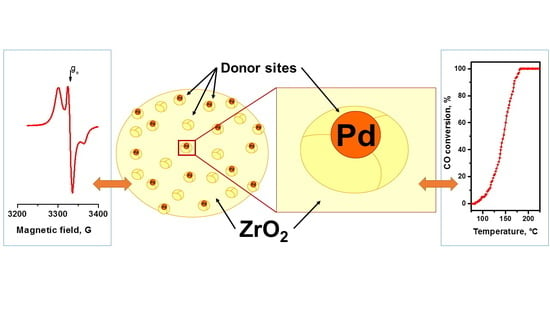
CO Oxidation over Pd/ZrO2 Catalysts: Role of Support′s Donor Sites
Abstract
:1. Introduction
2. Results and Discussion
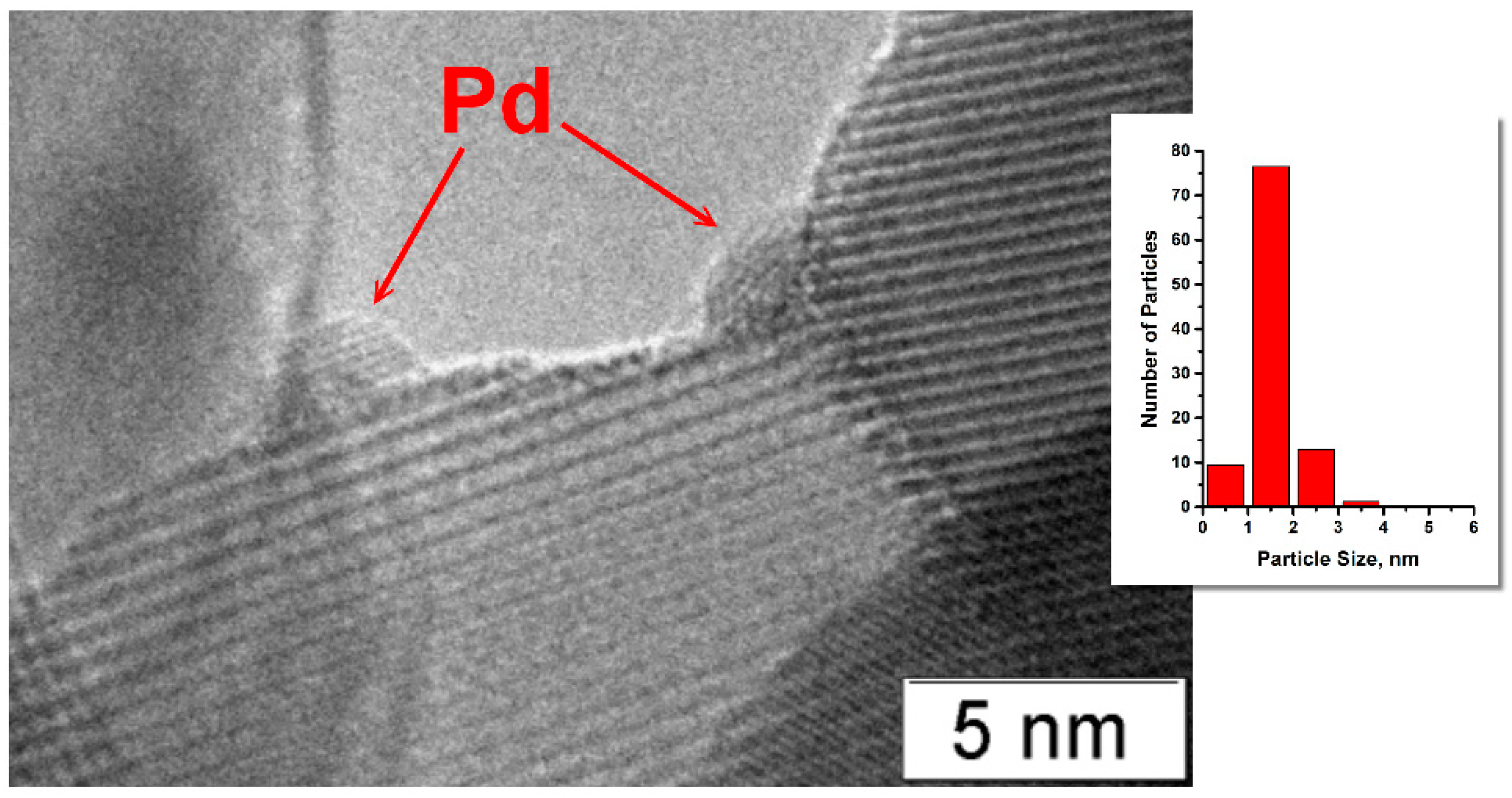
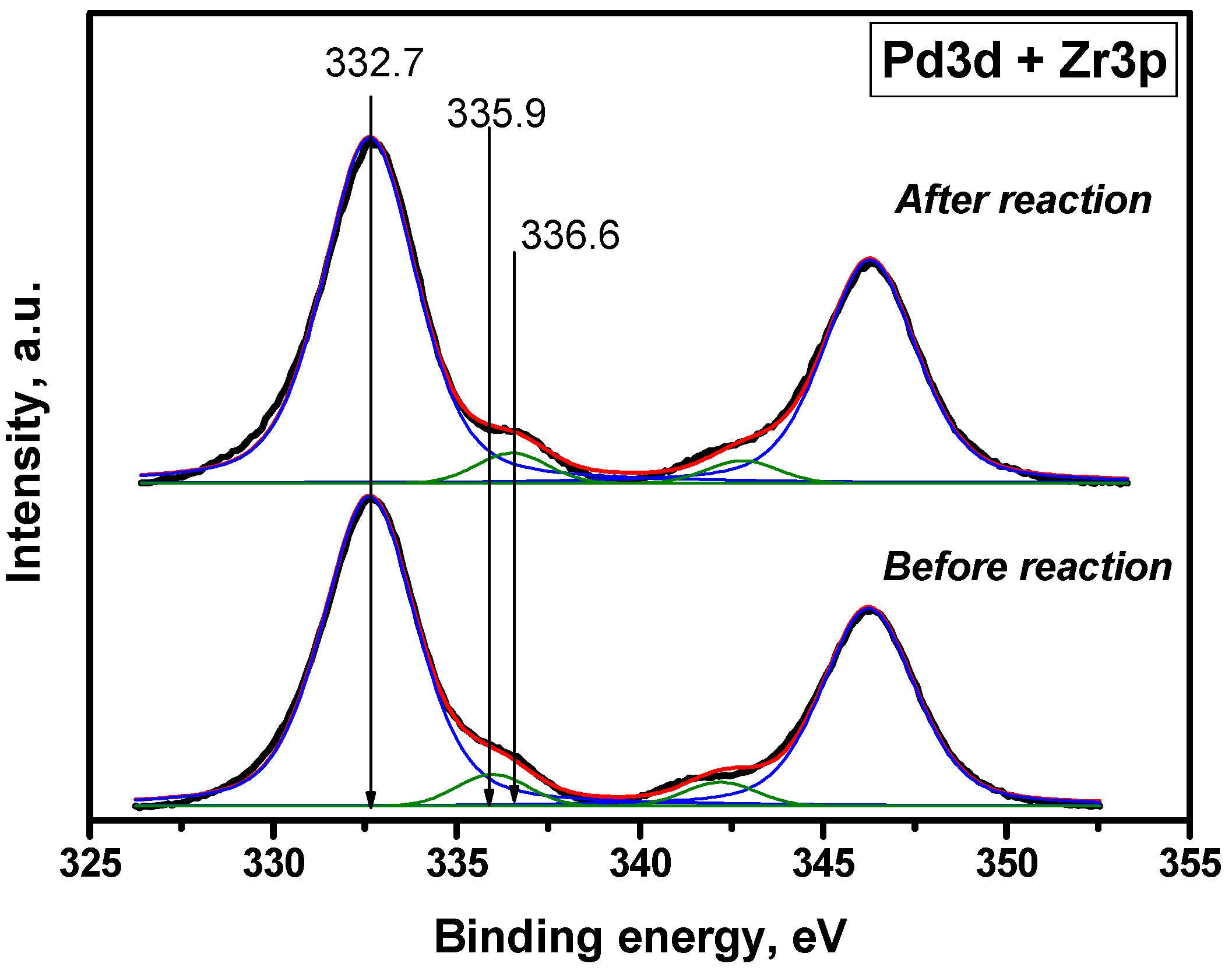
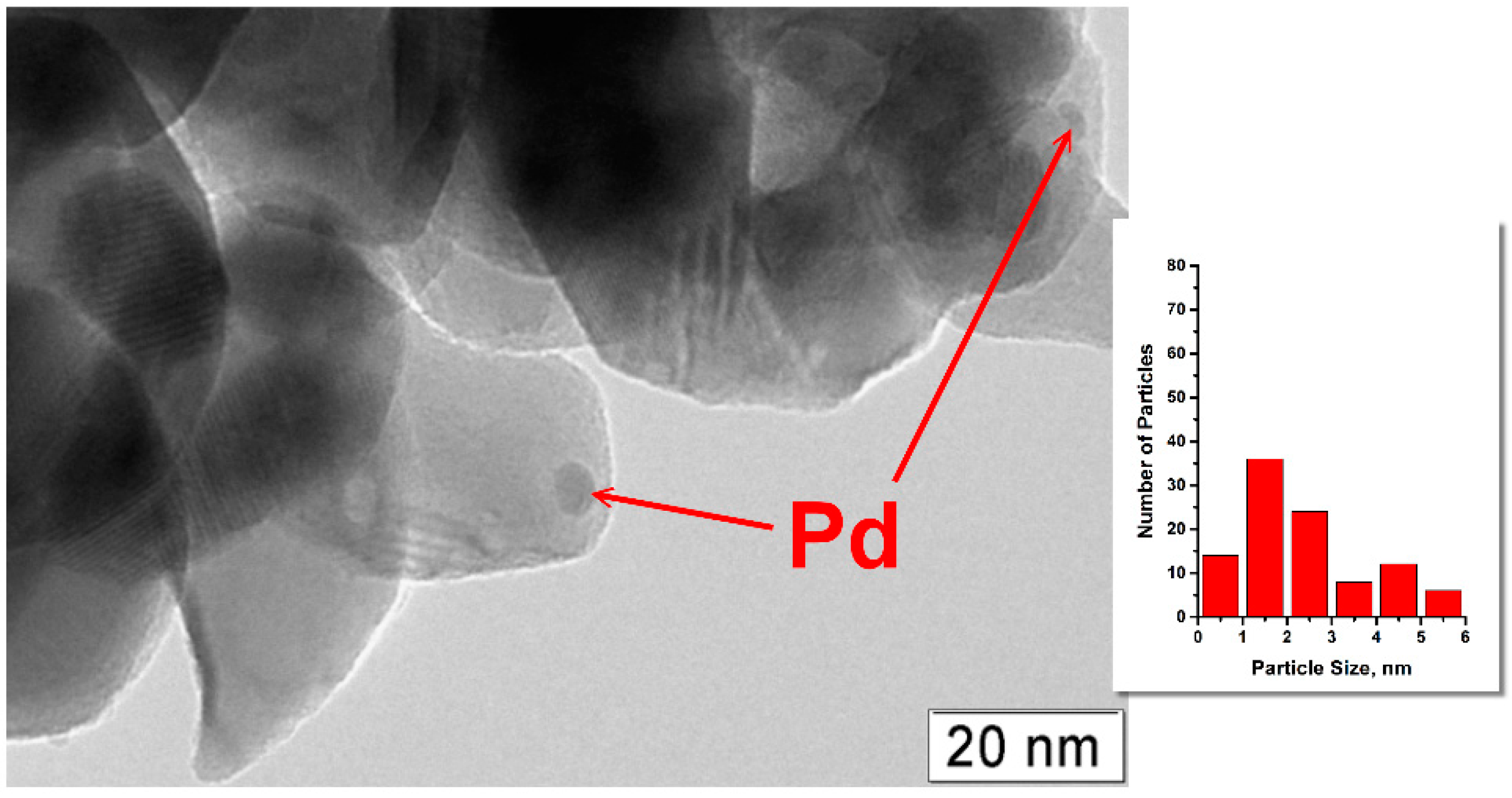
2.1. Characterization of Prepared Samples
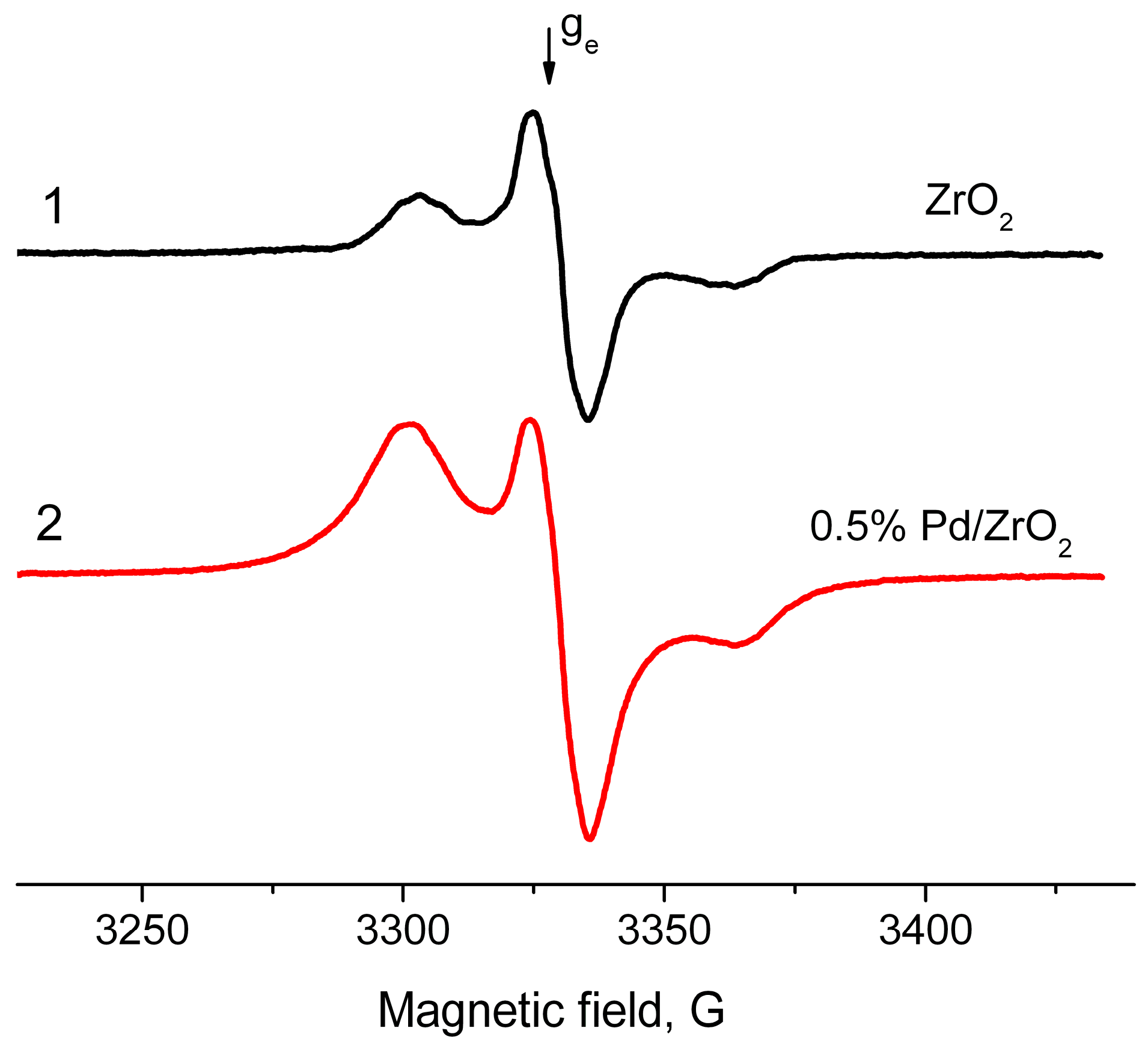
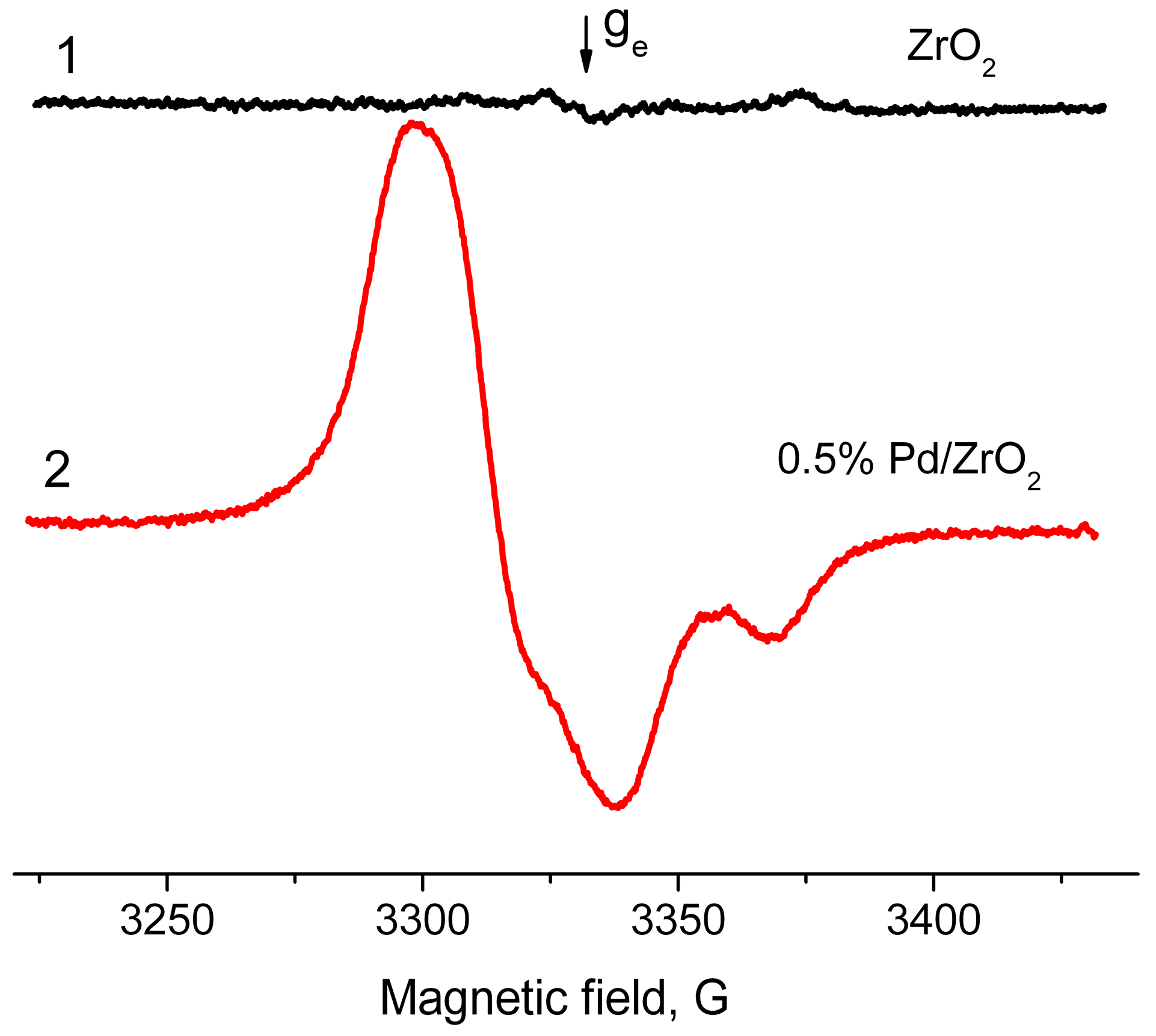
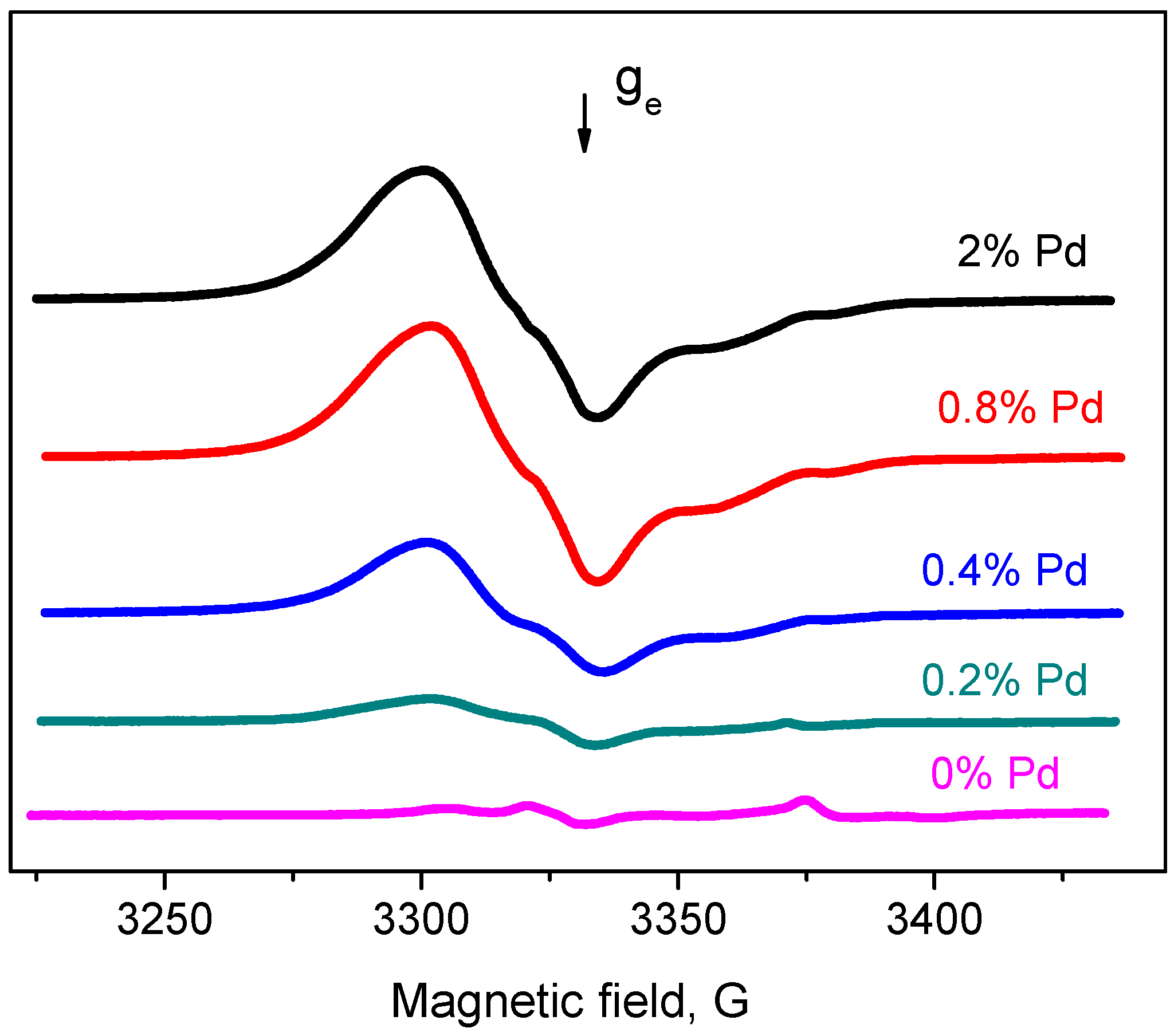
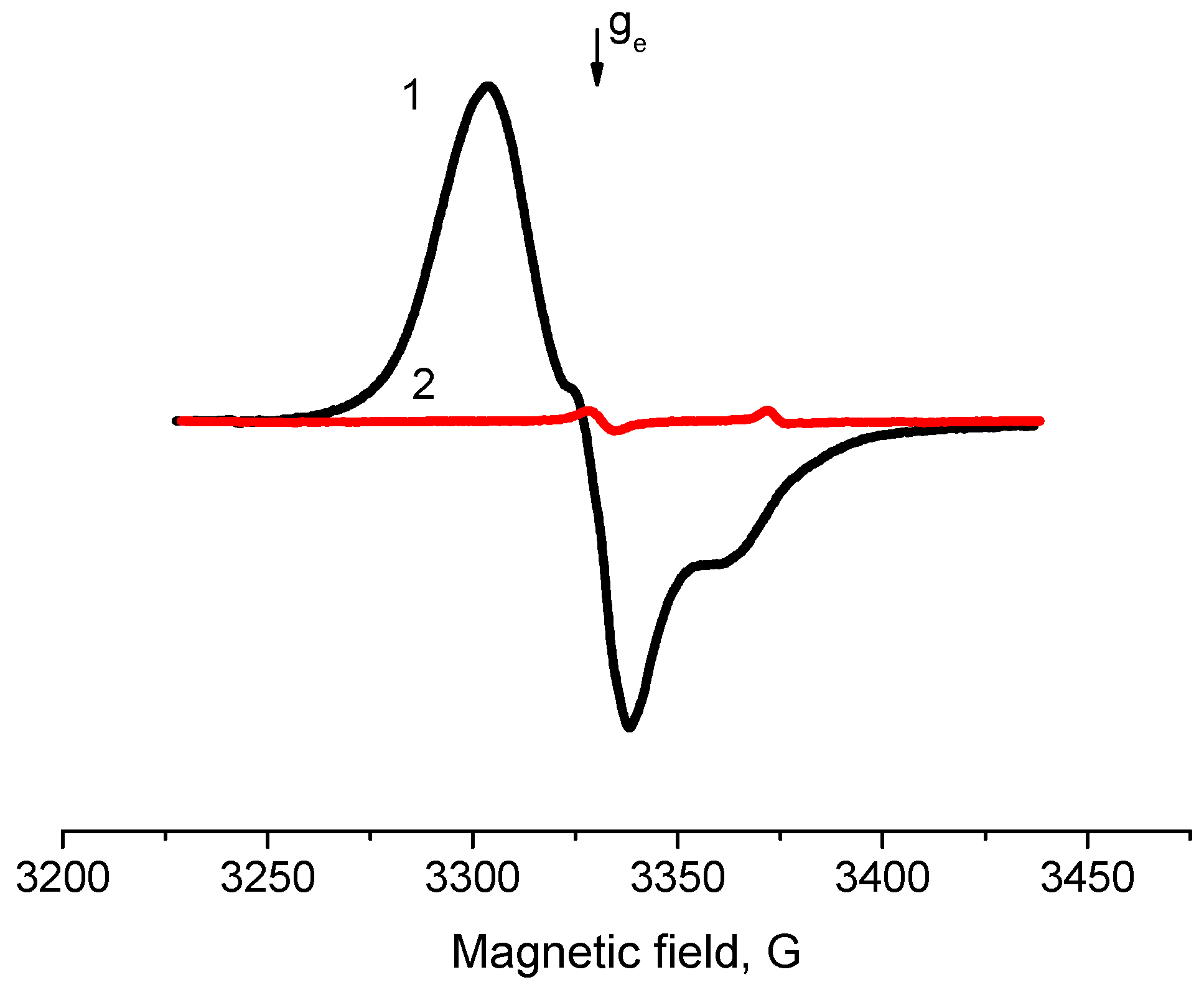
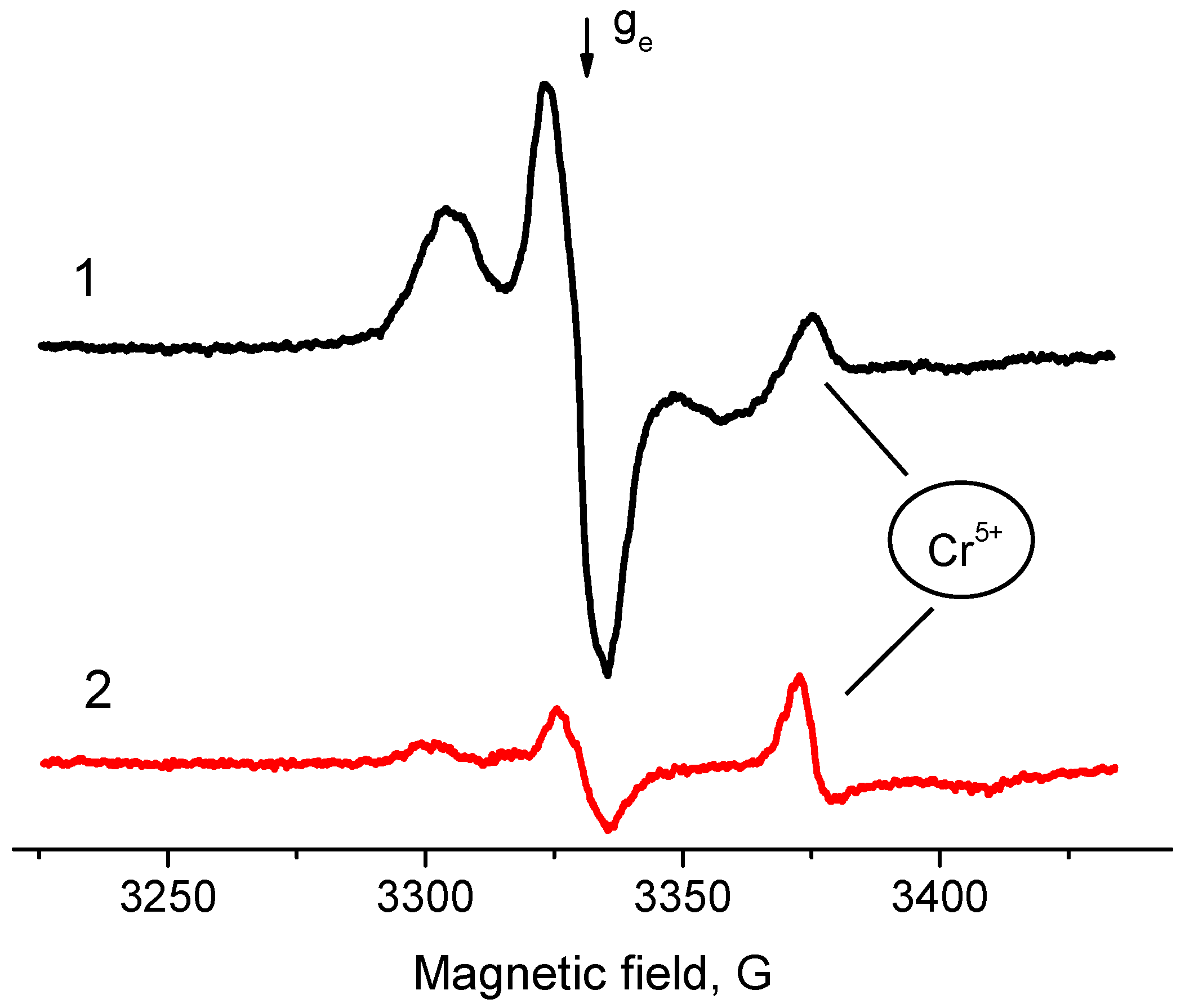
2.2. EPR Spectroscopy Spin Probe Method
- (1)
- Increase in average value of g-factor (gav) from 2.005 typical of “usual” radical anions up to 2.006 corresponding to “abnormal” ones, which can be detected only for a Pd/γ-Al2O3 system activated at low temperatures;
- (2)
- Reduction of the constant value Azz from 31 Gs for “usual” radical anions to 26.5 Gs for “abnormal” ones.
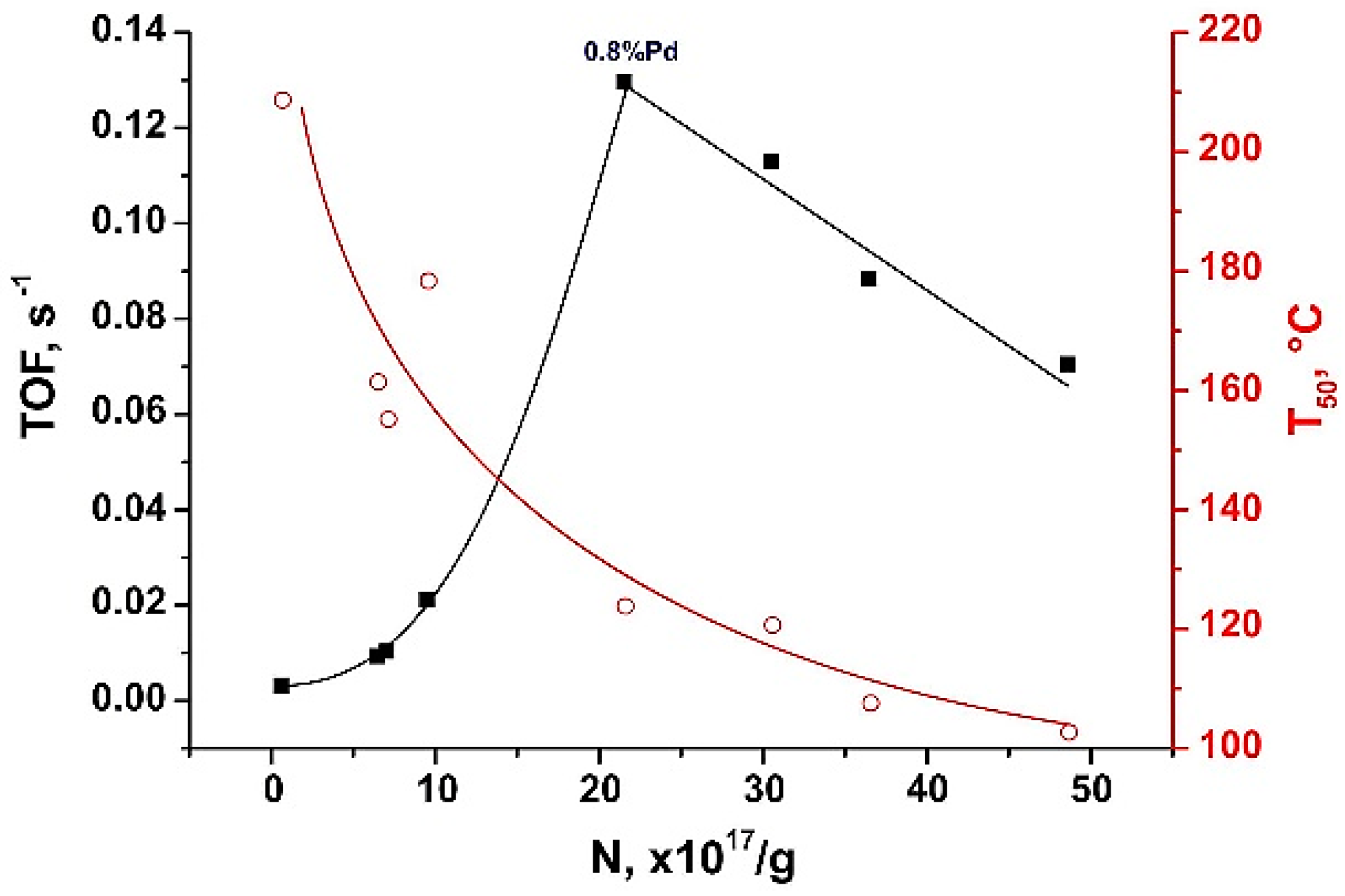
2.3. Testing the Catalytic Behavior in CO Oxidation Correlation with Spin Probe Data
3. Experimental Section
3.1. Preparation of the Samples
3.2. Characterization of the Samples
3.3. EPR Spectroscopy with Spin Probes
