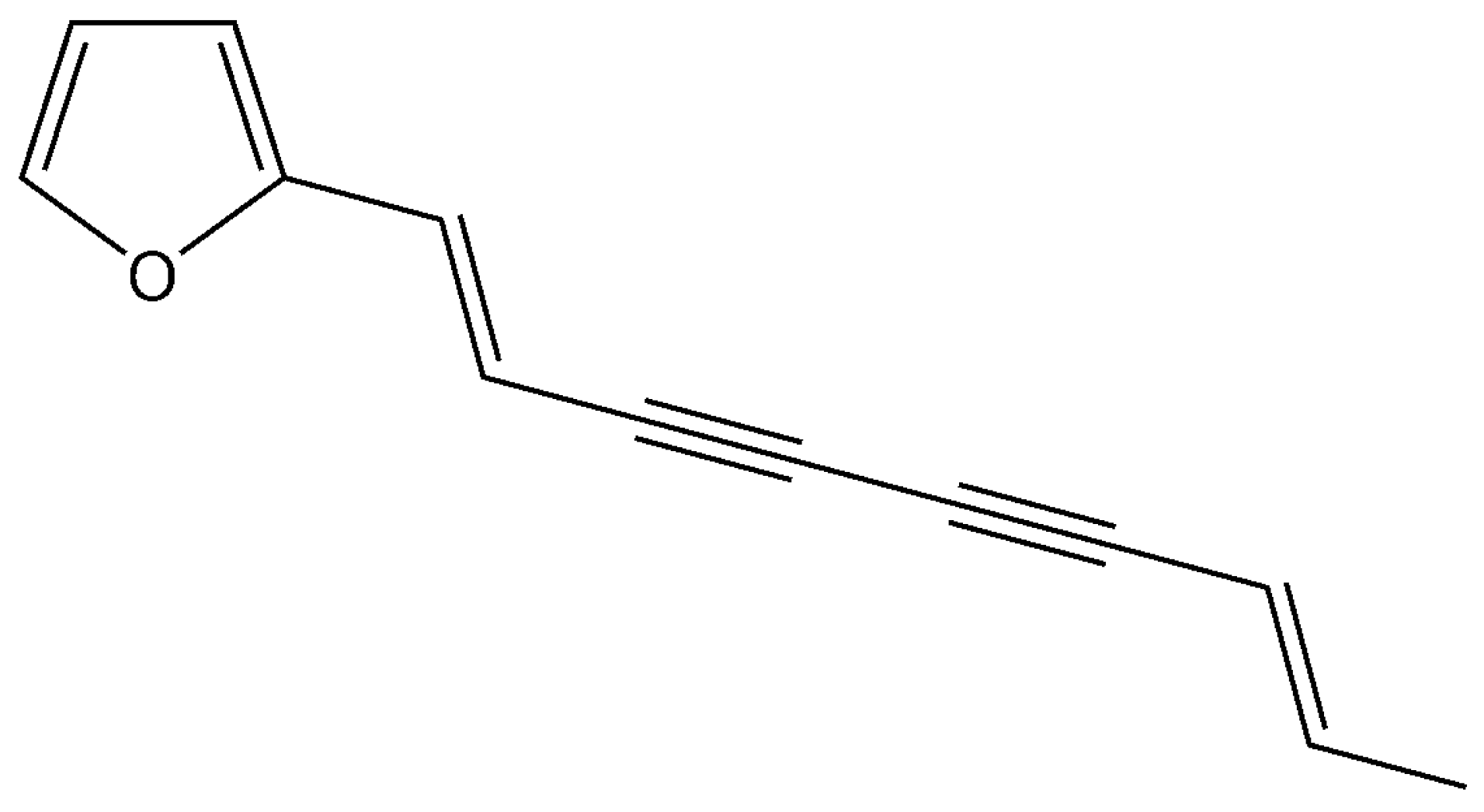
Atractylodin Inhibits Interleukin-6 by Blocking NPM-ALK Activation and MAPKs in HMC-1
Abstract
:1. Introduction
2. Results
2.1. The Effects of Atractylodin on PMA Plus A23187-Induced ALK Pathway Activation
2.2. The Effects of Atractylodin on PMA Plus A23187-Induced MAPKs Activation
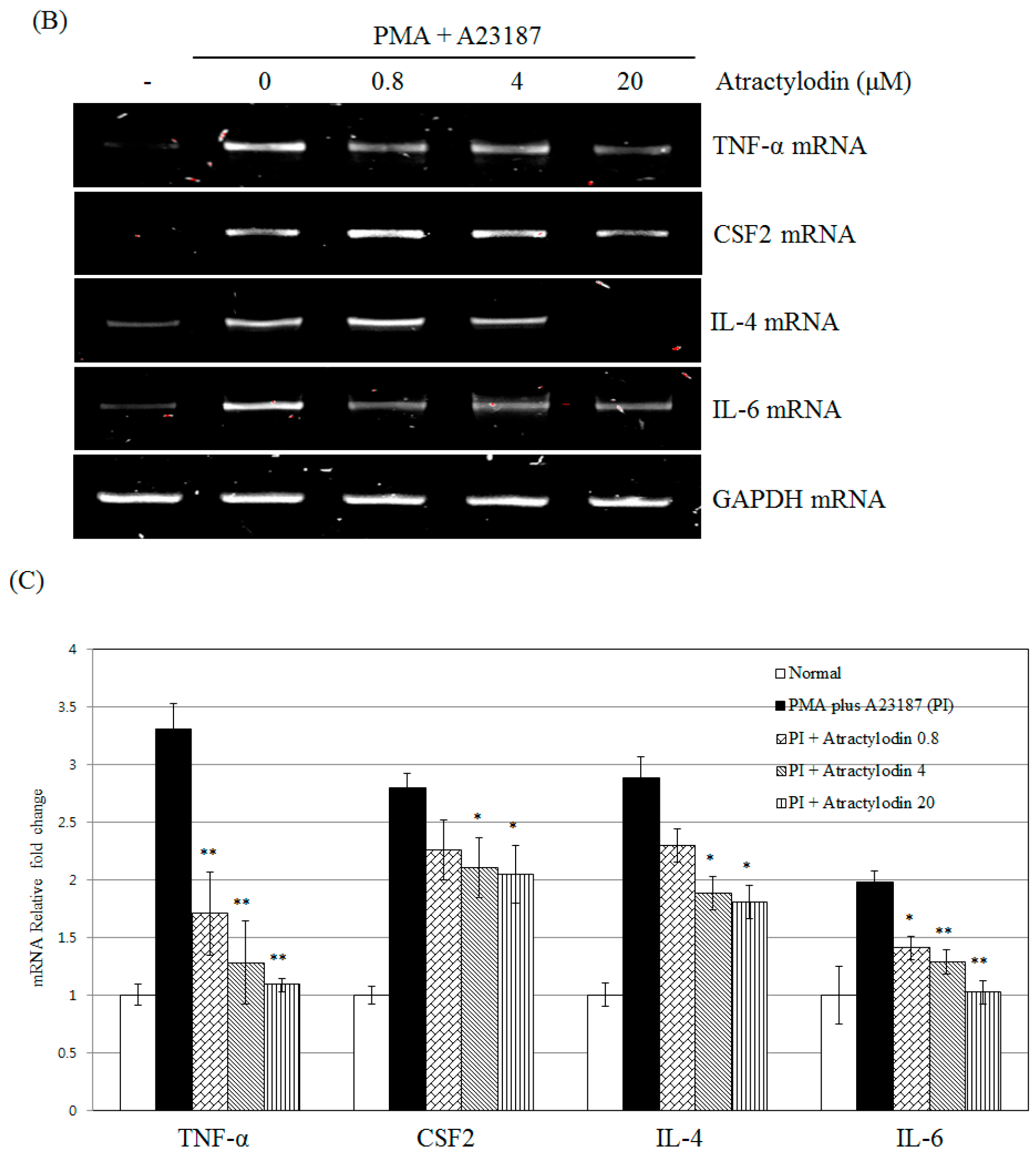
2.3. The Effects of Atractylodin on PMA Plus A23187-Induced Multiple Molecular Targets Activation
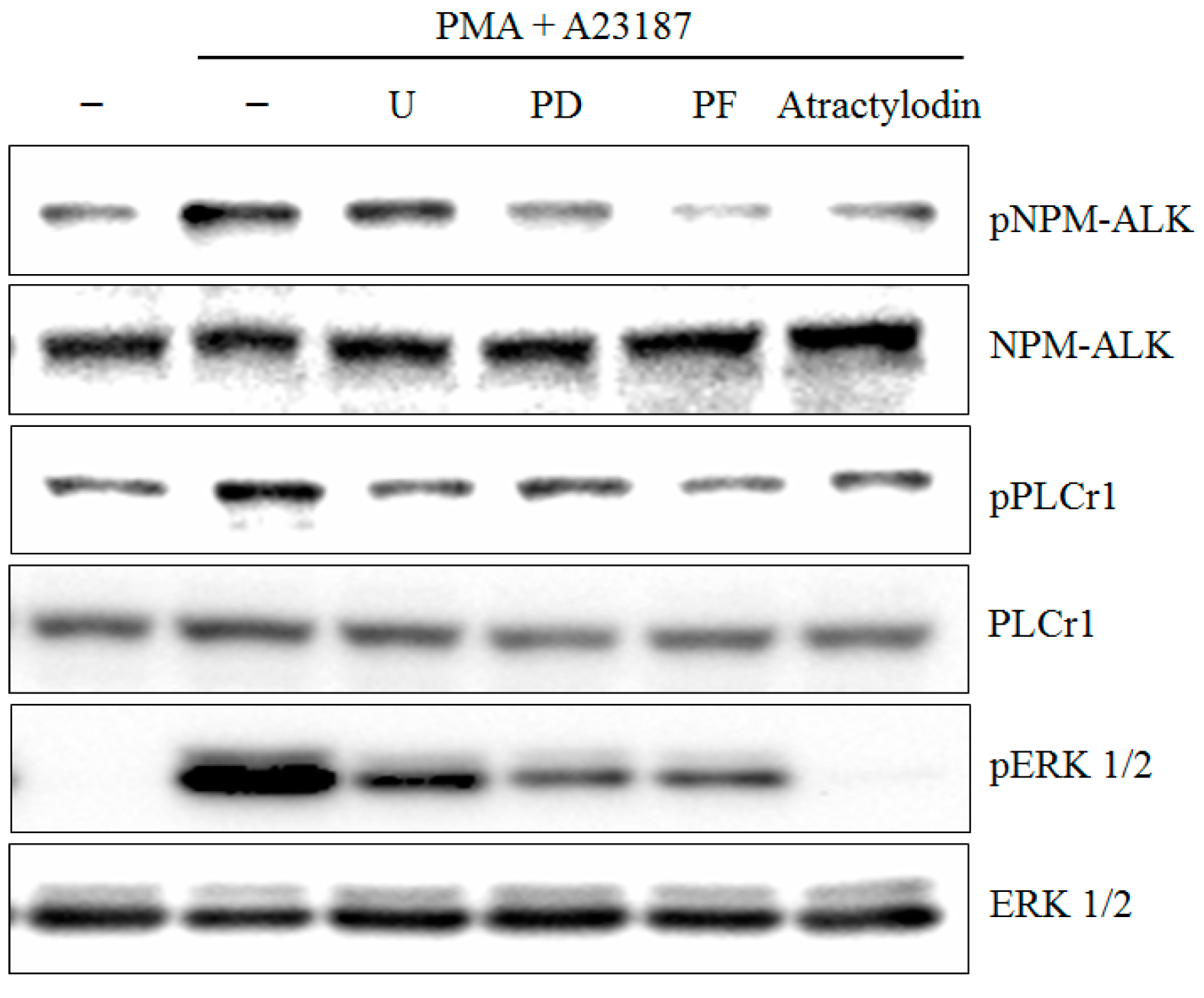
2.4. PMA Plus A23187 Induced Phosphorylation of PLCγ1 and ERK is Mediated by NPM-ALK
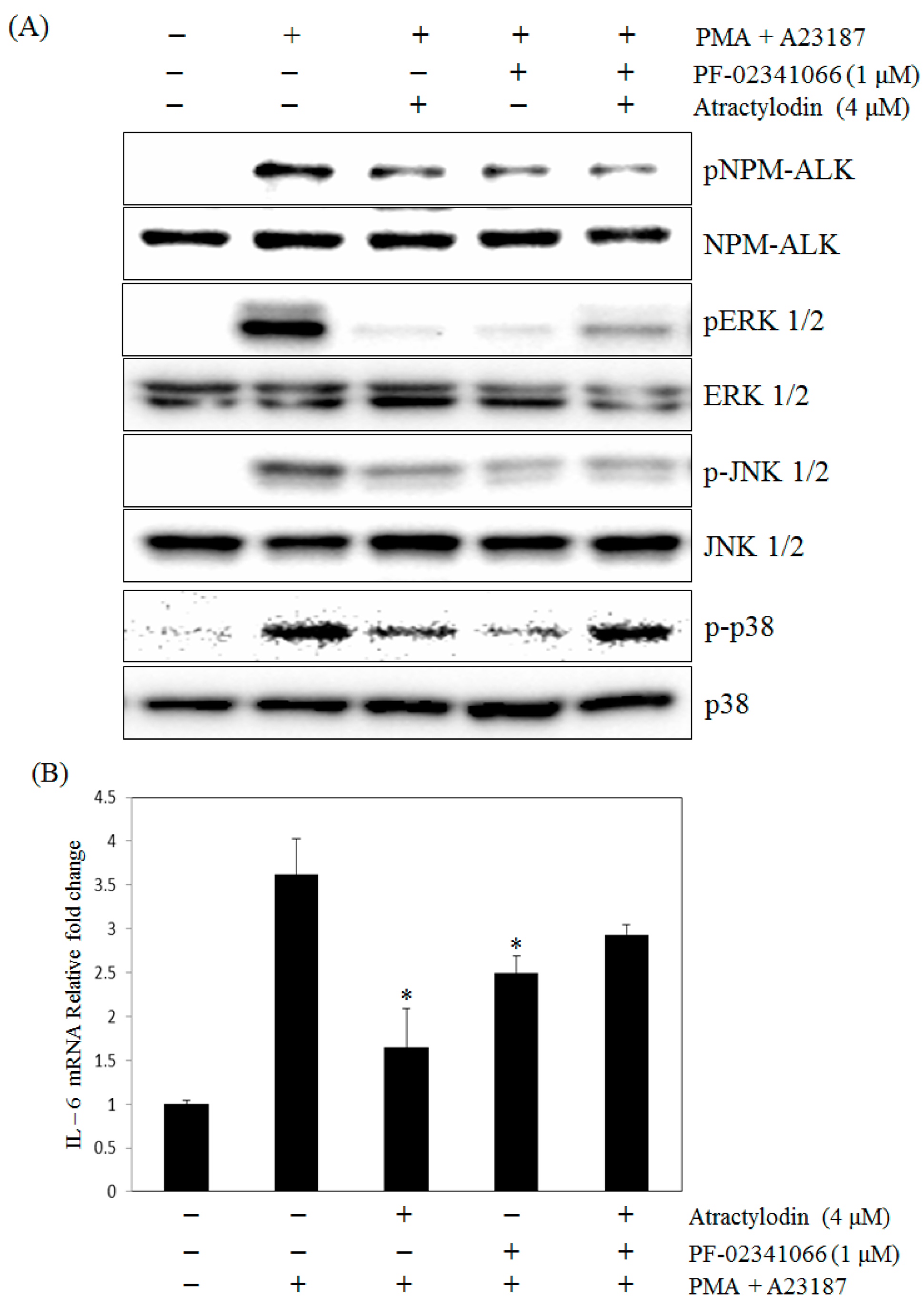
2.5. The Effects of Atractylodin with Selective ALK Inhibitor on PMA Plus A23187-Induced Human Mast Cell-1
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Drugs and Chemicals
4.3. Extraction and Isolation
4.4. Determination of Interleukin-6 Levels
4.5. Immunoblot Analysis
4.6. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.7. Quantitative Real-Time RT-PCR
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, H.; Han, T.; Sun, L.N.; Huang, B.K.; Chen, Y.F.; Zheng, H.C.; Qin, L.P. Regulative effects of essential oil from Atractylodes lancea on delayed gastric emptying in stress-induced rats. Phytomedicine 2008, 15, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Nogami, M.; Nishimura, M.; Moriura, T.; Arichi, S. Origins, processing, and qualities of crude drugs (1). Preventive effects of a Chinese crude drug, Zhu, on experimental stomach ulcer and its pharmacological evaluation. Yakugaku Zasshi 1983, 103, 442–448. [Google Scholar] [PubMed]
