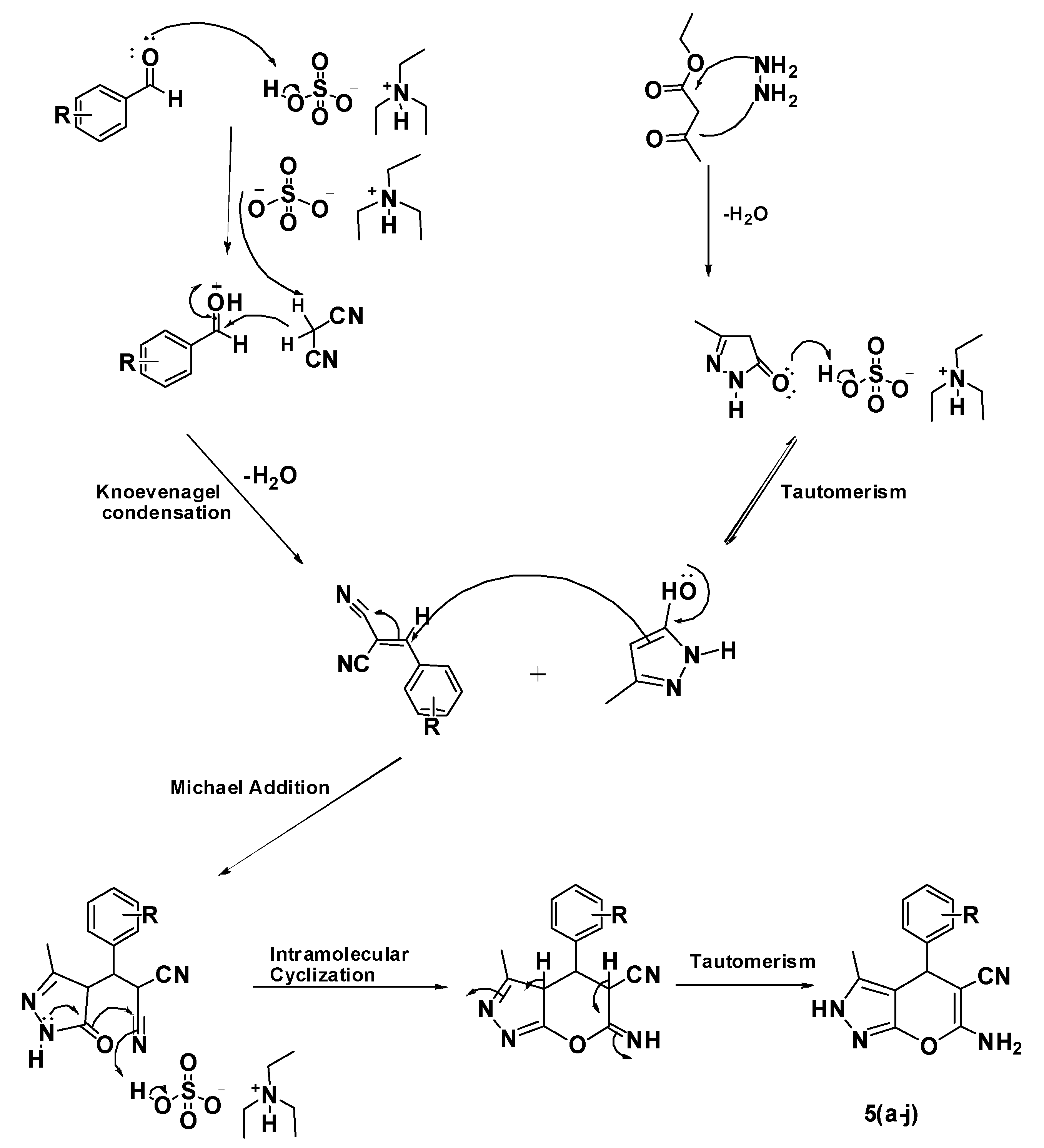
2.1. Chemistry
2.2. In Vitro Anticancer Activity
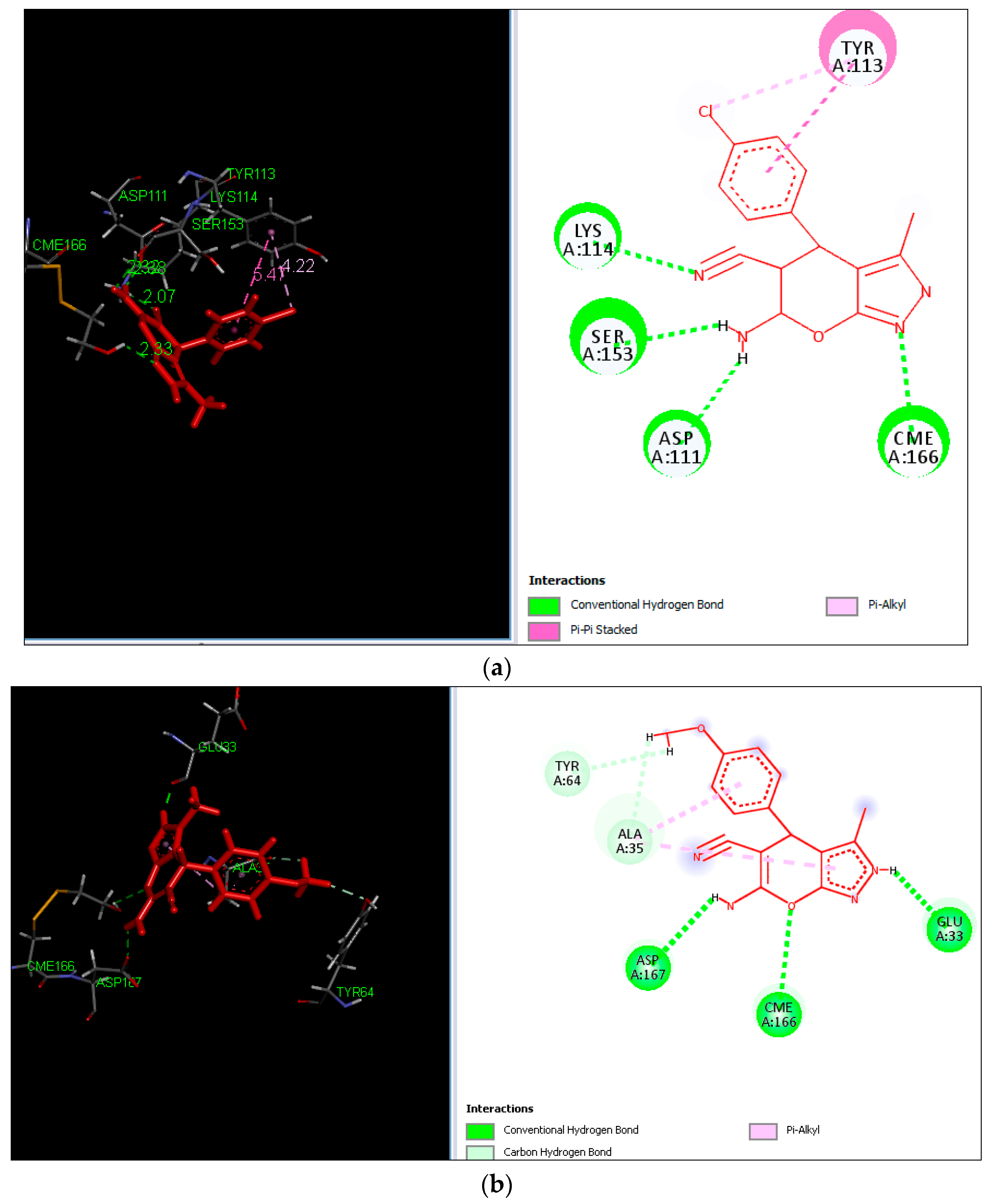
2.3. Molecular Docking
2.4. Prediction of ADMET Properties
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Synthesis of [Et3NH][HSO4]
4.3. Synthesis of 6-Amino-4-(substituted phenyl)-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles
4.4. Evaluation of In Vitro Anticancer Activity
4.5. Molecular Docking Study
4.6. In Silico ADMET Prediction
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Bertram, G.; Katzung, L. Basic and Clinical Pharmacology; McGraw-Hill Medical Publication Division: New York, NY, USA, 2010; Volume 11, pp. 1091–1116. [Google Scholar]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, A.B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Karim, S.S.; Anwar, M.M.; Mohamed, N.A.; Nasr, T.; Elseginy, S.A. Design, synthesis, biological evaluation and molecular docking studies of novel benzofuran-pyrazole derivatives as anticancer agents. Bioorg. Chem. 2015, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.H.; Srour, A.M.; Ismail, M.A.; Khater, M.A.; Serrya, R.A.; El-Manawaty, M.A. Design and synthesis of some new tri-substituted pyrazole derivatives as anticancer agents. Res. Chem. Intermed. 2016, 42, 6881–6892. [Google Scholar] [CrossRef]
- Fahmy, H.H.; Khalifa, N.M.; Ismail, M.M.; El-Sahrawy, H.M.; Nossier, E.S. Biological validation of novel polysubstituted pyrazole candidates with in vitro anticancer activities. Molecules 2016, 21, 271. [Google Scholar] [CrossRef] [PubMed]
- Rahmi, K.U. Design, synthesis, characterization, and anti-proliferative activity of novel pyrazole-3-carboxylic acid derivatives. Monatsh. Chem. 2015, 146, 1743–1749. [Google Scholar]
- Anam, A.; Abad, A.; Mohd, A.; Shamsuzzaman. Review: Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar]
- Lehmann, T.P. Cell-specific cytotoxic effect of pyrazole derivatives on breast cancer cell lines MCF7 and MDA-MB-231. J. Physiol. Pharmacol. 2017, 68, 201–207. [Google Scholar] [PubMed]
- El-Gamal, M.I.; Abdel-Maksoud, M.S.; Gamal El-Din, M.M.; Shin, J.S.; Lee, K.T.; Ho Yoo, K.; Oh, C.H. Synthesis, in vitro Anti-proliferative and Anti-inflammatory Activities, and Kinase Inhibitory effects of New 1,3,4-triarylpyrazole Derivatives. Anti-Cancer Agents Med. Chem. 2017, 17, 75–84. [Google Scholar]
- Elguero, J.; Goya, P.; Jagerovic, N.; Silva, A.M.S. Pyrazoles as drugs: Facts and fantasies. In Targets in Heterocyclic Systems; Attanasi, O.A., Spinelli, D., Eds.; Royal Society of Chemistry: Cambridge, UK, 2002; Volume 6, pp. 52–98. [Google Scholar]
