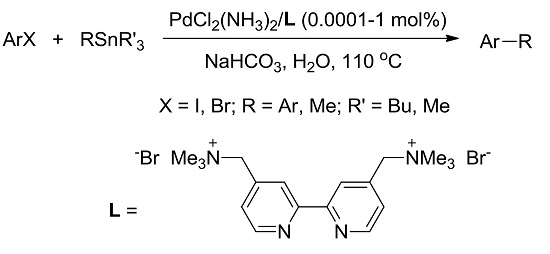
A Highly Efficient and Reusable Palladium(II)/Cationic 2,2’-Bipyridyl-Catalyzed Stille Coupling in Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Stille Coupling Conditions
2.2. Reuse Studies of the Residual Aqueous Solution
2.3. Scope of Substrates and Loading Amounts of Catalyst
3. Experimental Section
3.1. General Information
3.2. Typical Stille Coupling Procedure
3.3. Procedure for Reuse of the Catalytic Aqueous Solution
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stille, J.K. The palladium-catalyzed cross-coupling reactions of organotin reagents with organic electrophiles [new synthetic methods (58)]. Angew. Chem. Int. Ed. 1986, 25, 508–524. [Google Scholar] [CrossRef]
- Espinet, P.; Echavarren, A.M. The mechanisms of the Stille reaction. Angew. Chem. Int. Ed. 2004, 43, 4704–4734. [Google Scholar]
- Cordovilla, C.; Bartolomé, C.; Martínez-Ilarduya, J.M.; Espinet, P. The Stille reaction, 38 years later. ACS Catal. 2015, 5, 3040–3053. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Chakraborty, T.K.; Piscopio, A.D.; Minowa, N.; Bertinato, P. Total synthesis of rapamycin. J. Am. Chem. Soc. 1993, 115, 4419–4420. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Piscopio, A.D.; Bertinato, P.; Chakraborty, T.K.; Minowa, N.; Koide, K. Total synthesis of rapamycin. Chem. Eur. J. 1995, 1, 318–333. [Google Scholar] [CrossRef]
- Shair, M.D.; Yoon, T.Y.; Mosny, K.K.; Chou, T.C.; Danishefsky, S.J. The total synthesis of dynemicin A leading to development of a fully contained bioreductively activated enediyne prodrug. J. Am. Chem. Soc. 1996, 118, 9509–9525. [Google Scholar] [CrossRef]
- Amans, D.; Bareille, L.; Bellosta, V.; Cossy, J. Synthesis of the monomeric counterpart of marinomycin A. J. Org. Chem. 2009, 74, 7665–7674. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, W.P.; Gallagher, K.A.; Jean, M.; Schmidt, J.P.; Diorazio, L.J.; Taylor, R.J.K. Direct imine acylation: Synthesis of the proposed structures of ‘upenamide. Org. Lett. 2013, 15, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Souris, C.; Frébault, F.; Patel, A.; Audisio, D.; Houk, K.N.; Maulide, N. Stereoselective synthesis of dienyl-carboxylate building blocks: Formal synthesis of inthomycin C. Org. Lett. 2013, 15, 3242–3245. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Hashemi, E.; Azimian, F. Recent developments of the Stille reaction as a revolutionized method in total synthesis. Tetrahedron 2014, 70, 7–21. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; King, N.P.; Finlay, M.R.V.; He, Y.; Roschangar, F.; Vourloumis, D.; Vallberg, H.; Sarabia, F.; Ninkovic, S.; Hepworth, D. Total synthesis of epothilone E and related side-chain modified analogues via a Stille coupling based strategy. Bioorg. Med. Chem. 1999, 7, 665–697. [Google Scholar] [CrossRef]
- Ragan, J.A.; Raggon, J.W.; Hill, P.D.; Jones, B.P.; McDermott, R.E.; Munchhof, M.J.; Marx, M.A.; Casavant, J.M.; Copper, B.A.; Doty, J.L.; Lu, Y. Cross-coupling methods for the large-scale preparation of an imidazole-thienopyridine: Synthesis of [2-(3-methyl-3H-imidazol-4-yl)-thieno[3,2-b]pyridin-7-yl]-(2-methyl-1H-indol-5-yl)-amine. Org. Proc. Res. Dev. 2003, 7, 676–683. [Google Scholar] [CrossRef]
