Triterpenes for Well-Balanced Scar Formation in Superficial Wounds
Abstract
:1. Introduction
1.1. Medically Effective Triterpenes from Natural Sources
1.2. Triterpenes for Clinical Use: Topical Betulin Gel
1.3. Accelerating Wound Closure by Triterpenes
1.4. Conditioning of Scar Formation by Triterpenes
1.5. Does Accelerated Healing Accompany Discreet Scars?
2. Results and Discussion
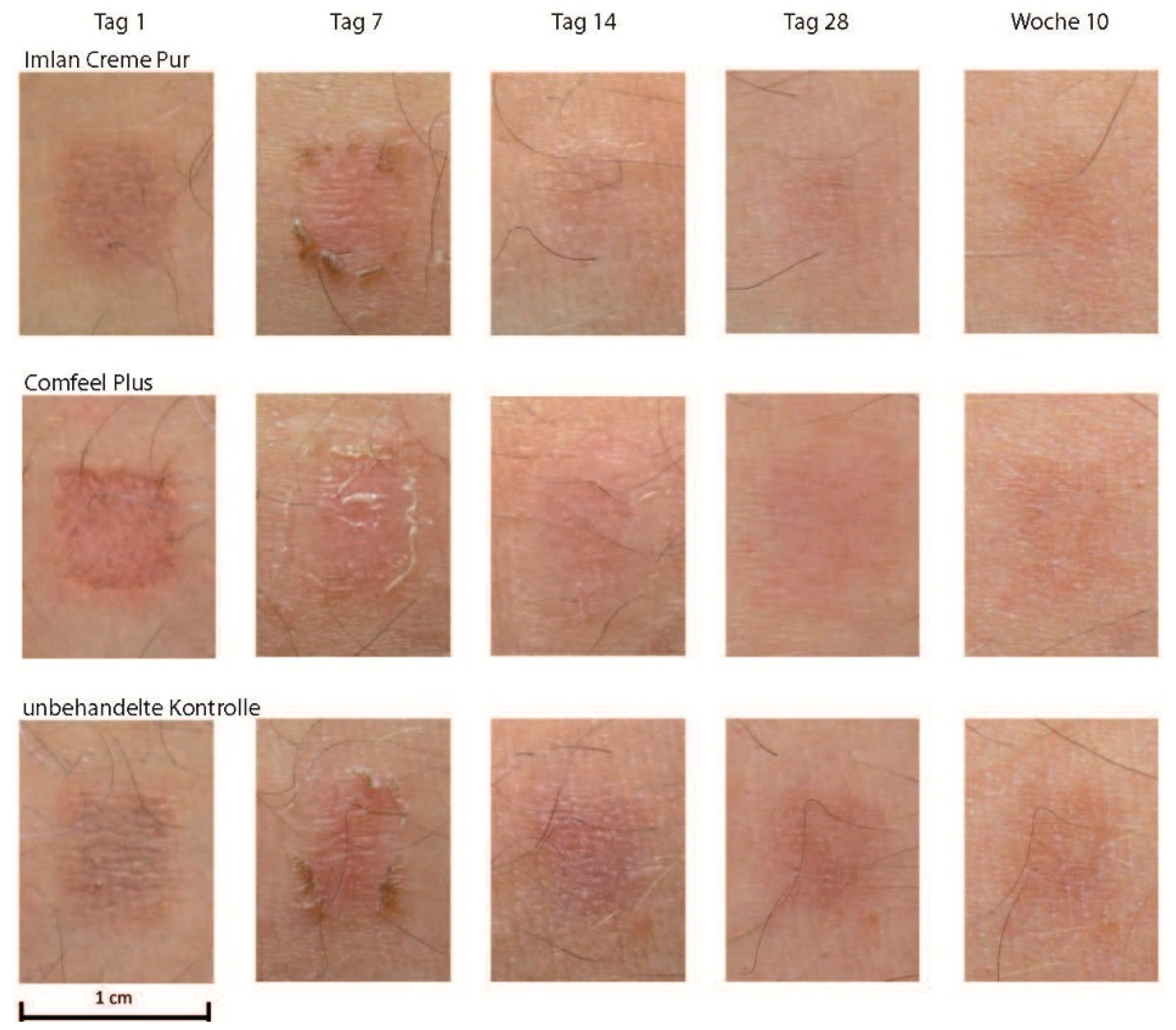
2.1. Investigating Scar Formation by TBG in Skin Grafting Wounds
2.2. Typical Clinical Course of Scar Formation
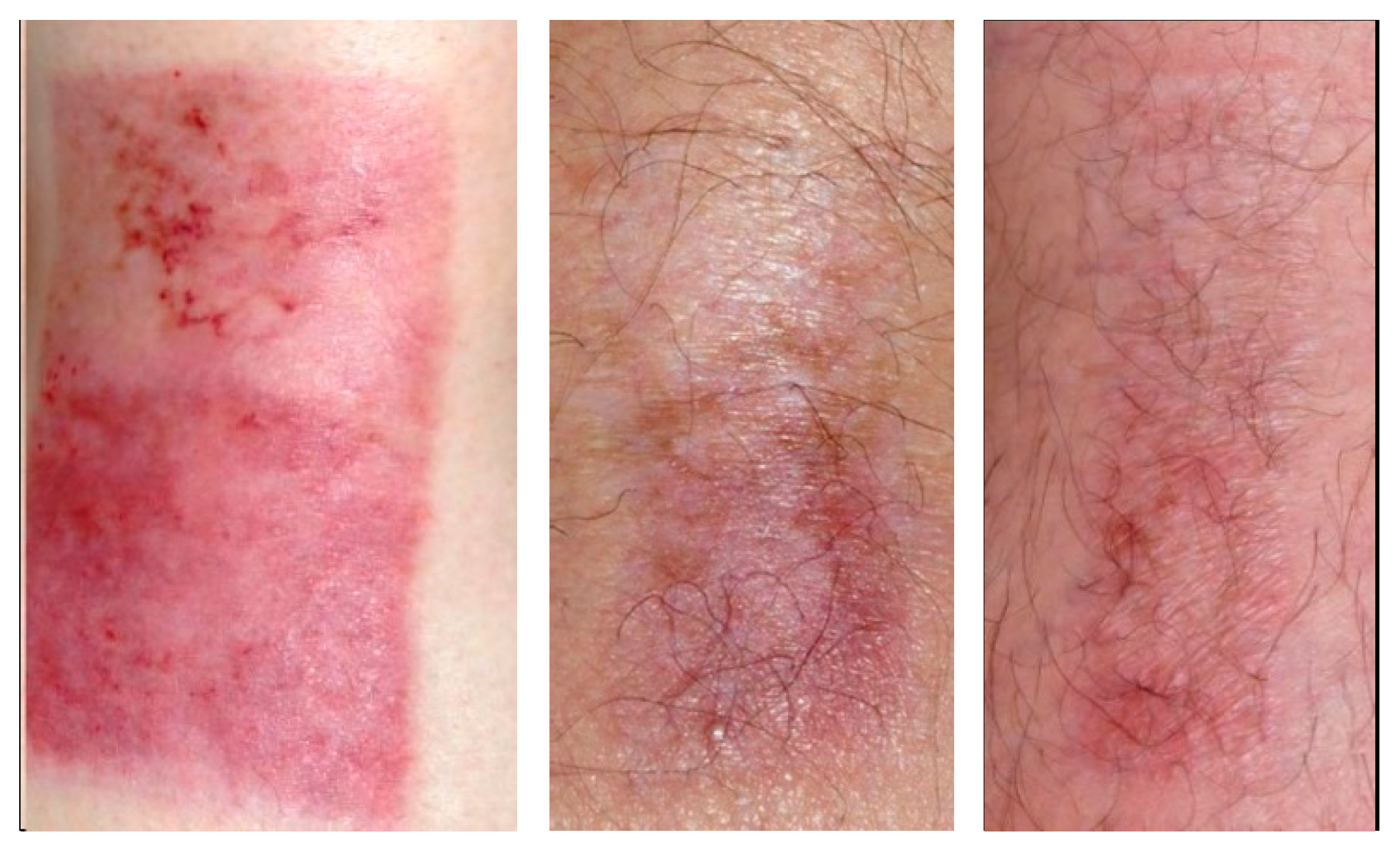
2.3. Variety of Scar Formation
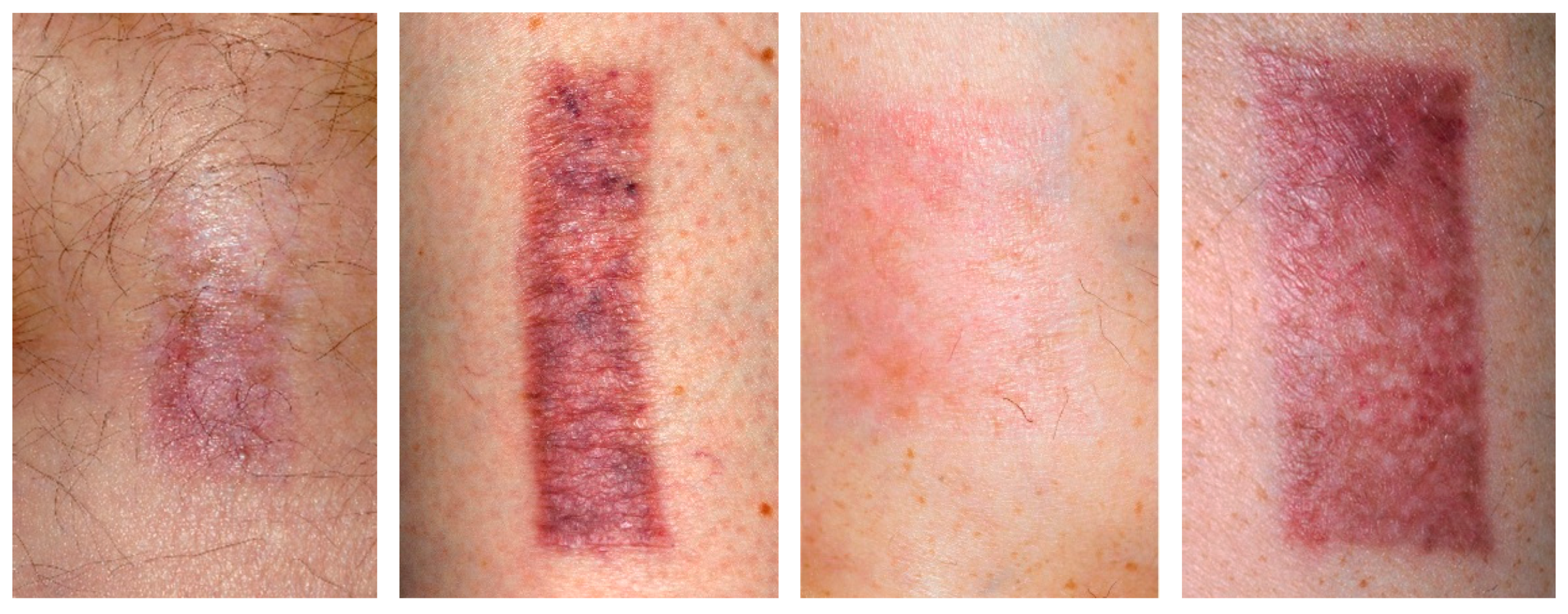
2.4. Scar Formation in Progress (3 Months)
2.5. Matured Scars (12 Months)
2.6. Discussing Triterpenes as the Active Ingredient of TBG
2.7. Discussing TBG Subject to Wound Dressing Procedure
3. Material and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Metelmann, H.-R.; Brandner, J.M.; Schumann, H.; Bross, F.; Fimmers, R.; Böttger, K.; Scheffler, A.; Podmelle, F. Accelerated Reepithelialization by Triterpenes: Proof of Concept in the Healing of Surgical Skin Lesions. Skin Pharmacol. Physiol. 2015, 28. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.-R.; Brandner, J.M.; Schumann, H.; Brossd, F.; Hoffmannd, M.; Podmelle, F. Accelerating the aesthetic benefit of wound healing by triterpene. J. Craniomaxillofac. Surg. 2012, 40, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Ekman, R. The suberin monomers and triterpenoids from the outer bark of Betula verruosa ehrh. Holzforschung 1983, 37, 205–211. [Google Scholar] [CrossRef]
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C.M. Physical, chemical and pharmacological characterization of a new oleogel-forming triterpene extract from the outer bark of birch (Betulae cortex). Planta Med. 2006, 72, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Kandefer-Szerszen, M. Protective effects of betulin and betulinic acid against ethanol-induced cytotoxicity in HepG2 cells. Pharmacol. Rep. 2005, 57, 588–595. [Google Scholar] [PubMed]