- Singh, S.K.; Reddy, P.G.; Rao, K.S.; Lohray, B.B.; Misra, P.; Rajjak, S.A.; Rao, Y.K. Venkatewarlu, A. Polar substitutions in the benzenesulfonamide ring of celecoxib afford a potent 1,5-diarylpyrazole class of COX-2 inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Qvortrup, K.; Jensen, J.F.; Sørensen, M.S.; Kouskoumvekaki, I.; Petersen, R.K.; Taboureau, O.; Kristiansen, K.; Nielsen, T.E. Synthesis and biological evaluation of dihydropyrano[2,3-c]pyrazoles as a new class of PPARγ partial agonists. PLoS ONE 2017, 12, e0162642. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kaur, A.; Mankotia, S.; Singh, H.; Singh, A.; Singh, J.; Gupta, M.; Sharma, S.; Nepali, K.; Singh Bedi, P.M. Synthesis, screening and docking of fused pyrano[3,2-d]pyrimidine derivatives as xanthine oxidase inhibitor. Eur. J. Med. Chem. 2017, 131, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Liu, D.; Zhang, Z.J.; Shan, S.; Han, X.; Srinivasula, S.M.; Croce, C.M.; Alnemri, E.S.; Huang, Z. Structure-based discovery of an organic compound that bind Bc1-2 protein and induces apoptosis of tumor cells. Proc. Natl. Acad. Sci. USA 2000, 97, 7124–7129. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.R.; Khaireldin, N.Y.; Fahmyb, A.F.; El-Sayeda, A.A.F. Facile synthesis of fused nitrogen containing heterocycles as anticancer agents. Der. Pharm. Chem. 2010, 2, 400–417. [Google Scholar]
- Erugu, Y.; Sangepu, B.; Varre, K.; Pamanji, R.; Bomma, Y.; Janapala, V.R.; Srinivasarao, V.; Tigulla, P.; Jetti, V.R. Design, an efficient ecofriendly synthesis of spirooxindole derivatives and their anticancer activity supported by molecular docking studies. World J. Pharm. Pharm. Sci. 2014, 3, 1895–1914. [Google Scholar]
- Soad, K.S.; Magda, F.M.; Ahmed, F.D.; Ahmed, H.M.E.; Ismail, A.A. Molecular docking simulation and anticancer assessment on human breast carcinoma cell line using novel bis(1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile)andis(1,4-dihydropyrazolo[4′,3′:5,6]pyrano[2,3-b]pyridine-6-carbonitrile) derivatives. Bioorg. Chem. 2017. [Google Scholar] [CrossRef]
- Pantsar, T.; Singha, P.; Tapio, J.N.; Koshevoy, I.; Leppänen, J.; Poso, A.; Juha, M.A.N.; Pasonen-Seppänen, S.; Savinainen, J.R.; Laitinen, T.; et al. Design, synthesis, and biological evaluation of 2,4-dihydropyrano[2,3-c]pyrazole derivatives as autotaxin inhibitors. Eur. J. Pharm. Sci. 2017. [Google Scholar] [CrossRef]
- Adibi, H.; Hosseinzadeh, L.; Farhadi, S.; Ahmadi, F. Synthesis and cytotoxic evaluation of6-amino-4-aryl-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-carbonitrile derivatives using borax with potential anticancer effects. J. Rep. Pharm. Sci. 2013, 2, 116–124. [Google Scholar]
