Synthesis and Preliminary Biological Evaluation of Indol-3-yl-oxoacetamides as Potent Cannabinoid Receptor Type 2 Ligands
Abstract
:1. Introduction
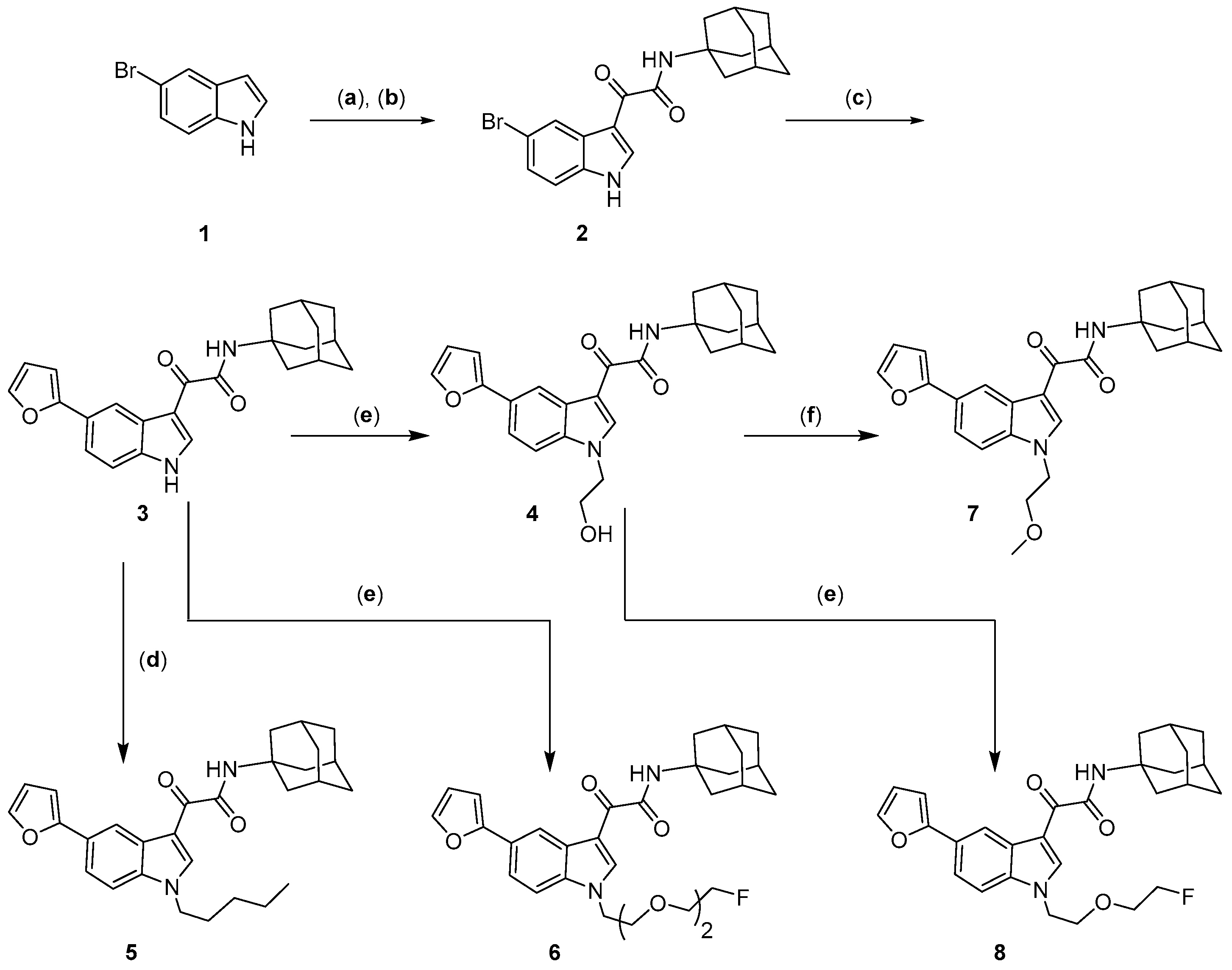
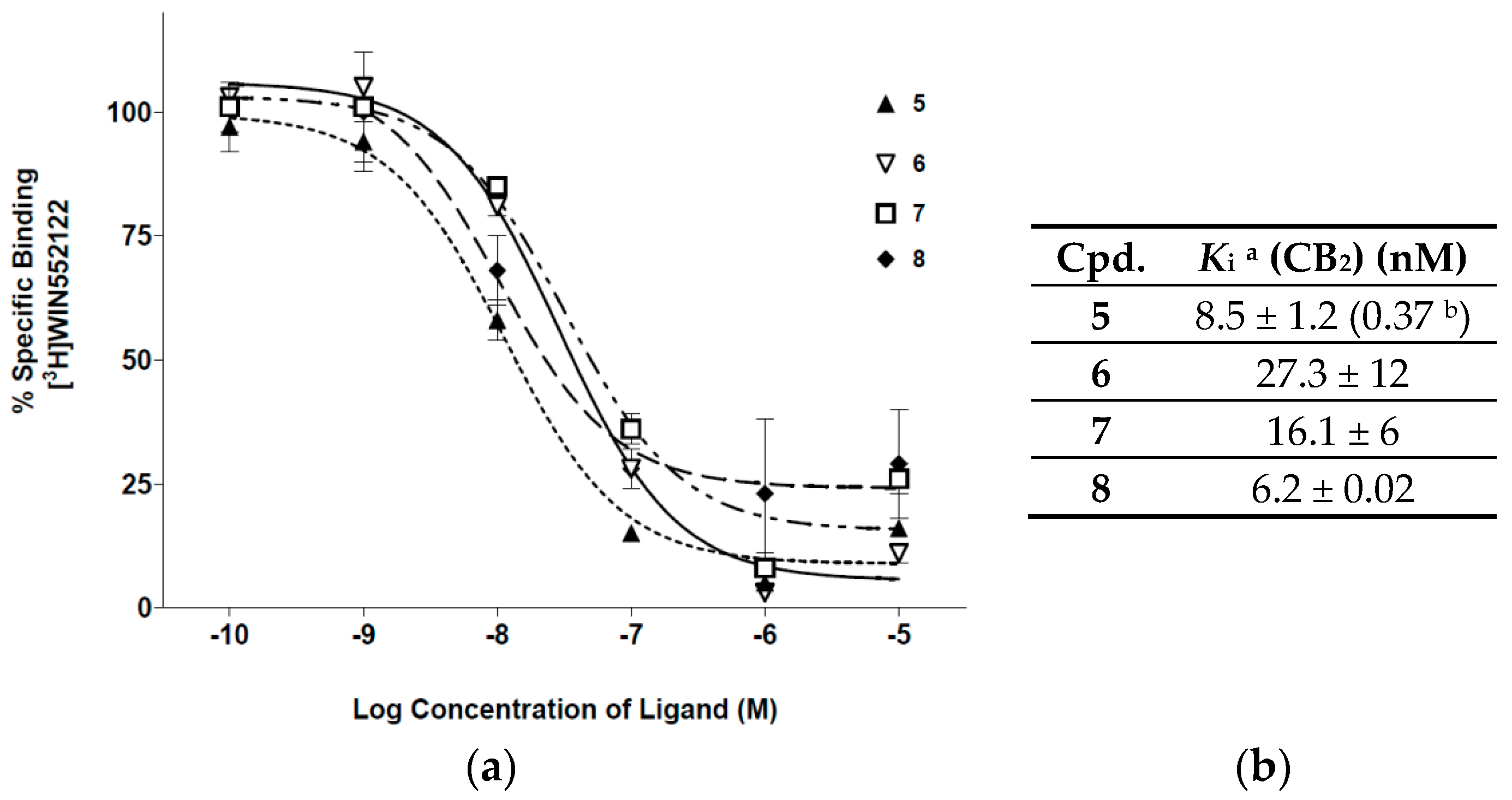
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Procedures and Compound Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CB2 | cannabinoid receptors type 2 |
| CB1 | cannabinoid receptors type 1 |
| PET | positron emission tomography |
| DMF | N,N-dimethylformamide |
| TBAB | tetrabutylammonium bromide |
| Et3N | triethylamine |
| CHO | Chinese Hamster Ovary |
| EA | ethyl acetate |
| Et2O | diethyl ether |
| DCM | dichloromethane |
| Pd(PPh3)4 | tetrakis(triphenylphosphine)palladium(0) |
| n-BuBr | 1-bromobutane |
| MeI | methyl iodide |
| Hex | hexane |
References and Notes
- Gaoni, Y.; Mechoulam, R. Isolation structure and partial synthesis of active constituent of hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, E.; Kathmann, M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 2001, 22, 565–572. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. The endocannabinoid signaling system in the CNS: A primer. Int. Rev. Neurobiol. 2015, 125, 1–47. [Google Scholar] [PubMed]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal structure of the human cannabinoid receptor CB1. Cell 2016, 167, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Yin, J.; Chapman, K.; Grzemska, M.; Clark, L.; Wang, J.; Rosenbaum, D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 2016. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Ferreira, G.A.; Jamerson, M.J. Endocannabinoids and the immune system in health and disease. Handb. Exp. Pharmacol. 2015, 231, 185–211. [Google Scholar] [PubMed]
- Stella, N. Cannabinoid signaling in glial cells. Glia 2004, 48, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Palazuelos, J.; Aguado, T.; Egia, A.; Mechoulam, R.; Guzman, M.; Galve-Roperh, I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006, 20, 2405–2407. [Google Scholar] [CrossRef] [PubMed]