3.4. Measurement of Palladium Surface Atoms
3.5. Testing the Catalytic Behavior in CO Oxidation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hackett, S.F.J.; Brydson, R.M.; Gass, M.H.; Harvey, I.; Newman, A.D.; Wilson, K.; Lee, A.F. High-Activity, Single-Site Mesoporous Pd/Al2O3 Catalysts for Selective Aerobic Oxidation of Allylic Alcohols. Angew. Chem. Int. Ed. 2007, 46, 8593–8596. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Accounts Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.K.; Nair, N.N. Rh1/γ-Al2O3 Single-Atom Catalysis of O2 Activation and CO Oxidation: Mechanism, Effects of Hydration, Oxidation State, and Cluster Size. ChemCatChem 2013, 5, 1811–1821. [Google Scholar] [CrossRef]
- Bruix, A.; Lykhach, Y.; Matolnova, I.; Neitzel, A.; Skála, T.; Tsud, N.; Vorokhta, M.; Stetsovych, V.; Ševčíková, K.; Mysliveček, J.; et al. Maximum Noble-Metal Efficiency in Catalytic Materials: Atomically Dispersed Surface Platinum. Angew. Chem. Int. Ed. 2014, 53, 10525–10530. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Hu, J.; Mei, D.; Yi, C.W.; Kim, D.H.; Peden, C.H.F.; Allard, L.F.; Szanyi, J. Coordinatively Unsaturated Al3+ Centers as Binding Sites for Active Catalyst Phases of Platinum on γ-Al2O3. Science 2009, 325, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.H.; Kwak, J.H.; Hu, J.Z.; Cho, S.J.; Szanyi, J.; Allard, L.F.; Peden, C.H.F. Unique Role of Anchoring Penta-Coordinated Al3+ Sites in the Sintering of γ-Al2O3-Supported Pt Catalysts. J. Phys. Chem. Lett. 2010, 1, 2688–2691. [Google Scholar] [CrossRef]
- Peterson, E.J.; DeLaRiva, A.T.; Lin, S.; Johnson, R.S.; Guo, H.; Miller, J.T.; Kwak, J.H.; Peden, C.H.F.; Kiefer, B.; Allard, L.F.; et al. Low-temperature Carbon Monoxide Oxidation Catalysed by Regenerable Atomically Dispersed Palladium on Alumina. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Sicolo, S.; Pacchioni, G. Charging and Stabilization of Pd Atoms and Clusters on an Electron-rich MgO Surface. Surf. Sci. 2008, 602, 2801–2807. [Google Scholar] [CrossRef]
- Flockhart, B.D.; Leith, I.R.; Pink, R.C. Electron-transfer at Alumina Surfaces. Part 2—Electron-donor Properties of Aluminas. Trans. Faraday Soc. 1969, 65, 542–551. [Google Scholar] [CrossRef]
- Flockhart, B.D.; Leith, I.R.; Pink, R.C. Electron-transfer at Alumina Surfaces. Part 3—Reduction of Aromatic Nitro-compounds. Trans. Faraday Soc. 1970, 66, 469–476. [Google Scholar] [CrossRef]