- Kamel, M.M. Convenient synthesis, characterization, cytotoxicity and toxicity of pyrazole derivatives. Acta Chim. Slov. 2015, 62, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.V.; Seijas, J.A.; Vazquez-Tato, M.P.; Sarkate, A.P.; Lokwani, D.K.; Nikalje, A.G. Ultrasound mediated one-pot, three component synthesis, docking and ADME prediction of novel 5-amino-2-(4-chlorophenyl)-7-substitutedphenyl-8,8a-dihydro-7H-(1,3,4)thiadiazolo(3,2)pyrimidine-6-carbonitrile derivatives as anticancer agents. Molecules 2016, 21, 894. [Google Scholar] [CrossRef] [PubMed]
- Nikalje, A.G.; Tiwari, S.V.; Tupe, J.G.; Vyas, V.K.; Qureshi, G. Ultrasound assisted-synthesis and biological evaluation of piperazinylprop-1-en-2-yloxy-2h-chromen-2-ones as cytotoxic agents. Lett. Drug Des. Discov. 2017, 14. [Google Scholar] [CrossRef]
- Tiwari, S.V.; Siddiqui, S.; Seijas, J.A.; Vazquez-Tato, M.P.; Sarkate, A.P.; Lokwani, D.K.; Nikalje, A.G. Microwave-assisted facile synthesis, anticancer evaluation and docking study of N-((5-(substituted methylene amino)-1,3,4-thiadiazol-2-yl)methyl)benzamide derivatives. Molecules 2017, 22, 995. [Google Scholar] [CrossRef] [PubMed]
- Junek, H.; Aigner, H. Synthesenmit Nitrilen, XXXV. Reaktionen von Tetracyanathylen mit Heterocyclen. Eur. J. Inorg. Chem. 1973, 106, 914–921. [Google Scholar]
- Sharanin, Y.A.; Sharanina, L.G.; Puzanova, V.V. Nitrile cyclization reactions. VII. Synthesis of 6-amino-4-aryl-3-methyl-5-cyano-1H,4H-pyrazolo[3,4-B]pyrans. Z. Chem. Inform. 1983, 19, 2609–2615. [Google Scholar] [CrossRef]
- Sharanin, Y.A.; Sharanina, L.G.; Puzanova, V.V. Nitrile cyclization reactions. VII. synthesis of 6-amino-4-aryl-3-methyl-5-cyano-1H,4H-pyrazolo(3,4-B) pyrans. J. Org. Chem. USSR (Engl. Transl.) 1983, 19, 221. [Google Scholar] [CrossRef]
- Peng, Y.; Song, G.; Dou, R. Surface cleaning under combined microwave and ultrasound irradiation: Flash synthesis of 4H-pyrano[2,3-c]pyrazoles in aqueous media. Green Chem. 2006, 8, 573–575. [Google Scholar] [CrossRef]
- Vasuki, G.; Kumaravel, K. Rapid four component reaction in water: Synthesis of pyranopyrazoles. Tetrahedron Lett. 2008, 49, 5636–5638. [Google Scholar] [CrossRef]
- Lehmann, F.; Holm, S.L.; Laufer, M.S. Three-component combinatorial synthesis of novel dihydropyrano[2,3-c]pyrazoles. J. Comb. Chem. 2008, 10, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Ghods, A.; Derikvand, F.; Bakhtiari, K.; Bammoharram, F.F. H14 [NaP 5W 30 O 110] catalyzed one-pot three-component synthesis of dihydropyrano[2,3-c]pyrazole and pyrano[2,3-d]pyrimidine derivatives. J. Iran. Chem. Soc. 2010, 7, 615–620. [Google Scholar] [CrossRef]
- Reddy, M.B.M.; Jayashankara, V.P.; Pasha, M.A. Glycine-catalyzed efficient synthesis of pyranopyrazoles via one-pot multicomponent reaction. Synth. Commun. 2010, 40, 2930–2934. [Google Scholar] [CrossRef]
- Kanagaraj, K.; Pitchumani, K. Solvent-free multicomponent synthesis of pyrano-pyrazoles: Per-6-amino-β-cyclodextrin as a remarkable catalyst and host. Tetrahedron Lett. 2010, 51, 3312–3316. [Google Scholar] [CrossRef]