- Molina-Holgado, E.; Vela, J.M.; Arevalo-Martin, A.; Almazan, G.; Molina-Holgado, F.; Borrell, J.; Guaza, C. Cannabinoids promote oligodendrocyte progenitor survival: Involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J. Neurosci. 2002, 22, 9742–9753. [Google Scholar] [PubMed]
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 2005, 95, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kim, J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience 2015, 311, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, M.L.; Muzzio, D.O.; Ehrhardt, J.; Franchi, A.M.; Zygmunt, M.; Jensen, F. Expression analysis of cannabinoid receptors 1 and 2 in B cells during pregnancy and their role on cytokine production. J. Reprod. Immunol. 2016, 116, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, T.K.; Meissler, J.J. Effects of cannabinoids on T-cell function and resistance to infection. J. Neuroimmune Pharmacol. 2015, 10, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Oddi, S.; Piccoli, A.; Fazio, D.; Maccarrone, M. Type-2 cannabinoid receptors in neurodegeneration. Pharmacol. Res. 2016, 111, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.C.; Glass, M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr. Neuropharmacol. 2007, 5, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; Romero, J.P.; Tolon, R.M.; Clemente, D.; Docagne, F.; Hillard, C.J.; Guaza, C.; Romero, J. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J. Neurosci. 2007, 27, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; de Ceballos, M.L.; Gomez del Pulgar, T.; Rueda, D.; Corbacho, C.; Velasco, G.; Galve-Roperh, I.; Huffman, J.W.; Ramon y Cajal, S.; Guzman, M. Inhibition of glioma growth in vivo by selective activation of the CB2 cannabinoid receptor. Cancer Res. 2001, 61, 5784–5789. [Google Scholar] [PubMed]
- Fernandez-Ruiz, J.; Romero, J.; Ramos, J.A. Endocannabinoids and neurodegenerative disorders: Parkinson’s Disease, Huntington’s Chorea, Alzheimer’s Disease, and others. Handb. Exp. Pharmacol. 2015, 231, 233–259. [Google Scholar] [PubMed]