- Suksamrarn, S.; Panseeta, P.; Kunchanawatta, S.; Distaporn, T.; Ruktasing, S.; Suksamrarn, A. Ceanothane- and lupane-type triterpenes with antiplasmodial and antimycobacterial activities from Ziziphus cambodiana. Chem. Pharm. Bull. 2006, 54, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Eiznhamer, D.A.; Xu, Z.Q. Betulinic acid: A promising anticancer candidate. IDrugs 2004, 7, 359–373. [Google Scholar] [PubMed]
- Liu, J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Alakurtti, S.; Makela, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29. [Google Scholar] [CrossRef] [PubMed]
- Manez, S.; Recio, M.C.; Giner, R.M.; Rios, J.L. Effect of selected triterpenoids on chronic dermal inflammation. Eur. J. Pharmacol. 1997, 334, 103–105. [Google Scholar] [CrossRef]
- Geetha, T.; Varalakshmi, P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J. Ethnopharmacol. 2001, 76, 77–80. [Google Scholar] [CrossRef]
- De la Puerta, R.; Martinez-Dominguez, E.; Ruiz-Gutierrez, V. Effect of minor components of virgin olive oil on topical antiinflammatory assays. Z. Naturforsch. 2000, 55, 814–819. [Google Scholar]
- Metelmann, H.R. Topisches Betulin-Gel zum beschleunigten Wundverschluss bei plastischen Operationen. Z. Phytother. 2016, 37, 54–58. [Google Scholar] [CrossRef]
- Harish, B.G. Wound healing activity and docking of glycogensynthase-kinase-3-β-protein with isolated triterpenoid lupeol in rats. Phytomedicine 2008, 15, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, S.; Laszczyk, M.N.; Scheffler, A. A preliminary pharmacokinetic study of betulin, the main pentacyclic triterpene from extract of outer bark of birch (Betulae alba cortex). Molecules 2008, 13, 3224–3235. [Google Scholar] [CrossRef] [PubMed]
- Cichewicz, R.H.; Kouzi, S.A. Chemistry, Biological Activity, and Chemotherapeutic Potential of Betulinic Acid for the Prevention and Treatment of Cancer and HIV Infections. Med. Res. Rev. 2004, 24, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, L.; Rodriguez, J.A.; Schmeda-Hirschmann, G. Gastroprotective activity of oleanolic acid derivatives on experimentally induced gastric lesions in rats and mice. J. Pharm. Pharmacol. 2002, 54, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Singh, G.B.; Singh, S.; Bani, S.; Gupta, B.D.; Banerjee, S.K. Anti-inflammatory activity of oleanolic acid in rats and mice. J. Pharm. Pharmacol. 1992, 44, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Udeani, G.O.; Zhao, G.M.; Shin, Y.G.; Cooke, B.P.; Graham, J.; Beecher, C.W.W.; Kinghorn, A.D.; Pezzuto, J.M. Pharmacokinetics and tissue distribution of betulinic acid in CD-1 mice. Biopharm. Drug Dispos. 1999, 20, 379–383. [Google Scholar] [CrossRef]