- Samant, S.D.; Patil, N.R.; Kshirsagar, S.W. Mg-Al Hydrotalcite as a first heterogeneous basic catalyst for the synthesis of 4H-pyrano[2,3-c]pyrazoles through a four-component reaction. Synth. Commun. 2011, 41, 1320–1325. [Google Scholar]
- Babaie, M.; Sheibani, H. Nanosized magnesium oxide as a highly effective heterogeneous base catalyst for the rapid synthesis of pyranopyrazoles via a tandem four-component reaction. Arab. J. Chem. 2011, 4, 159–162. [Google Scholar] [CrossRef]
- Mecadon, H.; Rohman, M.R.; Kharbangar, I.; Laloo, B.M.; Kharkongor, I.; Rajbangshi, M.; Myrboh, B. L-Proline as an efficient catalyst for the multi-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano[2,3-F]pyrazole-5-carbonitriles in water. Tetrahedron Lett. 2011, 52, 3228–3231. [Google Scholar] [CrossRef]
- Mecadon, H.; Rohman, M.R.; Rajbangshi, M.; Myrboh, B. γ-Alumina as a recyclable catalyst for the four-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles in aqueous medium. Tetrahedron Lett. 2011, 52, 2523–2525. [Google Scholar] [CrossRef]
- Kiyani, H.; Samimi, H.A.; Ghorbani, F.; Esmaieli, S. One-pot, four-component synthesis of pyrano[2,3-c]pyrazoles catalyzed by sodium benzoate in aqueous medium. Curr. Chem. Lett. 2013, 2, 197–206. [Google Scholar] [CrossRef]
- Bihani, M.; Bora, P.P.; Bez, G.; Askari, H. Amberlyst A21 catalyzed chromatography free method for multicomponent synthesis of dihydropyrano[2,3-F]pyrazoles in ethanol. ACS Sustain. Chem. Eng. 2013, 1, 440–447. [Google Scholar] [CrossRef]
- Wu, M.; Feng, Q.; Wan, D.; Ma, J. CTACl as catalyst for four-component, one-pot synthesis of pyranopyrazole derivatives in aqueous medium. Synth. Commun. 2013, 43, 1721–1726. [Google Scholar] [CrossRef]
- Yakaiah, S.; Buchappa, G.; Durgaprasad, K.; Ravibabu, K.; Aparna, P. Ionic liquid catalyzed one-pot three component synthesis of dihydropyrano[2,3-c]pyrazole under green condtion. Asian J. Chem. 2016, 28, 2441–2445. [Google Scholar] [CrossRef]
- Chaudhari, M.A.; Gujar, J.B.; Kawade, D.S.; Jogdand, N.R.; Shingare, M.S. A highly efficient and sustainable synthesis of dihydropyrano[2,3-c]pyrazoles using polystyrene-supported p-toluenesulfonic acid as reusable catalyst. Cogent Chem. 2015, 1, 1063830. [Google Scholar] [CrossRef]
- Brahmachari, G.; Banerjee, B. Facile and one-pot access to diverse and densely functionalized2-amino-3-cyano-4h-pyrans and pyran-annulated heterocyclic scaffolds via an eco-friendly multicomponent reaction at room temperature using urea as a novel organo-catalyst. ACS Sustain. Chem. Eng. 2014, 2, 411–422. [Google Scholar] [CrossRef]