- Nogami, M.; Moriura, T.; Kubo, M.; Tani, T. Studies on the origin, processing and quality of crude drugs. II. Pharmacological evaluation of the Chinese crude drug “zhu” in experimental stomach ulcer. (2). Inhibitory effect of extract of Atractylodes lancea on gastric secretion. Chem. Pharm. Bull. 1986, 34, 3854–3860. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.W.; Kiyohara, H.; Matsumoto, T.; Yang, H.C.; Yamada, H. Characterization of pectic polysaccharides having intestinal immune system modulating activity from rhizomes of Atractylodes lancea DC. Carbohydr. Polym. 2001, 46, 125–134. [Google Scholar] [CrossRef]
- Duan, J.A.; Wang, L.; Qian, S.; Su, S.; Tang, Y. A new cytotoxic prenylated dihydrobenzofuran derivative and other chemical constituents from the rhizomes of Atractylodes lancea DC. Arch. Pharm. Res. 2008, 31, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Tseng-Crank, J.; Corneliusen, B.; Yimam, M.; Hodges, M.; Hong, M.; Maurseth, C.; Oh, M.; Kim, H.; Chu, M.; et al. Lipase inhibition and antiobesity effect of Atractylodes lancea. Planta Med. 2014, 80, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.W.; Kirstein, M.N.; Valentine, M.B.; Dittmer, K.G.; Shapiro, D.N.; Saltman, D.L.; Look, A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1994, 263, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Stoica, G.E.; Kuo, A.; Aigner, A.; Sunitha, I.; Souttou, B.; Malerczyk, C.; Caughey, D.J.; Wen, D.; Karavanov, A.; Riegel, A.T.; et al. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J. Biol. Chem. 2001, 276, 16772–16779. [Google Scholar] [CrossRef] [PubMed]