- Jäger, S.; Winkler, K.; Pfüller, U.; Scheffler, A. Solubility Studies of Oleanolic Acid and Betulinic Acid in Aqueous Solutions and Plant extracts of Viscum album L. Planta Med. 2007, 73, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.K.; Lee, H.S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Dispos. 2007, 28, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Strickley, R.G. Solubilizing excipients in oral and injectable formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, U.; Laszczyk, M.N.; Kraus, M.; Leuner, K.; Kersten, A.; Simon-Haarhaus, B.; Scheffler, A.; Martin, S.F.; Müller, W.E.; Nashan, D.; et al. Triterpenes promote keratinocyte differentation in vitro, ex vivo and in vivo. J. Investig. Dermatol. 2010, 130, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Huyke, C.; Laszczyk, M.; Scheffler, A.; Ernst, R.; Schempp, C.M. Treatment of actinic keratoses with birch bark extract: A pilot study. J. Dtsch. Dermatol. Ges. 2006, 4, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Huyke, C.; Reuter, J.; Rodig, M.; Kersten, A.; Laszczyk, M.; Scheffler, A.; Nashan, D.; Schempp, C. Treatment of actinic keratoses with a novel betulin-based oleogel. A prospective, randomized, comparative pilot study. J. Dtsch. Dermatol. Ges. 2008, 7, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.-R.; Podmelle, F.; Waite, P.D. Long-term cosmetic benefit of wound healing by betuline. Am. J. Cosm. Surg. 2012, 29, 19–24. [Google Scholar] [CrossRef]
- Debats, I.B.; Koeneman, M.M.; Booi, D.I.; Bekers, O.; van der Hulst, R.R. Intravenous arginine and human skin graft donor site healing: A randomized controlled trial. Burns 2011, 37, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, D.A.; Barrow, R.E.; Rutan, R.L.; Broemeling, L.; Herndon, D.N. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Ann. Surg. 1994, 220, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ottomann, C.; Hartmann, B.; Tyler, J.; Maier, H.; Thiele, R.; Schaden, W.; Stojadinovic, A. Prospective randomized trial of accelerated reepithelization of skin graft donor sites using extracorporeal shock wave therapy. J. Am. Coll. Surg. 2010, 211, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Ottomann, C.; Stojadinovic, A.; Lavin, P.T.; Gannon, F.H.; Heggeness, M.H.; Thiele, R.; Schaden, W.; Hartmann, B. Prospective randomized phase II trial of accelerated reepithelialization of superficial second degree burn wounds using extracorporeal shock wave therapy. Ann. Surg. 2012, 255, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.-R.; Vu, T.T.; Le, T.N.B.; Hoang, T.H.A.; Phi, T.T.T.; Luong, T.M.I.; Doan, V.T.; Nguyen, T.T.H.; Nguyen, T.L.; L, D.Q.; et al. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment. Clin. Plasma Med. 2013, 1, 30–35. [Google Scholar] [CrossRef]
- Barna, M.; Kucera, A.; Hladicova, M.; Kucera, M. Wound healing effects of a Symphytum herb extract cream (Symphytum x uplandicum Nyman): Results of a randomized, controlled double-blind study. Wien Med. Wochenschr. 2007, 157, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Jull, A.B.; Rodgers, A.; Walker, N. Honey as a topical treatment for wounds. Cochrane Database Syst. Rev. 2008, 8. [Google Scholar]
- Dat, A.D.; Poon, F.; Pham, K.B.; Doust, J. Aloe vera for treating acute and chronic wounds. Cochrane Database Syst. Rev. 2012, 2. [Google Scholar] [CrossRef]
- Metelmann, H.R.; Mueller-Debus, C.; Podmelle, F. Imlan® Creme Pur in der Hautregeneration nach Laserskin Resurfacing. Kosm. Med. 2010, 6, 30–36. [Google Scholar]
- Barret, J.P.; Podmelle, F.; Lipový, B.; Rennekampf, H.-O.; Schumann, H.; Schwieger-Briel, A.; Zahn, T.; Metelmann, H.-R. Accelerated wound closure of split-thickness skin graft donor sites by a topical betulin gel: Results of two randomized, controlled, open-label phase III clinical trials. Medicine 2016. under revision. [Google Scholar]
- Likert, R. A Technique for the Measurement of Attitudes; The Science Press: New York, NY, USA, 1932. [Google Scholar]
- Metelmann, B.; Metelmann, C. M-Health in Prehospital Emergency Medicine: Experiences from the EU funded Project LiveCity. In M-Health Innovations for Patient-Centered Care; Moumtzoglou, A., Ed.; Medical Information Science Reference: Hershey, PA, USA, 2016; pp. 197–212. [Google Scholar]