- Mandha, S.R.; Siliverib, S.; Allaa, M.; Bommenaa, V.R.; Bomminenib, M.R.; Balasubramanian, S. Eco-friendly synthesis and biological evaluation of substituted pyrano[2,3-c]pyrazoles. Bioorg. Med. Chem. Lett. 2012, 22, 5272–5278. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.N.; Khan, K. [Et3NH][HSO4]-catalyzed efficient, eco-friendly, and sustainable synthesis of quinoline derivatives via knoevenagel condensation. ACS Sustain. Chem. Eng. 2014, 2, 1187–1194. [Google Scholar] [CrossRef]
- Hailes, H.C. Reaction solvent selection: The potential of water as a solvent for organic transformations. Org. Process Res. Dev. 2007, 11, 114–120. [Google Scholar] [CrossRef]
- El-Tamany, E.S.; El-Shahed, F.A.; Mohamed, B.H. Synthesis and biological activity of some pyrazole derivatives. J. Serb. Chem. Soc. 1999, 64, 9–18. [Google Scholar]
- Lei, Z.; Dai, C.; Chen, B. Gas solubility in ionic liquids. Chem. Rev. 2014, 114, 1289–1326. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, M.V.; Kornyshev, A.A. Ionic liquids at electrified interfaces. Chem. Rev. 2014, 114, 2978–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Wang, C.; Li, H.; Wang, Y. Preparation of dialkoxypropanes in simple ammonium ionic liquids. Green Chem. 2006, 8, 1076–1079. [Google Scholar] [CrossRef]
- Wang, C.; Guo, L.; Li, H.; Wang, Y.; Weng, J.; Wu, L. Preparation of simple ammonium ionic liquids and their application in the cracking of dialkoxypropanes. Green Chem. 2006, 8, 603–607. [Google Scholar] [CrossRef]
- Weng, J.; Wang, C.; Li, H.; Wang, Y. Novel quaternary ammonium ionic liquids and their use as dual solvent-catalysts in the hydrolytic reaction. Green Chem. 2006, 8, 96–99. [Google Scholar] [CrossRef]
- Ganeshpure, P.A.; George, G.; Das, J. Brønsted acidic ionic liquids derived from alkylamines as catalysts andmediums for Fischer esterification: Study of structure-activity relationship. J. Mol. Catal. A Chem. 2008, 279, 182–186. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, W.; Li, H.; Guo, L. Solvent-free synthesis of unsaturated ketones by Saucy-Marbet reaction with simple ammonium ionic liquid as the catalyst. Green Chem. 2009, 11, 843–847. [Google Scholar] [CrossRef]
- Rajendran, A.; Raghupathy, D.; Priyadarshini, M.A. Domino Green Synthesis of Bis (indolyl) methane catalyzed by ionic liquid [Et3NH][HSO4]. Int. J. ChemTech. Res. 2011, 3, 298–302. [Google Scholar]
- Kermani, E.T.; Khabazzadeh, H.; Jazinizadeh, T. Friedländer synthesis of poly-substituted quinolines in the presence of triethylammonium hydrogen sulfate [Et3NH][HSO4] as a highly efficient, and cost effective acidic ionic liquid catalyst. J. Heterocycl. Chem. 2011, 48, 1192–1196. [Google Scholar] [CrossRef]
- Suryawanshi, N.S.; Jain, P.; Singhal, M.; Khan, I. Mannich synthesis under ionic liquid [Et3NH][HSO4]. Catal. J. Appl. Chem. 2012, 1, 18–23. [Google Scholar] [CrossRef]