- Iwahara, T.; Fujimoto, J.; Wen, D.; Cupples, R.; Bucay, N.; Arakawa, T.; Mori, S.; Ratzkin, B.; Yamamoto, T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 1997, 14, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Wellmann, A.; Doseeva, V.; Butscher, W.; Raffeld, M.; Fukushima, P.; Stetler-Stevenson, M.; Gardner, K. The activated anaplastic lymphoma kinase increases cellular proliferation and oncogene up-regulation in rat 1a fibroblasts. FASEB J. 1997, 11, 965–972. [Google Scholar] [PubMed]
- Zhang, Q.; Nowak, I.; Vonderheid, E.C.; Rook, A.H.; Kadin, M.E.; Nowell, P.C.; Shaw, L.M.; Wasik, M.A. Activation of Jak/STAT proteins involved in signal transduction pathway mediated by receptor for interleukin 2 in malignant T lymphocytes derived from cutaneous anaplastic large T-cell lymphoma and Sezary syndrome. Proc. Natl. Acad. Sci. USA 1996, 93, 9148–9153. [Google Scholar] [CrossRef] [PubMed]
- Cussac, D.; Greenland, C.; Roche, S.; Bai, R.Y.; Duyster, J.; Morris, S.W.; Delsol, G.; Allouche, M.; Payrastre, B. Nucleophosmin-anaplastic lymphoma kinase of anaplastic large-cell lymphoma recruits, activates, and uses pp60c-src to mediate its mitogenicity. Blood 2004, 103, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.Y.; Ouyang, T.; Miething, C.; Morris, S.W.; Peschel, C.; Duyster, J. Nucleophosmin-anaplastic lymphoma kinase associated with anaplastic large-cell lymphoma activates the phosphatidylinositol 3-kinase/AKT antiapoptotic signaling pathway. Blood 2000, 96, 4319–4327. [Google Scholar] [PubMed]