- Lee, D.-H.; Jung, J.-Y.; Jin, M.-J. Highly active and recyclable silica gel-supported palladium catalyst for mild cross-coupling reactions of unactivated heteroaryl chlorides. Green Chem. 2010, 12, 2024–2029. [Google Scholar] [CrossRef]
- McAfee, S.M.; McCahill, J.S.J.; Macaulay, C.M.; Hendsbee, A.D.; Welch, G.C. Utility of a heterogeneous palladium catalyst for the synthesis of a molecular semiconductor via Stille, Suzuki, and direct heteroarylation cross-coupling reactions. RSC Adv. 2015, 5, 26097–26106. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Lofù, A.; Mastrorilli, P.; Mucciante, V.; Nobile, C.F. Stille coupling reactions catalysed by a polymer supported palladium complex. J. Organomet. Chem. 2006, 691, 131–137. [Google Scholar] [CrossRef]
- Bahari, S.; Mohammadi-Aghdam, B.; Molaei, R.; Gharibi, Z. Diphenylphosphinoethane-functionalized polystyrene resin-supported Pd(0) complex—A highly active and recyclable catalyst for the Stille reaction under aerobic conditions. Can. J. Chem. 2012, 90, 784–789. [Google Scholar] [CrossRef]
- Jin, M.-J.; Lee, D.-H. A practical heterogeneous catalyst for the Suzuki, Sonogashira, and Stille coupling reactions of unreactive aryl chlorides. Angew. Chem. Int. Ed. 2010, 49, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani-Choghamarani, A.; Norouzi, M. Palladium supported on modified magnetic nanoparticles: A phosphine-free and heterogeneous catalyst for Suzuki and Stille reactions. Appl. Organomet. Chem. 2016, 30, 140–147. [Google Scholar] [CrossRef]
- Saha, D.; Sen, R.; Maity, T.; Koner, S. Anchoring of palladium onto surface of porous metal−organic framework through post-synthesis modification and studies on Suzuki and Stille coupling reactions under heterogeneous condition. Langmuir 2013, 29, 3140–3151. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Urgaonkar, S.; McLaughlin, P.A.; Verkade, J.G. Highly active palladium catalysts supported by bulky proazaphosphatrane ligands for Stille cross-coupling: Coupling of aryl and vinyl chlorides, room temperature coupling of aryl bromides, coupling of aryl triflates, and synthesis of sterically hindered biaryls. J. Am. Chem. Soc. 2004, 126, 16433–16439. [Google Scholar] [PubMed]
- Zhao, H.; Wang, Y.; Sha, J.; Sheng, S.; Cai, M. MCM-41-supported bidentate phosphine palladium(0) complex as an efficient catalyst for the heterogeneous Stille reaction. Tetrahedron 2008, 64, 7517–7523. [Google Scholar] [CrossRef]
- Jana, S.; Haldar, S.; Koner, S. Heterogeneous Suzuki and Stille coupling reactions using highly efficient palladium(0) immobilized MCM-41 catalyst. Tetrahedron Lett. 2009, 50, 4820–4823. [Google Scholar] [CrossRef]
- Cai, M.; Zheng, G.; Ding, G. The first heterogeneous carbonylative Stille coupling of organostannanes with aryl iodides catalyzed by MCM-41-supported bidentate phosphine palladium(0) complex. Green Chem. 2009, 11, 1687–1693. [Google Scholar] [CrossRef]