- Aizpurua-Olaizola, O.; Elezgarai, I.; Rico-Barrio, I.; Zarandona, I.; Etxebarria, N.; Usobiaga, A. Targeting the endocannabinoid system: Future therapeutic strategies. Drug. Discov. Today 2016. [Google Scholar] [CrossRef] [PubMed]
- Brust, P.; van den Hoff, J.; Steinbach, J. Development of [18F]-labeled radiotracers for neuroreceptor imaging with positron emission tomography. Neurosci. Bull. 2014, 30, 777–811. [Google Scholar] [CrossRef]
- Horti, A.G.; Raymont, V.; Terry, G.E. PET imaging of endocannabinoid system. In PET and SPECT of Neurobiological Systems; Dierckx, R.A.J.O., Otte, A., de Vries, E.F.J., van Waarde, A., Luiten, P.G.M., Eds.; Springer: Berlin. Germany, 2014; pp. 249–319. [Google Scholar]
- Evens, N.; Bormans, G.M. Non-invasive imaging of the type 2 cannabinoid receptor, focus on positron emission tomography. Curr. Top. Med. Chem. 2010, 10, 1527–1543. [Google Scholar] [CrossRef]
- Ory, D.; Celen, S.; Verbruggen, A.; Bormans, G. PET radioligands for in vivo visualization of neuroinflammation. Curr. Pharm. Des. 2014, 20, 5897–5913. [Google Scholar] [CrossRef]
- (a) Ahmad, R.; Postnov, A.; Bormans, G.; Versijpt, J.; Vandenbulcke, M.; van Laere, K. Decreased in vivo availability of the cannabinoid type 2 receptor in Alzheimer’s disease. E. J. Nucl. Med. Mol. Imaging 2016, 1–9; (b) Slavik, R.; Muller Herde, A.; Haider, A.; Kramer, S. D.; Weber, M.; Schibli, R.; Ametamey, S.M.; Mu, L. Discovery of a fluorinated 4-oxo-quinoline derivative as a potential positron emission tomography radiotracer for imaging cannabinoid receptor type 2. J. Neurochem. 2016, 874–886; (c) Haider, A.; Müller Herde, A.; Slavik, R.; Weber, M.; Mugnaini, C.; Ligresti, A.; Schibli, R.; Mu, L.; Mensah Ametamey, S. Synthesis and Biological Evaluation of Thiophene-Based Cannabinoid Receptor Type 2 Radiotracers for PET Imaging. Front. Neurosci. 2016, 10:350; (d) Slavik, R.; Herde, A.M.; Bieri, D.; Weber, M.; Schibli, R.; Kramer, S.D.; Ametamey, S. M.; Mu, L. Synthesis, radiolabeling and evaluation of novel 4-oxo-quinoline derivatives as PET tracers for imaging cannabinoid type 2 receptor. Eur. J. Med. Chem. 2015, 92c, 554–564.