- Khabazzadeh, H.; Kermani, E.T.; Jazinizadeh, T. An efficient synthesis of 3,4-dihydropyrimidin-2(1H)-ones catalyzed by molten [Et3NH][HSO4]. Arab. J. Chem. 2012, 5, 485–488. [Google Scholar] [CrossRef]
- Zhou, Z.; Deng, X. [Et3NH][HSO4] Catalyzed efficient and green synthesis of 1,8-dioxo-octahydroxanthenes. J. Mol. Catal. A Chem. 2013, 367, 99–102. [Google Scholar] [CrossRef]
- Malla, A.M.; Parveen, M.; Ahmad, F.; Azaz, S.; Alam, M. [Et3NH][HSO4]-catalyzed eco-friendly and expeditious synthesis of thiazolidine and oxazolidine derivatives. RSC Adv. 2015, 5, 19552–19569. [Google Scholar] [CrossRef]
- Han, X.X.; Du, H.; Hung, C.T.; Liu, L.L.; Wu, P.H.; Ren, D.H.; Huang, S.J.; Liu, S.B. Syntheses of novel halogen-free Brønsted—Lewis acidic ionic liquid catalysts and their applications for synthesis of methyl caprylate. Green Chem. 2015, 17, 499–508. [Google Scholar] [CrossRef]
- Subhedar, D.D.; Shaikh, M.H.; Arkile, M.A.; Yeware, A.; Sarkar, D.; Shingate, B.B. Facile synthesis of 1,3-thiazolidin-4-ones as antitubercular agents. Bioorg. Med. Chem. Lett. 2016, 26, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y. Multicomponent reactions in unconventional solvents: State of the art. Green Chem. 2012, 14, 2091–2128. [Google Scholar] [CrossRef]
- Prasanna, P.; Perumal, S.; Menéndez, J.C. Chemodivergent, multicomponent domino reactions in aqueous media: L-proline catalyzed assembly of densely functionalized 4H-pyrano[2,3-c]pyrazoles and bispyrazolylpropanoates from simple, acyclic starting materials. Green Chem. 2013, 15, 1292–1299. [Google Scholar] [CrossRef]
- Tiwari, S.V.; Nikalje, A.G.; Lokwani, D.K.; Sarkate, A.P.; Jamir, K. Synthesis, biological evaluation, molecular docking study and acute oral toxicity study of coupled Imidazolyl-Pyrimidine derivatives. Lett. Drug Des. Discov. 2017, 14. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, U.D.; Nikalje, A.G.; Netankar, P.D.; Lingampalle, D.L. Ionic liquid mediated synthesis of 5-arylidine-2,4-thiazolidinedionesand antibacterial evaluation. J. Med. Chem. Drug Discov. 2015, 1, 331–342. [Google Scholar]
- Shaikh, M.H.; Subhedar, D.D.; Khan, F.A.K.; Sangshetti, J.N.; Shingate, B.B. [Et3NH][HSO4]-catalyzed one-pot, solvent-free synthesis and biological evaluation of a-amino phosphonates. Res. Chem. Intermed. 2016, 2, 5115–5131. [Google Scholar] [CrossRef]
- Lin, C.M.; Ho, H.H.; Pettit, G.R.; Hamel, E. Antimitotic natural products combretastatin A-4 and combretastatin A-2: Studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry 1989, 28, 6984–6991. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.A.; Gilmartin, M.E.; Hall, J.L.; Cowan, N.J. Three expressed sequences within the human betatubulin multigene family each define a distinct isotype. J. Mol. Biol. 1985, 182, 11–20. [Google Scholar] [CrossRef]