- Bhunia, S.; Koner, S. Heterogeneous Stille and Sonogashira cross-coupling reactions over palladium anchored mesoporous silica catalyst. Indian J. Chem. Sect. A 2011, 50, 1380–1387. [Google Scholar]
- Ghorbani-Choghamarani, A.; Nikpour, F.; Ghorbani, F.; Havasi, F. Anchoring of Pd(II) complex in functionalized MCM-41 as an efficient and recoverable novel nano catalyst in C–C, C–O and C–N coupling reactions using Ph3SnCl. RSC Adv. 2015, 5, 33212–33220. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, G.; Hao, W.; Cai, M. MCM-41-supported mercapto palladium(0) complex: An efficient and recyclable catalyst for the heterogeneous Stille coupling reaction. Appl. Organomet. Chem. 2010, 24, 92–98. [Google Scholar] [CrossRef]
- Choudary, B.M.; Madhi, S.; Chowdari, N.S.; Kantam, M.L.; Sreedhar, B. Layered double hydroxide supported nanopalladium catalyst for Heck-, Suzuki-, Sonogashira-, and Stille-type coupling reactions of chloroarenes. J. Am. Chem. Soc. 2002, 124, 14127–14136. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, J.C.; Lezutekong, R.; Crooks, R.M. Dendrimer-encapsulated Pd nanoparticles as aqueous, room-temperature catalysts for the Stille reaction. J. Am. Chem. Soc. 2005, 127, 5097–5103. [Google Scholar] [CrossRef] [PubMed]
- Calò, V.; Nacci, A.; Monopoli, A.; Montingelli, F. Pd nanoparticles as efficient catalysts for Suzuki and Stille coupling reactions of aryl halides in ionic liquids. J. Org. Chem. 2005, 70, 6040–6044. [Google Scholar] [CrossRef] [PubMed]
- Tatumi, R.; Akita, T.; Fujihara, H. Synthesis of small palladium nanoparticles stabilized by bisphosphine BINAP bearing an alkyl chain and their palladium nanoparticle–catalyzed carbon–carbon coupling reactions under room-temperature. Chem. Commun. 2006, 3349–3351. [Google Scholar] [CrossRef] [PubMed]
- Pacardo, D.B.; Sethi, M.; Jones, S.E.; Naik, R.R.; Knecht, M.R. Biomimetic synthesis of Pd nanocatalysts for the Stille coupling reaction. ACS Nano 2009, 3, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Sawoo, S.; Srimani, D.; Dutta, P.; Lahiri, R.; Sarkar, A. Size controlled synthesis of Pd nanoparticles in water and their catalytic application in C–C coupling reactions. Tetrahedron 2009, 65, 4367–4374. [Google Scholar] [CrossRef]
- Bernechea, M.; de Jesús, E.; López-Mardomingo, C.; Terreros, P. Dendrimer-encapsulated Pd nanoparticles versus palladium acetate as catalytic precursors in the Stille reaction in water. Inorg. Chem. 2009, 48, 4491–4496. [Google Scholar] [CrossRef] [PubMed]
- Pacardo, D.B.; Slocik, J.M.; Kirk, K.C.; Naik, R.R.; Knecht, M.R. Interrogating the catalytic mechanism of nanoparticle mediated Stille coupling reactions employing bio-inspired Pd nanocatalysts. Nanoscale 2011, 3, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Sarkar, A. Palladium nanoparticles immobilized on chemically modified silica gel: Efficient heterogeneous catalyst for Suzuki, Stille and Sonogashira cross-coupling reactions. Adv. Synth. Catal. 2011, 353, 2814–2822. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.; Tao, Z. A mild and recyclable nano-sized nickel catalyst for the Stille reaction in water. Catal. Sci. Technol. 2012, 2, 707–710. [Google Scholar] [CrossRef]