- Konovalova, T.A.; Volodin, A.M. Photo-induced Generation of Radicals from m-Dinitrobenzene Adsorbed on γ-Al2O3: Direct Evidence for the Formation of Electron Donor-acceptor (EDA) Complexes with Participation of Solvent Molecules. React. Kinet. Catal. Lett. 1993, 51, 227–232. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Mishakov, I.V.; Medvedev, D.A.; Noskov, A.S. Characterization of Active Sites of Pd/Al2O3 Model Catalysts with Low Pd Content by Luminescence, EPR and Ethane Hydrogenolysis. Appl. Catal. B Environ. 2011, 103, 397–403. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Gavrilov, M.S.; Volodin, A.M.; Stoyanovskii, V.O.; Slavinskaya, E.M.; Mishakov, I.V.; Shubin, Y.V. Catalytic Purification of Exhaust Gases Over Pd-Rh Alloy Catalysts. Top. Catal. 2013, 56, 1008–1014. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Kenzhin, R.M.; Slavinskaya, E.M.; Mishakov, I.V.; Plyusnin, P.E.; Shubin, Y.V. Stabilization of Active Sites in Alloyed Pd-Rh Catalysts on γ-Al2O3 Support. Catal. Today 2014, 238, 80–86. [Google Scholar] [CrossRef]