- Bai, R.Y.; Dieter, P.; Peschel, C.; Morris, S.W.; Duyster, J. Nucleophosmin-anaplastic lymphoma kinase of large-cell anaplastic lymphoma is a constitutively active tyrosine kinase that utilizes phospholipase C-gamma to mediate its mitogenicity. Mol. Cell. Biol. 1998, 18, 6951–6961. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Kashiwakura, J.; Hong, H.; Yasudo, H.; Ando, T.; Maeda-Yamamoto, M.; Wu, D.; Kawakami, Y.; Kawakami, T. Phospholipase C-β3 regulates FcɛRI-mediated mast cell activation by recruiting the protein phosphatase SHP-1. Immunity 2011, 34, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bermejo, M.L.; Leskow, F.C.; Fujii, T.; Wang, Q.; Blumberg, P.M.; Ohba, M.; Kuroki, T.; Han, K.C.; Lee, J.; Marquez, V.E.; et al. Diacylglycerol (DAG)-lactones, a new class of protein kinase C (PKC) agonists, induce apoptosis in LNCaP prostate cancer cells by selective activation of PKCalpha. J. Biol. Chem. 2002, 277, 645–655. [Google Scholar] [CrossRef]
- Bacchiocchi, R.; Baldanzi, G.; Carbonari, D.; Capomagi, C.; Colombo, E.; van Blitterswijk, W.J.; Graziani, A.; Fazioli, F. Activation of alpha-diacylglycerol kinase is critical for the mitogenic properties of anaplastic lymphoma kinase. Blood 2005, 106, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, P.; Zhang, J.; Young, L.C.; Lai, R.; Li, L. Studies of phosphoproteomic changes induced by nucleophosmin-anaplastic lymphoma kinase (ALK) highlight deregulation of tumor necrosis factor (TNF)/Fas/TNF-related apoptosis-induced ligand signaling pathway in ALK-positive anaplastic large cell lymphoma. Mol. Cell Proteom. 2010, 9, 1616–1632. [Google Scholar] [CrossRef] [PubMed]