- Pacardo, D.B.; Knecht, M.R. Exploring the mechanism of Stille C–C coupling via peptide-capped Pd nanoparticles results in low temperature reagent selectivity. Catal. Sci. Technol. 2013, 3, 745–753. [Google Scholar] [CrossRef]
- Yano, H.; Nakajima, Y.; Obora, Y. N,N-Dimethylformamide-stabilized palladium nanoclusters as catalyst for Migita-Kosugi-Sstille cross-coupling reactions. J. Organomet. Chem. 2013, 745–746, 258–261. [Google Scholar] [CrossRef]
- Sun, J.; Bao, M.; Feng, X.; Yu, X.; Yamamoto, Y.; Almansour, A.I.; Arumugam, N.; Kumar, R.S. Carboxylative coupling reaction of five-membered (chloromethyl) heteroarenes with allyltributylstannane catalyzed by palladium nanoparticles. Tetrahedron Lett. 2015, 56, 6747–6750. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Wang, K.; Fu, Y.; Du, Z. Microwave-assisted Stille cross-coupling reaction catalysed by in situ formed palladium nanoparticles. J. Chem. Res. 2015, 39, 399–402. [Google Scholar]
- Nasrollahzadeh, M.; Sajadi, S.M.; Honarmand, E.; Maham, M. Preparation of palladium nanoparticles using Euphorbia thymifolia L. leaf extract and evaluation of catalytic activity in the ligand-free Stille and Hiyama cross-coupling reactions in water. New J. Chem. 2015, 39, 4745–4752. [Google Scholar] [CrossRef]
- Li, X.; Zhu, T.; Shao, Z.; Li, Y.; Chang, H.; Gao, W.; Zhang, Y.; Wei, W. Newly-generated Al(OH)3-supported Pd nanoparticles-catalyzed Stille and Kumada coupling reactions of diazonium salts, (Het)aryl chlorides. Tetrahedron 2016, 72, 69–75. [Google Scholar] [CrossRef]
- Handy, S.T.; Zhang, X. Organic synthesis in ionic liquids: The Stille coupling. Org. Lett. 2001, 3, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Chiappe, C.; Imperato, G.; Napolitano, E.; Pieraccini, D. Ligandless Stille cross-coupling in ionic liquids. Green Chem. 2004, 6, 33–36. [Google Scholar] [CrossRef]
- Hao, W.; Xi, Z.; Cai, M. A practical synthesis of biaryls and aromatic acetylenes by Stille coupling in room-temperature ionic liquids. Synth. Commun. 2012, 42, 2396–2406. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Wang, K.-H.; Wang, J.-X. Atom-efficient, palladium-catalyzed Stille coupling reactions of tetraphenylstannane with aryl iodides or aryl bromides in polyethylene glycol 400 (PEG-400). Adv. Synth. Catal. 2009, 351, 1378–1382. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Wang, K.-H.; Wang, J.-X. Pd(PPh3)4-PEG 400 catalyzed protocol for the atom-efficient Stille cross-coupling reaction of organotin with aryl bromides. J. Org. Chem. 2009, 74, 5599–5602. [Google Scholar] [CrossRef] [PubMed]
- Iranpoor, N.; Firouzabadi, H.; Davan, E.E.; Rostami, A.; Nematollahi, A. Triphenyltin chloride as a new source of phenyl group for C-heteroatom and C-C bond formation. J. Organomet. Chem. 2013, 740, 123–130. [Google Scholar] [CrossRef]