- Volodin, A.M.; Bolshov, V.A.; Konovalova, T.A. Photostimulated Formation of Radicals on Oxide Surfaces. Mol. Eng. 1994, 4, 201–226. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Rybinskaya, A.A.; Kenzhin, R.M.; Volodin, A.M.; Bedilo, A.F. Characterization of Electron Donor Sites on Al2O3 Surface. Phys. Chem. Chem. Phys. 2012, 14, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Iizuka, T.; Hattori, H.; Tanabe, K. Surface Properties of Zirconium Oxide and its Catalytic Activity for Isomerization of 1-Butene. J. Catal. 1979, 57, 1–10. [Google Scholar] [CrossRef]
- Bedilo, A.F.; Plotnikov, M.A.; Mezentseva, N.V.; Volodin, A.M.; Zhidomirov, G.M.; Rybkin, I.M.; Klabunde, K.J. Superoxide Radical Anions on the Surface of Zirconia and Sulfated Zirconia: Formation Mechanisms, Properties and Structure. Phys. Chem. Chem. Phys. 2005, 7, 3059–3069. [Google Scholar] [CrossRef] [PubMed]
- Kenzhin, R.M.; Volodin, A.M.; Bedilo, A.F. Reactivity of O2− Radical Anions on Hydrated ZrO2 Surface. Res. Chem. Intermediat. 2013, 39, 1789–1797. [Google Scholar] [CrossRef]