- Otranto, M.; do Nascimento, A.P.; Monte-Alto-Costa, A. Effects of supplementation with different edible oils on cutaneous wound healing. Wound Repair Regen. 2010, 18, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.L.; Cardoso, C.C.; Caputo, L.R.; Carvalho, J.C.T.; Fiorini, J.E.; Schneedorf, J.M. Cicatrizing and antimicrobial properties of an ozonised oil from sunflower seeds. Inflammopharmacology 2004, 12, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, S.; Naumann, K.; Pollok, S.; Wardecki, T.; Vidal-y-Sy, S.; Nascimento, J.M.; Boerries, M.; Schmidt, G.; Brandner, J.M.; Merfort, I. From a traditional medicinal plant to a rational drug: understanding the clinically proven wound healing efficacy of birch bark extract. PLoS ONE 2014, 9, e86147. [Google Scholar] [CrossRef] [PubMed]
- Bogle, M.A.; Yadav, G.; Arndt, K.A.; Dover, J.S. Wrinkles and Acne Scars: Ablative and Nonablative Facial Resurfacing. In Laser and IPL Technology in Dermatology and Aesthetic Medicine; Raulin, C., Karsai, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 289–297. [Google Scholar]
- Ortiz, A.E.; Tingey, C.; Yu, Y.E.; Ross, E.V. Topical steroids implicated in postoperative infection following ablative laser resurfacing. Lasers Surg. Med. 2012, 44. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, Z.; Vinciullo, C.; Elliott, T. Antibiotic prophylaxis for full-face laser resurfacing: Is it necessary? Arch. Dermatol. 2001, 137, 313–315. [Google Scholar] [PubMed]
- Walia, S.; Alster, T.S. Cutaneous CO2 laser resurfacing infection rate with and without prophylactic antibiotics. Dermatol. Surg. 1999, 25, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Madden, M.R.; Nolan, E.; Finkelstein, J.L.; Yurt, R.V.; Smeland, J.; Goodwin, C.W.; Hefton, J.; Staiano-Coico, L. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J. Trauma 1989, 29, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Collawn, S.S. Occlusion following laser resurfacing promotes reepithelialization and wound healing. Plast. Reconstr. Surg. 2000, 105, 2180–2189. [Google Scholar] [CrossRef] [PubMed]