- Kasprzycka, M.; Marzec, M.; Liu, X.; Zhang, Q.; Wasik, M.A. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT3. Proc. Natl. Acad. Sci. USA 2006, 103, 9964–9969. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.H.; Vernersson, E.; Grabbe, C.; Hallberg, B. Anaplastic lymphoma kinase: Signalling in development and disease. Biochem J. 2009, 420, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.; Perez-Atayde, A.; Hibbard, M.K.; Rubin, B.P.; Dal Cin, P.; Pinkus, J.L.; Pinkus, G.S.; Xiao, S.; Yi, E.S.; Fletcher, C.D.; et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am. J. Pathol. 2000, 157, 377–384. [Google Scholar] [CrossRef]
- Webb, T.R.; Slavish, J.; George, R.E.; Look, A.T.; Xue, L.; Jiang, Q.; Cui, X.; Rentrop, W.B.; Morris, S.W. Anaplastic lymphoma kinase: Role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev. Anticancer Ther. 2009, 9, 331–356. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, T.K.; Schmitt, E.M.; Ivashkiv, L.B. Inhibition of cytokines and JAK-STAT activation by distinct signaling pathways. Proc. Natl. Acad. Sci. USA 1996, 93, 9499–9504. [Google Scholar] [CrossRef] [PubMed]
- Decker, T.; Kovarik, P. Serine phosphorylation of STATs. Oncogene 2000, 19, 2628–2637. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Kwon, O.K.; Oh, S.R.; Lee, H.K.; Ahn, K.S.; Chin, Y.W. Mangosteen xanthones mitigate ovalbumin-induced airway inflammation in a mouse model of asthma. Food Chem. Toxicol. 2012, 50, 4042–4050. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Naka, T.; Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007, 7, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, L.; Jeromin, A.; Volpicelli-Daley, L.; De Camilli, P.; Holowka, D.; Baird, B. The β- and γ-isoforms of type I PIP5K regulate distinct stages of Ca2+ signaling in mast cells. J. Cell Sci. 2009, 122, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Zamo, A.; Chiarle, R.; Piva, R.; Howes, J.; Fan, Y.; Chilosi, M.; Levy, D.E.; Inghirami, G. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene 2002, 21, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Kühn, U.; Brand, P.; Willemsen, J.; Jonuleit, H.; Enk, A.H.; van Brandwijk-Petershans, R.; Saloga, J.; Knop, J.; Becker, D. Induction of tyrosine phosphorylation in human MHC class II-positive antigen-presenting cells by stimulation with contact sensitizers. J. Immunol. 1998, 160, 667–673. [Google Scholar] [PubMed]
- Slupianek, A.; Nieborowska-Skorska, M.; Hoser, G.; Morrione, A.; Majewski, M.; Xue, L.; Morris, S.W.; Wasik, M.A.; Skorski, T. Role of phosphatidylinositol 3-kinase-Akt pathway in nucleophosmin/anaplastic lymphoma kinase-mediated lymphomagenesis. Cancer Res. 2001, 61, 2194–2199. [Google Scholar] [PubMed]
- Ahmed, N.N.; Grimes, H.L.; Bellacosa, A.; Chan, T.O.; Tsichlis, P.N. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Kadin, M.E.; Carpenter, C. Systemic and primary cutaneous anaplastic large cell lymphomas. Semin. Hematol. 2003, 40, 244–256. [Google Scholar] [CrossRef]