- Park, J.H.; Cho, J.H.; Kim, Y.J.; Kim, E.S.; Han, H.S.; Shin, C.H. Hydrothermal Stability of Pd/ZrO2 Catalysts for High Temperature Methane Combustion. Appl. Catal. B Environ. 2014, 160, 135–143. [Google Scholar] [CrossRef]
- Ohtsuka, H. The Oxidation of Methane at Low Temperatures Over Zirconia-Supported Pd, Ir and Pt Catalysts and Deactivation by Sulfur Poisoning. Catal. Lett. 2011, 141, 413–419. [Google Scholar] [CrossRef]
- Wang, Q.; Li, C.; Guo, M.; Hu, C.; Xie, Y. Controllable synthesis of zirconia nano-powders using vapor-phase hydrolysis and theoretical analysis. J. Mater. Chem. A 2014, 2, 1346–1352. [Google Scholar] [CrossRef]
- Lieske, H.; Voelter, J. Palladium redispersion by spreading of palladium (II) oxide in oxygen treated palladium/alumina. J. Phys. Chem. 1985, 89, 1841–1842. [Google Scholar] [CrossRef]
- Khramtsov, V.V.; Vainer, L.M. Photon Transfer Reactions in Free Radicals. Spin pH Probes. Russ. Chem. Rev. 1988, 57, 824–838. [Google Scholar] [CrossRef]
- Occhiuzzi, M.; Cordischi, D.; Dragone, R. Intrinsic and Extrinsic Paramagnetic Centers in Zirconia. J. Phys. Chem. B 2002, 106, 12464–12469. [Google Scholar] [CrossRef]
- Powder Diffraction File. PDF-2/Release 2009; International Centre for Diffraction Data: Newtown Square, PA, USA, 2009.