- D’Ambra, T.E.; Estep, K.G.; Bell, M.R.; Eissenstat, M.A.; Josef, K.A.; Ward, S.J.; Haycock, D.A.; Baizman, E.R.; Casiano, F.M. Conformationally restrained analogs of pravadoline: Nanomolar potent, enantioselective, (aminoalkyl)indole agonists of the cannabinoid receptor. J. Med. Chem. 1992, 35, 124–135. [Google Scholar] [CrossRef]
- Eissenstat, M.A.; Bell, M.R.; D’Ambra, T.E.; Alexander, E.J.; Daum, S.J.; Ackerman, J.H.; Gruett, M.D.; Kumar, V.; Estep, K.G. Aminoalkylindoles: Structure-activity relationships of novel cannabinoid mimetics. J. Med. Chem. 1995, 38, 3094–3105. [Google Scholar] [CrossRef]
- Pasquini, S.; Mugnaini, C.; Ligresti, A.; Tafi, A.; Brogi, S.; Falciani, C.; Pedani, V.; Pesco, N.; Guida, F.; Luongo, L.; et al. Design, synthesis, and pharmacological characterization of indol-3-ylacetamides, indol-3-yloxoacetamides, and indol-3-ylcarboxamides: Potent and selective CB2 cannabinoid receptor inverse agonists. J. Med. Chem. 2012, 55, 5391–5402. [Google Scholar] [CrossRef]
- Aung, M.M.; Griffin, G.; Huffman, J.W.; Wu, M.-J.; Keel, C.; Yang, B.; Showalter, V.M.; Abood, M.E.; Martin, B.R. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend. 2000, 60, 133–140. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- (a) Moldovan, R.-P.; Teodoro, R.; Gao, Y.; Deuther-Conrad, W.; Kranz, M.; Wang, Y.; Kuwabara, H.; Nakano, M.; Valentine, H.; Fischer, S.; et al. Development of a high-affinity PET radioligand for imaging cannabinoid subtype 2 receptor. J. Med. Chem. 2016, 59, 7840–7855; (b) Savonenko, A.V.; Melnikova, T.; Wang, Y.; Ravert, H.; Gao, Y.; Koppel, J.; Lee, D.; Pletnikova, O.; Cho, E.; Sayyida, N.; et al. Cannabinoid CB2 receptors in a mouse model of Aβ amyloidosis: immunohistochemical analysis and suitability as a PET biomarker of neuroinflammation. PLoS ONE 2015, 10, e0129618; (c) Horti, A.G.; Gao, Y.; Ravert, H.T.; Finley, P.; Valentine, H.; Wong, D.F.; Endres, C.J.; Savonenko, A.V.; Dannals, R.F. Synthesis and biodistribution of [11C]A-836339, a new potential radioligand for PET imaging of cannabinoid type 2 receptors (CB2). Bioorg. Med. Chem. 2010, 18, 5202–5207.