- Nicoletti, M.I.; Valoti, G.; Giannakakou, P.; Zhan, Z.; Kim, J.H.; Lucchini, V.; Landoni, F.; Mayo, J.G.; Giavazzi, R.; Fojo, T. Expression of beta-tubulin isotypes in human ovarian carcinoma xenografts and in a sub-panel of human cancer cell lines from the NCI-Anticancer Drug Screen: Correlation with sensitivity to microtubule active agents. Clin. Cancer Res. 2001, 7, 2912–2922. [Google Scholar] [PubMed]
- McKean, P.G.; Vaughan, S.; Gull, K. The extended tubulin superfamily. J. Cell Sci. 2001, 114, 2723–2733. [Google Scholar] [PubMed]
- Perez, E.A. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol. Cancer Ther. 2009, 8, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Kavallaris, M. Cancer. Nat. Rev. 2010, 10, 1–12. [Google Scholar]
- Chaudhary, A.; Sharma, P.P.; Bhardwaj, G.; Jain, V.; Bharatam, P.V.; Shrivastav, B.; Roy, R.K. Synthesis, biological evaluation, and molecular modeling studies of novel heterocyclic compounds as anti-proliferative agents. Med. Chem. Res. 2013, 12, 5654–5669. [Google Scholar] [CrossRef]
- Huang, Y.; Hickey, R.P.; Yeh, J.L.; Liu, D.; Dadak, A.; Young, L.H.; Johnson, R.S.; Giordano, F.J. Cardiacmyocyte-specific HIF-1alpha deletion alters vascularization, energy availability, calcium flux, and contractility in the normoxic heart. FASEB J. 2004, 18, 1138–1140. [Google Scholar] [PubMed]
- Lagorce, D.; Sperandio, H.; Miteva, M.; Villoutreix, B.O. FAF-Drugs2: Free ADME/tox filtering tool to assist drug discovery and chemical biology project. BMC Biol. Inform. 2008, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Sangshetti, J.; Khan, F.; Chouthe, R.; Damale, M.; Shinde, D. Synthesis, docking and ADMET prediction of novel 5-((5-substituted-1-H-1,2,4-triazol-3-yl)methyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine as antifungal agents. Chin. Chem. Lett. 2014, 25, 1033–1038. [Google Scholar] [CrossRef]
- Lipinski, C.; Lombardo, F.; Dominy, B. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Abraham, M.H.; Lee, J.; Hersey, A.; Luscombe, C.N.; Beek, G.; Sherborne, B.; Cooper, I. Rate-limited steps of human oral absorption and QSAR studies. Pharm. Res. 2002, 19, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3747. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
| Solvent | Temperature (°C) | Time (min) | Yield (%) |
|---|---|---|---|
| Polyethylene glycol (PEG) | 80 | 60 | 72 |
| Deep eutectic solvent of cholinechloride:urea | 80 | 20 | 92 |
| Ionic liquid (N-methylpyridiniumtosylate) | 120 | 75 | 62 |
| Ionic liquid (triethylammonium hydrogen sulphate[Et3NH][HSO4]) | R.T. | 15 | 94 |
| [Et3NH][HSO4] mol % | Time (min) | Yield (%) |
|---|---|---|
| - | 60 | 00 |
| 5 | 50 | 50 |
| 10 | 45 | 65 |