- Sinfelt, J.H.; Yates, D.J.C. Catalytic hydrogenolysis of ethane over the noble metals of Group VIII. J. Catal. 1967, 8, 82–90. [Google Scholar] [CrossRef]
- Sample Availability: All studied samples are available from the authors.
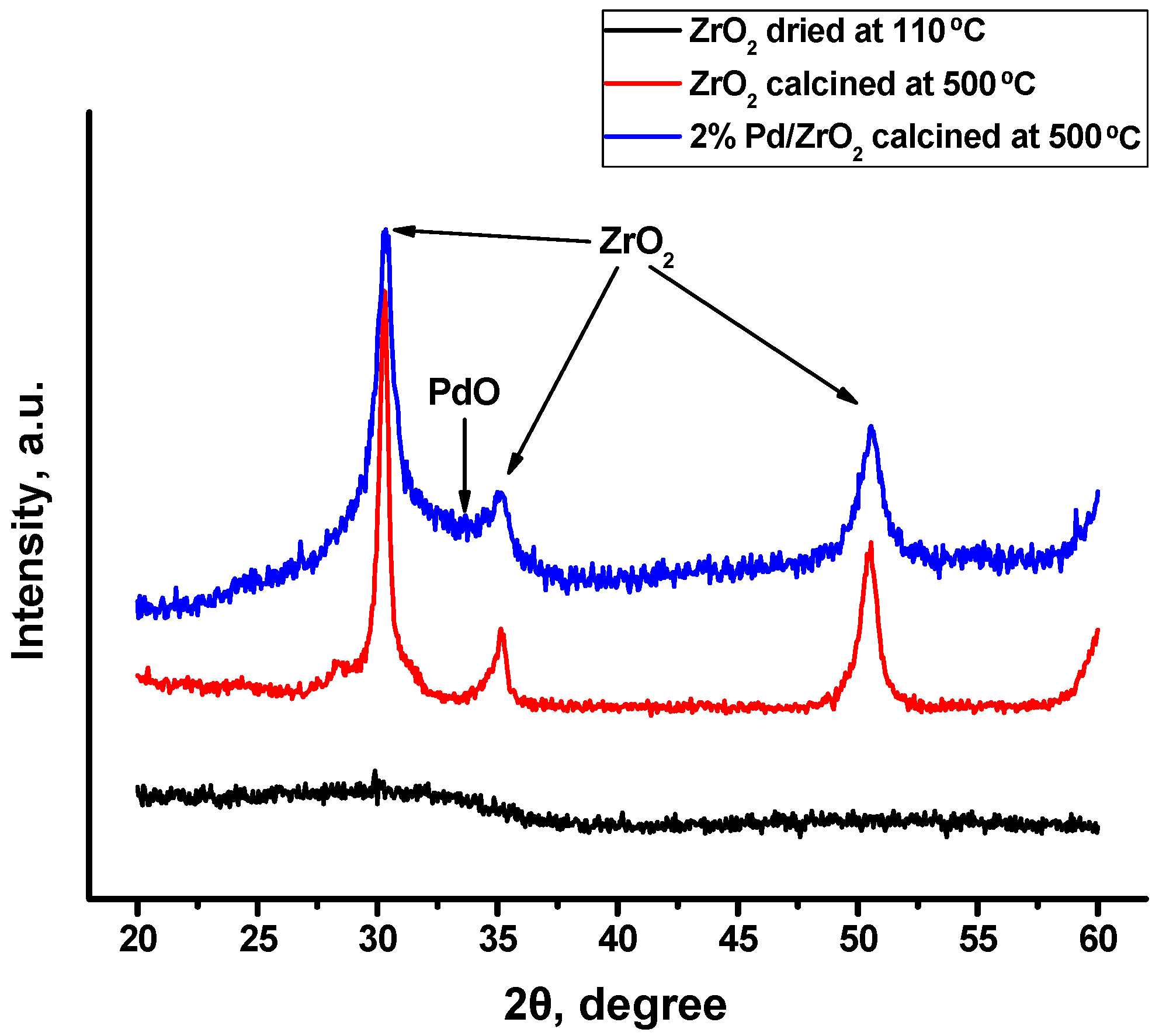
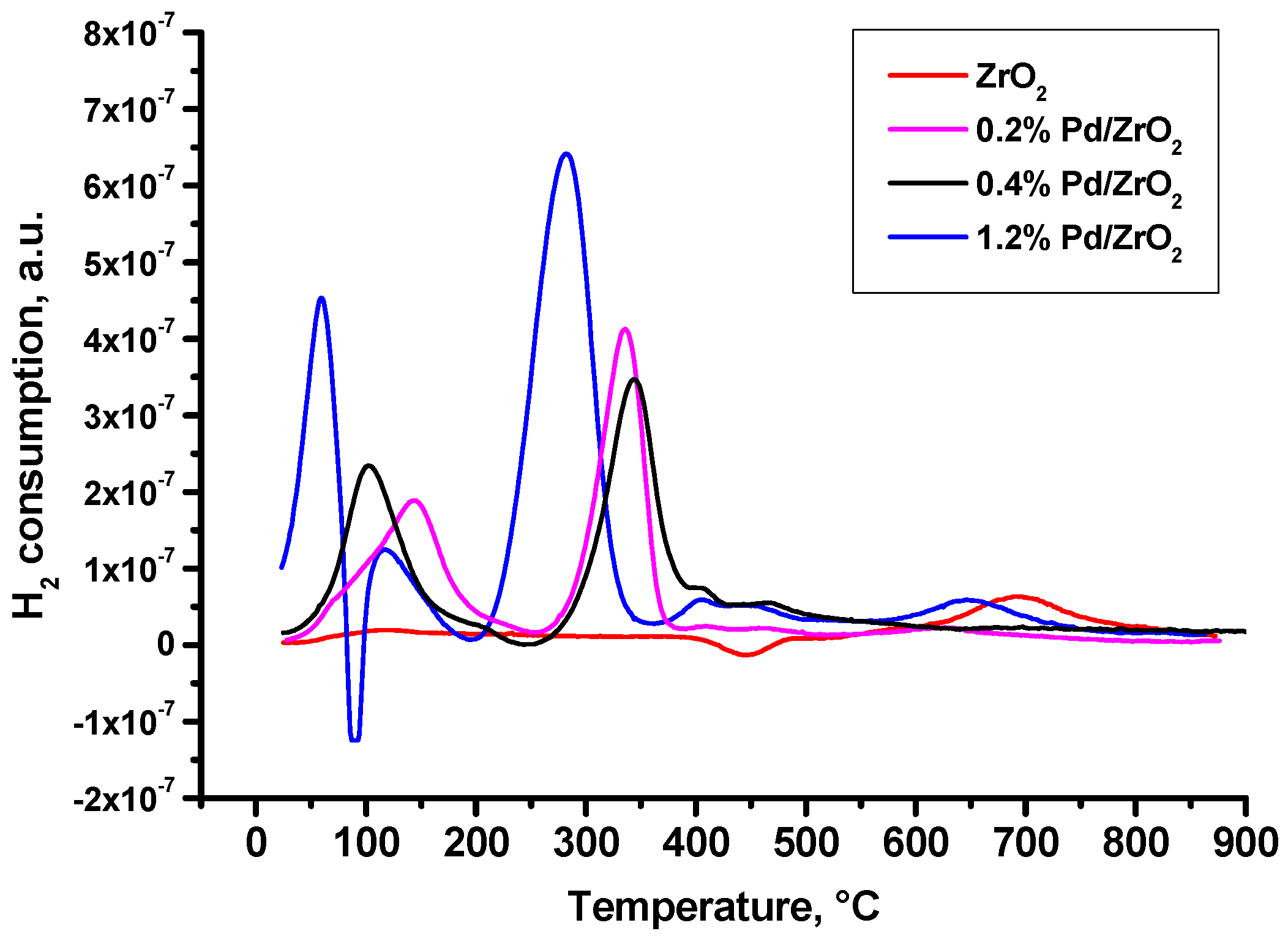
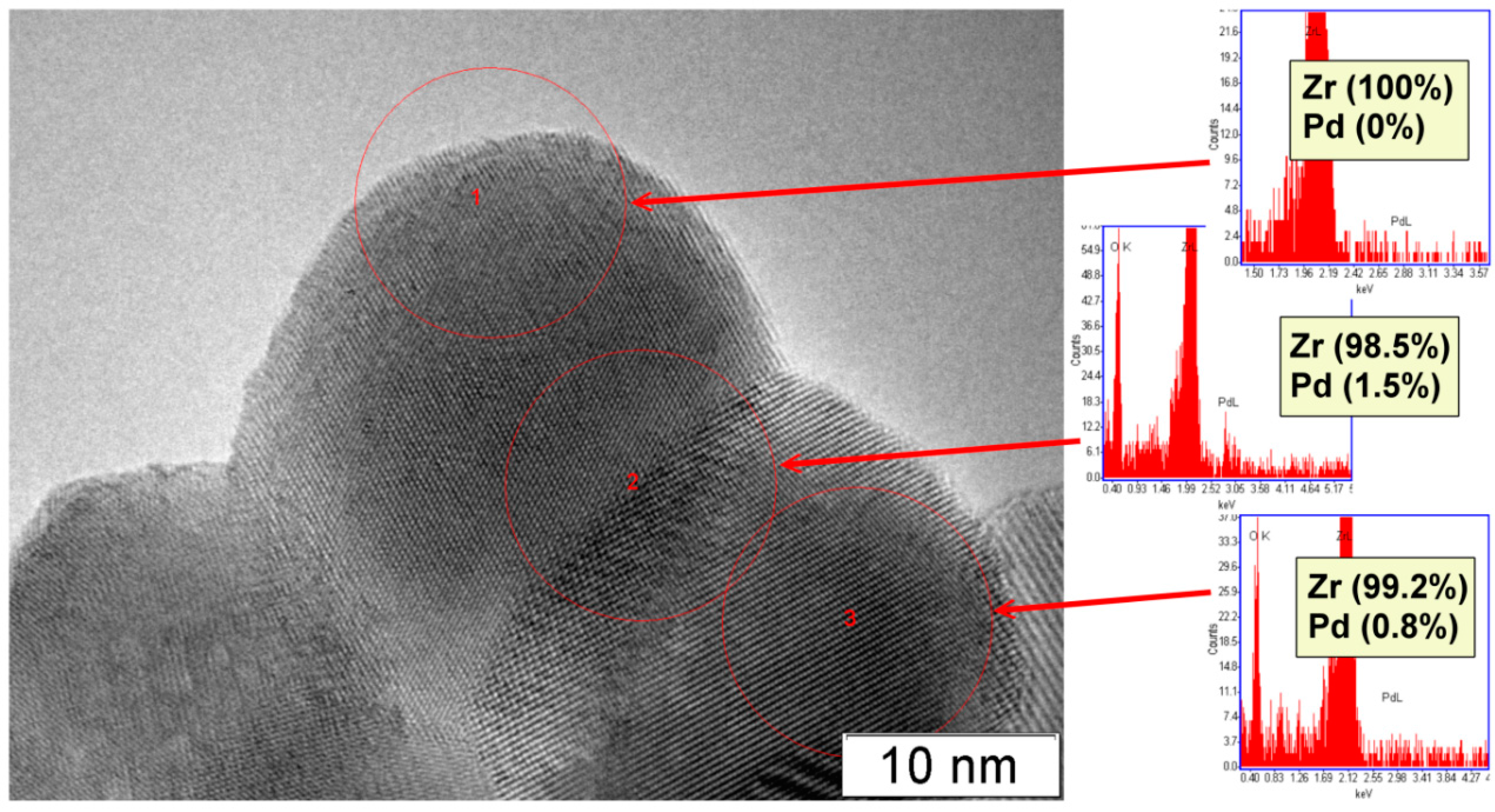



| Sample | Dried at 110 °C | Calcined at 500 °C | Sulphated and Calcined at 500 °C |
|---|---|---|---|
| ZrO2 | 273 | 126 | 83 |
| 0.2% Pd/ZrO2 | 275 | 124 | 83 |
| 2% Pd/ZrO2 | 268 | 123 | 80 |
| Sample | T50, °C | ||
|---|---|---|---|
| 1st Cycle | 2nd Cycle | 3rd Cycle | |
| 0.2% Pd/ZrO2 | 208.6 | 211.7 | 212.9 |
| 0.4% Pd/ZrO2 | 161.3 | 169.1 | 173.5 |
| 0.5% Pd/ZrO2 | 155.1 | 168.3 | 172.6 |
| 0.8% Pd/ZrO2 | 178.3 | 180.7 | 185.1 |
| 1.2% Pd/ZrO2 | 119.0 | 144.1 | 162.3 |
| 2.0% Pd/ZrO2 | 102.6 | 134.1 | 150.4 |
| Sample | Pd/Zr | Pd/C | Pd/O |
|---|---|---|---|
| Before reaction | 0.026 | 0.016 | 0.012 |