- Collawn, S.S. Re-epithelialization of the skin following CO2 laser resurfacing. J. Cosmet. Laser Ther. 2001, 3, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Kannon, G.A.; Garrett, A.B. Moist wound healing with occlusive dressings. A clinical review. Dermatol. Surg. 1995, 21, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Innes, M.E.; Umraw, N.; Fish, J.S.; Gomez, M.; Cartotto, R.C. The use of silver coated dressings on donor site wounds: A prospective, controlled matched pair study. Burns 2001, 27, 621–627. [Google Scholar] [CrossRef]
- Markl, P.; Prantl, L.; Schreml, S.; Babilas, P.; Landthaler, M.; Schwarze, H. Management of split-thickness donor sites with synthetic wound dressings. Ann. Plast. Surg. 2010, 65, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.S.; Mackie, I.P.; Greenwood, J.E. A randomised prospective study of split skin graft donor site dressings: AWBAT-D vs. Duoderm®. Burns 2012, 38, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Voineskos, S.H.; Ayeni, O.A.; McKnight, L. Systematic review of skin graft donor-site dressings. Plast. Reconstr. Surg. 2009, 124, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of TBG are commercially available as Episalvan®.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kindler, S.; Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; Müller-Debus, C.; Metelmann, H.-R.; et al. Triterpenes for Well-Balanced Scar Formation in Superficial Wounds. Molecules 2016, 21, 1129. https://doi.org/10.3390/molecules21091129
Kindler S, Schuster M, Seebauer C, Rutkowski R, Hauschild A, Podmelle F, Metelmann C, Metelmann B, Müller-Debus C, Metelmann H-R, et al. Triterpenes for Well-Balanced Scar Formation in Superficial Wounds. Molecules. 2016; 21(9):1129. https://doi.org/10.3390/molecules21091129
Chicago/Turabian StyleKindler, Stefan, Matthias Schuster, Christian Seebauer, Rico Rutkowski, Anna Hauschild, Fred Podmelle, Camilla Metelmann, Bibiana Metelmann, Charlotte Müller-Debus, Hans-Robert Metelmann, and et al. 2016. "Triterpenes for Well-Balanced Scar Formation in Superficial Wounds" Molecules 21, no. 9: 1129. https://doi.org/10.3390/molecules21091129
APA StyleKindler, S., Schuster, M., Seebauer, C., Rutkowski, R., Hauschild, A., Podmelle, F., Metelmann, C., Metelmann, B., Müller-Debus, C., Metelmann, H.-R., & Metelmann, I. (2016). Triterpenes for Well-Balanced Scar Formation in Superficial Wounds. Molecules, 21(9), 1129. https://doi.org/10.3390/molecules21091129