- Naghipour, A.; Ghorbani-Choghamarani, A.; Heidarizadi, F.; Notash, B. Synthesis, characterization and structural study of a phosphonium salt containing the [Pd2Br6]2− ion and its application as a novel, efficient and renewable heterogeneous catalyst for amination of aryl halides and the Stille cross-coupling reaction. Polyhedron 2016, 105, 18–26. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Naghipour, A.; Heidarizadi, F.; Shirkhani, R.; Notash, B. Bis[(2-methylacetatobenzyl)tri(p-tolyl)phosphonium] hexabromodipalladate(II); synthesis, characterization, structural study and application as a retrievable heterogeneous catalyst for the amination of aryl halides and Stille cross-coupling reaction. Inorg. Chim. Acta 2016, 446, 97–102. [Google Scholar] [CrossRef]
- Roshchin, A.I.; Bumagin, N.A.; Beletskaya, I.P. Palladium-catalyzed cross-coupling reaction of organostannoates with aryl halides in aqueous medium. Tetrahedron Lett. 1995, 36, 125–128. [Google Scholar] [CrossRef]
- Rai, R.; Aubrecht, K.B.; Collum, D.B. Palladium-catalyzed Stille couplings of aryl-, vinyl-, and alkyltrichlorostannanes in aqueous solution. Tetrahedron Lett. 1995, 36, 3111–3114. [Google Scholar] [CrossRef]
- Wolf, C.; Lerebours, R. Efficient Stille cross-coupling reaction using aryl chlorides or bromides in water. J. Org. Chem. 2003, 68, 7551–7554. [Google Scholar] [CrossRef] [PubMed]
- Ogo, S.; Takebe, Y.; Uehara, K.; Yamazaki, T.; Nakai, H.; Watanabe, Y.; Fukuzumi, S. pH-Dependent C-C coupling reactions catalyzed by water-soluble palladacyclic aqua catalysts in water. Organometallics 2006, 25, 331–338. [Google Scholar] [CrossRef]
- Li, J.-H.; Hu, X.-C.; Liang, Y.; Xie, Y.-X. PEG-400 promoted Pd(OAc)2/DABCO-catalyzed cross-coupling reactions in aqueous media. Tetrahedron 2006, 62, 31–38. [Google Scholar] [CrossRef]
- Susanto, W.; Chu, C.-Y.; Ang, W.J.; Chou, T.-C.; Lo, L.-C.; Lam, Y. Fluorous oxime palladacycle: A precatalyst for carbon−carbon coupling reactions in aqueous and organic medium. J. Org. Chem. 2012, 77, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.-P.; Cai, C.; Lipshutz, B.H. Stille couplings in water at room temperature. Green Chem. 2013, 15, 105–109. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Chen, S.-N.; Tsai, F.-Y. Recyclable and highly active cationic 2,2′-bipyridyl palladium(II) catalyst for Suzuki cross-coupling reaction in water. Tetrahedron Lett. 2006, 47, 9267–9270. [Google Scholar] [CrossRef]
- Chen, S.-N.; Wu, W.-Y.; Tsai, F.-Y. Hiyama reaction of aryl bromides with arylsiloxanes catalyzed by a reusable palladium(II)/cationic bipyridyl system in water. Tetrahedron 2008, 64, 8164–8168. [Google Scholar] [CrossRef]
- Chen, S.-N.; Wu, W.-Y.; Tsai, F.-Y. Homocoupling reaction of terminal alkynes catalyzed by a reusable cationic 2,2′-bipyridyl palladium(II)/CuI system in water. Green Chem. 2009, 11, 269–274. [Google Scholar] [CrossRef]
- Huang, S.-H.; Wu, T.-M.; Tsai, F.-Y. pH-Dependent conjugate addition of arylboronic acids to α,β-unsaturated enones catalyzed by a reusable palladium(II)/cationic 2,2′-bipyridyl system in water under air. Appl. Organomet. Chem. 2010, 24, 619–624. [Google Scholar] [CrossRef]