- Pasquini, S.; Mugnaini, C.; Brizzi, A.; Ligresti, A.; di Marzo, V.; Ghiron, C.; Corelli, F. Rapid Combinatorial Access to a Library of 1,5-Disubstituted-3-indole-N-alkylacetamides as CB2 Receptor Ligands. J. Comb. Chem. 2009, 11, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, S.; Ligresti, A.; Mugnaini, C.; Semeraro, T.; Cicione, L.; de Rosa, M.; Guida, F.; Luongo, L.; de Chiaro, M.; Cascio, M.G.; et al. Investigations on the 4-quinolone-3-carboxylic acid motif. 3. Synthesis, structure-affinity relationships, and pharmacological characterization of 6-substituted 4-quinolone-3-carboxamides as highly selective cannabinoid-2 receptor ligands. J. Med. Chem. 2010, 53, 5915–5928. [Google Scholar] [CrossRef] [PubMed]
- Mugnaini, C.; Brizzi, A.; Ligresti, A.; Allarà, M.; Lamponi, S.; Vacondio, F.; Silva, C.; Mor, M.; di Marzo, V.; Corelli, F. Investigations on the 4-Quinolone-3-carboxylic Acid Motif. 7. Synthesis and Pharmacological Evaluation of 4-Quinolone-3-carboxamides and 4-Hydroxy-2-quinolone-3-carboxamides as High Affinity Cannabinoid Receptor 2 (CB2R) Ligands with Improved Aqueous Solubility. J. Med. Chem. 2016, 59, 1052–1067. [Google Scholar] [PubMed]
- Friscourt, F.; Fahrni, C.J.; Boons, G.-J. A fluorogenic probe for the catalyst-free detection of azide-tagged molecules. J. Am. Chem. Soc. 2012, 134, 18809–18815. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wang, X.; Yu, P.; Zhang, J.; Li, Z.; Zhang, X.; Yang, Y.; Ono, M.; Jia, H.; Saji, H.; et al. Synthesis and evaluation of novel 18F labeled 2-pyridinylbenzoxazole and 2-pyridinylbenzothiazole derivatives as ligands for positron emission tomography (PET) imaging of β-amyloid plaques. J. Med. Chem. 2012, 55, 9283–9296. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A. XLV. Theory of ætherification. Philos. Mag. Ser. 1850, 37, 350–356. [Google Scholar]
- Rühl, T.; Deuther-Conrad, W.; Fischer, S.; Günther, R.; Hennig, L.; Krautscheid, H.; Brust, P. Cannabinoid receptor type 2 (CB2)-selective N-aryl-oxadiazolyl-propionamides: Synthesis, radiolabelling, molecular modelling and biological evaluation. Org. Med. Chem. Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, R.; Moldovan, R.-P.; Lueg, C.; Günther, R.; Donat, C.K.; Ludwig, F.-A.; Fischer, S.; Deuther-Conrad, W.; Wünsch, B.; Brust, P. Radiofluorination and biological evaluation of N-aryl-oxadiazolyl-propionamides as potential radioligands for PET imaging of cannabinoid CB2 receptors. Org. Med. Chem. Lett. 2013, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yung-Chi, C.; Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, R.-P.; Deuther-Conrad, W.; Horti, A.G.; Brust, P. Synthesis and Preliminary Biological Evaluation of Indol-3-yl-oxoacetamides as Potent Cannabinoid Receptor Type 2 Ligands. Molecules 2017, 22, 77. https://doi.org/10.3390/molecules22010077
Moldovan R-P, Deuther-Conrad W, Horti AG, Brust P. Synthesis and Preliminary Biological Evaluation of Indol-3-yl-oxoacetamides as Potent Cannabinoid Receptor Type 2 Ligands. Molecules. 2017; 22(1):77. https://doi.org/10.3390/molecules22010077
Chicago/Turabian StyleMoldovan, Rareş-Petru, Winnie Deuther-Conrad, Andrew G. Horti, and Peter Brust. 2017. "Synthesis and Preliminary Biological Evaluation of Indol-3-yl-oxoacetamides as Potent Cannabinoid Receptor Type 2 Ligands" Molecules 22, no. 1: 77. https://doi.org/10.3390/molecules22010077