| 15 | 15 | 70 |
| 20 | 15 | 94 |
| 25 | 15 | 85 |
| Run | Time (min) | Yield (%) |
|---|---|---|
| 1 | 15 | 94 |
| 2 | 15 | 82 |
| 3 | 15 | 78 |
| 4 | 15 | 75 |
| Compounds | GI50 Against Cancer Cell Line in µM | Total Score -Logki | Crash Score | Polar Score | |||
|---|---|---|---|---|---|---|---|
| MAD-MB-231 | HeLa | SK-MEL-2 | K-562 | ||||
| 5b | 0.74 | >100 | <0.1 | 11.2 | 6.4039 | −0.9801 | 2.0598 |
| 5d | 25.76 | >100 | <0.1 | 6.41 | 5.4734 | −1.3871 | 4.449 |
| 5g | 2.02 | >100 | 2.92 | 40.73 | 4.6919 | −0.4209 | 5.4948 |
| 5h | 49.84 | >100 | 0.12 | 4.57 | 4.5247 | −0.184 | 3.7291 |
| 5j | 66.65 | >100 | <0.1 | 9.47 | 4.6555 | −0.7369 | 3.5549 |
| ADR | <0.1 | 0.03 | <0.1 | <0.1 | NA | NA | NA |
| CL2 | NA | NA | NA | NA | 5.1969 | −0.8748 | 3.5552 |
| Lig_ID | %ABS (<100%) | MW (<500) | LogP (<5) | PSA (<150) | n-RotB (<10) | n-RigB (<25) | HBD (<5) | HBA (<10) | RatioH/C (<1) | Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 78.64 | 252.3 | 2.64 | 87.72 | 1 | 17 | 2 | 2 | 0.357 | Non Toxic |
| 5b | 78.64 | 286.7 | 3.29 | 87.72 | 1 | 17 | 2 | 2 | 0.428 | Non Toxic |
| 5c | 78.64 | 270.3 | 2.78 | 87.72 | 1 | 17 | 2 | 2 | 0.428 | Non Toxic |
| 5d | 75.55 | 282.3 | 2.65 | 96.95 | 2 | 17 | 2 | 3 | 0.4 | Non Toxic |
| 5e | 71.76 | 268.3 | 2.34 | 108 | 1 | 17 | 3 | 3 | 0.428 | Non Toxic |
| 5f | 68.57 | 298.3 | 2.35 | 117.2 | 2 | 17 | 3 | 4 | 0.466 | Non Toxic |
| 5g | 72.37 | 312.3 | 2.65 | 106.2 | 3 | 17 | 2 | 4 | 0.437 | Non Toxic |
| 5h | 62.93 | 297.3 | 3.07 | 127.7 | 2 | 18 | 2 | 4 | 0.571 | Non Toxic |
| 5i | 78.64 | 258.3 | 2.7 | 116 | 1 | 16 | 2 | 3 | 0.5 | Non Toxic |
| 5j | 75.55 | 358.4 | 4.22 | 96.95 | 4 | 23 | 2 | 3 | 0.5 | Non Toxic |
| CL2 | 75 | 252.3 | 2.64 | 87.72 | 1 | 17 | 2 | 2 | 0.357 | Non Toxic |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nimbalkar, U.D.; Seijas, J.A.; Vazquez-Tato, M.P.; Damale, M.G.; Sangshetti, J.N.; Nikalje, A.P.G. Ionic Liquid-Catalyzed Green Protocol for Multi-Component Synthesis of Dihydropyrano[2,3-c]pyrazoles as Potential Anticancer Scaffolds. Molecules 2017, 22, 1628. https://doi.org/10.3390/molecules22101628
Nimbalkar UD, Seijas JA, Vazquez-Tato MP, Damale MG, Sangshetti JN, Nikalje APG. Ionic Liquid-Catalyzed Green Protocol for Multi-Component Synthesis of Dihydropyrano[2,3-c]pyrazoles as Potential Anticancer Scaffolds. Molecules. 2017; 22(10):1628. https://doi.org/10.3390/molecules22101628
Chicago/Turabian StyleNimbalkar, Urja D., Julio A. Seijas, Maria Pilar Vazquez-Tato, Manoj G. Damale, Jaiprakash N. Sangshetti, and Anna Pratima G. Nikalje. 2017. "Ionic Liquid-Catalyzed Green Protocol for Multi-Component Synthesis of Dihydropyrano[2,3-c]pyrazoles as Potential Anticancer Scaffolds" Molecules 22, no. 10: 1628. https://doi.org/10.3390/molecules22101628