| After 3 catalytic cycles | 0.022 | 0.017 | 0.008 |
| Number of Catalytic Cycles | Specific Surface Area of Palladium, m2/g | |
|---|---|---|
| 0.4% Pd/ZrO2 | 1.2% Pd/ZrO2 | |
| 0 | 420 | 425 |
| 1 | 189 | 254 |
| 3 | 27 | 112 |
| 6 | 3 | 54 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vedyagin, A.A.; Volodin, A.M.; Kenzhin, R.M.; Chesnokov, V.V.; Mishakov, I.V. CO Oxidation over Pd/ZrO2 Catalysts: Role of Support′s Donor Sites. Molecules 2016, 21, 1289. https://doi.org/10.3390/molecules21101289
Vedyagin AA, Volodin AM, Kenzhin RM, Chesnokov VV, Mishakov IV. CO Oxidation over Pd/ZrO2 Catalysts: Role of Support′s Donor Sites. Molecules. 2016; 21(10):1289. https://doi.org/10.3390/molecules21101289
Chicago/Turabian StyleVedyagin, Aleksey A., Alexander M. Volodin, Roman M. Kenzhin, Vladimir V. Chesnokov, and Ilya V. Mishakov. 2016. "CO Oxidation over Pd/ZrO2 Catalysts: Role of Support′s Donor Sites" Molecules 21, no. 10: 1289. https://doi.org/10.3390/molecules21101289