- Huang, S.-H.; Chen, J.-R.; Tsai, F.-Y. Palladium(II)/cationic 2,2′-bipyridyl system as a highly efficient and reusable catalyst for the Mizoroki-Heck reaction in water. Molecules 2010, 15, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-W.; Huang, S.-H.; Lee, H.-S.; Tsai, F.-Y. A reusable palladium(II)/cationic 2,2′-bipyridyl catalytic system for hydroxycarbonylation of aryl iodides in water. J. Chin. Chem. Soc. 2013, 60, 769–772. [Google Scholar] [CrossRef]
- Ren, Y.; Dienes, Y.; Hettel, S.; Parvez, M.; Hoge, B.; Baumgartner, T. Highly fluorinated dithieno[3,2-b:2′,3′-d]phospholes with stabilized LUMO levels. Organometallics 2009, 28, 734–740. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Karami, K.; Pirisedigh, A. Application of dimeric orthopalladated complex in Suzuki–Miyaura cross coupling reaction under microwave irradiation and conventional heating. Inorg. Chim. Acta 2011, 370, 531–535. [Google Scholar] [CrossRef]
- Bernhardt, S.; Manolikakes, G.; Kunz, T.; Knochel, P. Preparation of solid salt-stabilized functionalized organozinc compounds and their application to cross-coupling and carbonyl addition reactions. Angew. Chem. Int. Ed. 2011, 50, 9205–9209. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhou, W.; Wang, F.; Wang, J.-X. In situ generation of palladium nanoparticles: Ligand-free palladium catalyzed ultrafast Suzuki-Miyaura cross-coupling reaction in aqueous phase at room temperature. Tetrahedron 2011, 67, 4914–4918. [Google Scholar] [CrossRef]
- Tsai, F.-Y.; Lin, B.-N.; Chen, M.-J.; Mou, C.-Y.; Liu, S.-T. Anchored palladium bipyridyl complex in nanosized MCM-41: A recyclable and efficient catalyst for the Kumada–Corriu reaction. Tetrahedron 2007, 63, 4304–4309. [Google Scholar] [CrossRef]
- Worm-Leonhard, K.; Meldal, M. Green catalysts: Solid-phase peptide carbene ligands in aqueous transition-metal catalysis. Eur. J. Org. Chem. 2008, 5244–5253. [Google Scholar] [CrossRef]
- Kuriyama, M.; Shimazawa, R.; Shirai, R. Design and synthesis of thioether-imidazolium chlorides as efficient ligands for palladium-catalyzed Suzuki–Miyaura coupling of aryl bromides with arylboronic acids. Tetrahedron 2007, 63, 9393–9400. [Google Scholar] [CrossRef]
- Schmidt, B.; Hölter, F. Suzuki–Miyaura cross coupling reactions with phenoldiazonium salts. Org. Biomol. Chem. 2011, 9, 4914–4920. [Google Scholar] [CrossRef] [PubMed]
- DeSchepper, R.E.; Swenton, J.S. Anodic oxidation studies of oxygenated biphenyls. Convenient synthetic routes to certain functionalized biphenyls. Tetrahedron Lett. 1985, 26, 4831–4834. [Google Scholar] [CrossRef]
- Cunningham, A.; Mokal-Parekh, V.; Wilson, C.; Woodward, S. On the use of mixtures of organotin species for catalytic enantioselective ketone allylation—A detective story. Org. Biomol. Chem. 2004, 2, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.-C.; Huang, K.-S.; Chen, H.-M.; Wu, C.-C.; Lin, G.-J. An efficient method for the preparation of nitriles via the dehydration of aldoximes with phthalic anhydride. J. Chin. Chem. Soc. 2004, 51, 619–627. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 3 and 6 are available from the authors.