- Powers, C.; Aigner, A.; Stoica, G.E.; McDonnell, K.; Wellstein, A. Pleiotrophin signaling through anaplastic lymphoma kinase is rate-limiting for glioblastoma growth. J. Biol. Chem. 2002, 277, 14153–14158. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Tsang, C.M.; Ip, W.K.; Lam, C.W. Molecular mechanisms for the release of chemokines from human leukemic mast cell line (HMC)-1 cells activated by SCF and TNF-alpha: Roles of ERK, p38 MAPK, and NF-kappaB. Allergy 2006, 61, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.H.; Jang, H.J.; Chae, H.S.; Oh, Y.C.; Choi, J.G.; Lee, Y.S.; Kim, J.H.; Kim, Y.C.; Sohn, D.H.; Park, H.; et al. Anti-inflammatory mechanisms of resveratrol in activated HMC-1 cells: Pivotal roles of NF-kappaB and MAPK. Pharmacol. Res. 2009, 59, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Denburg, J.A.; Dolovich, J.; Harnish, D. Basophil mast cell and eosinophil growth and differentiation factors in human allergic disease. Clin. Exp. Allergy 1989, 19, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Atractylodin is available from the authors.



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, H.-S.; Kim, Y.-M.; Chin, Y.-W. Atractylodin Inhibits Interleukin-6 by Blocking NPM-ALK Activation and MAPKs in HMC-1. Molecules 2016, 21, 1169. https://doi.org/10.3390/molecules21091169
Chae H-S, Kim Y-M, Chin Y-W. Atractylodin Inhibits Interleukin-6 by Blocking NPM-ALK Activation and MAPKs in HMC-1. Molecules. 2016; 21(9):1169. https://doi.org/10.3390/molecules21091169
Chicago/Turabian StyleChae, Hee-Sung, Young-Mi Kim, and Young-Won Chin. 2016. "Atractylodin Inhibits Interleukin-6 by Blocking NPM-ALK Activation and MAPKs in HMC-1" Molecules 21, no. 9: 1169. https://doi.org/10.3390/molecules21091169