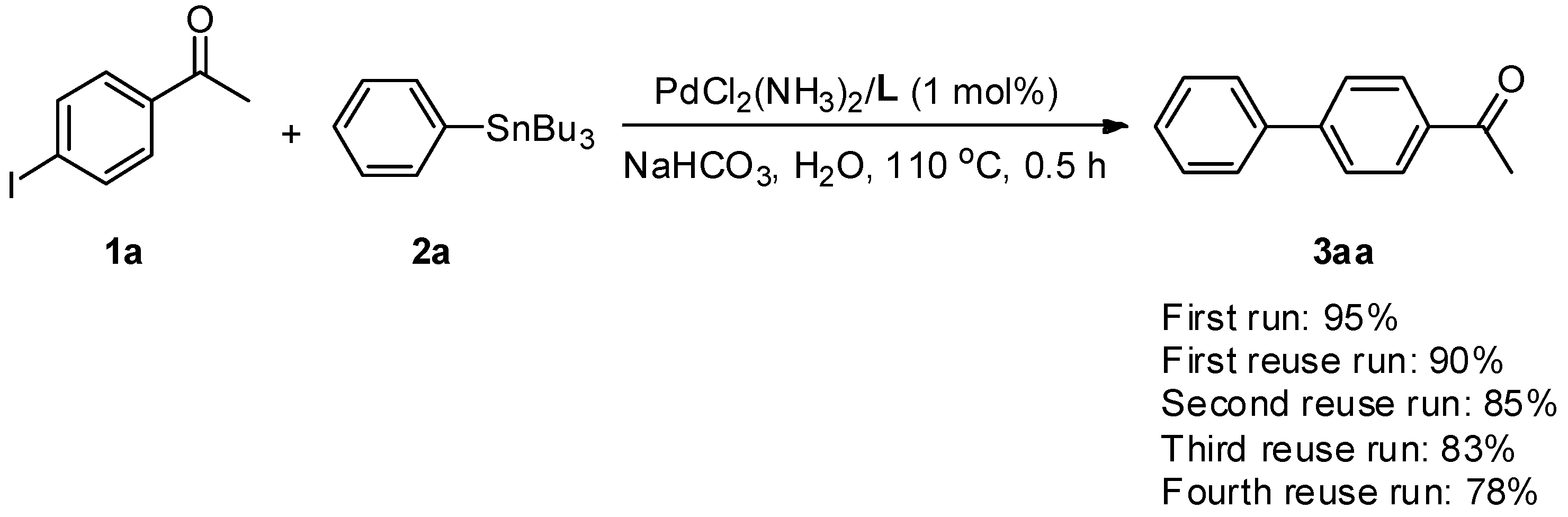
| Entry | Base | Yield (%) b |
|---|---|---|
| 1 | KOH | 70 |
| 2 | K3PO4 | 75 |
| 3 | K2CO3 | 85 |
| 4 | KF | 60 |
| 5 | KOAc | 74 |
| 6 | NaHCO3 | 95 |
| 7 c | NaHCO3 | 32 |
| 8 d | NaHCO3 | 35 |
| 9 e | NaHCO3 | 0 |
| 10 f | NaHCO3 | 90 |
| 11 g | NaHCO3 | 95 |
| Entry | Aryl Iodide | ArSnBu3 | [Pd] (mol %) | Duration (h) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | 4-IC6H4COMe 1a | C6H5SnBu3 2a | 0.01 | 3 | 3aa (98) |
| 2 c | 1a | 2a | 0.0001 | 48 | 3aa (82) |
| 3 | 1a | 4-FC6H5SnBu3 2b | 0.01 | 3 | 3ab (97) |
| 4 | 1a | 4-MeOC6H5SnBu3 2c | 0.01 | 3 | 3ac (93) |
| 5 c | 1a | 2c | 0.0001 | 48 | 3ac (72) |
| 6 | 4-IC6H4CN 1b | 2a | 0.01 | 3 | 3ba (91) |
| 7 c | 1b | 2a | 0.0001 | 48 | 3ba (75) |
| 8 | 1b | 2b | 0.01 | 3 | 3bb (93) |
| 9 | 1b | 2c | 0.01 | 3 | 3bc (90) |
| 10 | C6H5I 1c | 2a | 0.01 | 6 | 3ca (95) |
| 11 | 1c | 2b | 0.01 | 6 | 3cb (95) |
| 12 | 1c | 2c | 0.01 | 6 | 3cc (90) |
| 13 | 4-IC6H4Me 1d | 2a | 0.01 | 6 | 3da (89) |
| 14 c | 1d | 2a | 0.0001 | 48 | 3da (59) |
| 15 | 1d | 2b | 0.01 | 6 | 3db (82) |
| 16 | 1d | 2c | 0.01 | 6 | 3dc (88) |
| 17 | 4-IC6H4OMe 1e | 2a | 0.01 | 6 | 3cc (94) |
| 18 c | 1e | 2a | 0.0001 | 48 | 3cc (66) |
| 19 | 1e | 2b | 0.01 | 6 | 3eb (84) |
| 20 | 1e | 2c | 0.01 | 6 | 3ec (80) |
| Entry | Aryl Bromide | ArSnBu3 | [Pd] (mol %) | Duration (h) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | 4-BrC6H4COMe 4a | C6H5SnBu3 2a | 0.01 | 6 | 3aa (96) |
| 2 c | 4a | 2a | 0.0001 | 48 | 3aa (70) |
| 3 | 4a | 4-FC6H5SnBu3 2b | 0.01 | 6 | 3ab (96) |
| 4 | 4a | 2b | 0.001 | 12 | 3ab (91) |
| 5 | 4a | 4-MeOC6H5SnBu3 2c | 0.01 | 6 | 3ac (92) |
| 6 | 4a | 2c | 0.001 | 12 | 3ac (95) |
| 7 | 4-BrC6H4CN 4b | 2a | 0.01 | 6 | 3ba (93) |
| 8 | 4b | 2b | 0.01 | 8 | 3bb (90) |
| 9 | 4b | 2c | 0.01 | 8 | 3bc (88) |
| 10 | C6H5Br 4c | 2a | 1 | 24 | 3ca (92) |
| 11 | 4c | 2b | 1 | 36 | 3cb (96) |
| 12 | 4c | 2c | 1 | 36 | 3cc (94) |
| 13 | 4-BrC6H4OMe 4e | 2a | 1 | 24 | 3cc (84) |
| 14 | 4e | 2b | 1 | 24 | 3eb (83) |
| 15 | 4e | 2c | 1 | 36 | 3ec (97) |
| 16 d | 4-BrC6H4OH 4f | 2a | 1 | 3 | 3fa (90) |
| 17 d | 4f | 2a | 0.01 | 12 | 3fa (96) |
| 18 c,d | 4f | 2a | 0.0001 | 48 | 3fa (56) |
| 19 d | 4f | 2b | 0.01 | 12 | 3fb (94) |
| 20 d | 4f | 2c | 0.01 | 12 | 3fc (88) |
| Entry | Aryl Halide | Duration (h) | Yield (%) b |
|---|---|---|---|
| 1 c | 4-IC6H4COMe 1a | 24 | 6a (51) |
| 2 d | 1a | 24 | 6a (63) |
| 3 e | 1a | 24 | 6a (70) |
| 4 | 1a | 24 | 6a (91) |
| 5 | 4-IC6H4CN 1b | 24 | 6b (78) |
| 6 | 4-IC6H4OMe 1e | 36 | 6e (42) |
| 7 | 4-BrC6H4COMe 4a | 48 | 6a (40) |
| 8 | 4-BrC6H4CN 4b | 48 | 6b (31) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.-Y.; Liu, L.-J.; Chang, F.-P.; Cheng, Y.-L.; Tsai, F.-Y. A Highly Efficient and Reusable Palladium(II)/Cationic 2,2’-Bipyridyl-Catalyzed Stille Coupling in Water. Molecules 2016, 21, 1205. https://doi.org/10.3390/molecules21091205
Wu W-Y, Liu L-J, Chang F-P, Cheng Y-L, Tsai F-Y. A Highly Efficient and Reusable Palladium(II)/Cationic 2,2’-Bipyridyl-Catalyzed Stille Coupling in Water. Molecules. 2016; 21(9):1205. https://doi.org/10.3390/molecules21091205
Chicago/Turabian StyleWu, Wei-Yi, Ling-Jun Liu, Fen-Ping Chang, Yu-Lun Cheng, and Fu-Yu Tsai. 2016. "A Highly Efficient and Reusable Palladium(II)/Cationic 2,2’-Bipyridyl-Catalyzed Stille Coupling in Water" Molecules 21, no. 9: 1205. https://doi.org/10.3390/molecules21091205