Three Novel Triterpenoids from Taraxacum officinale Roots
Abstract
:1. Introduction
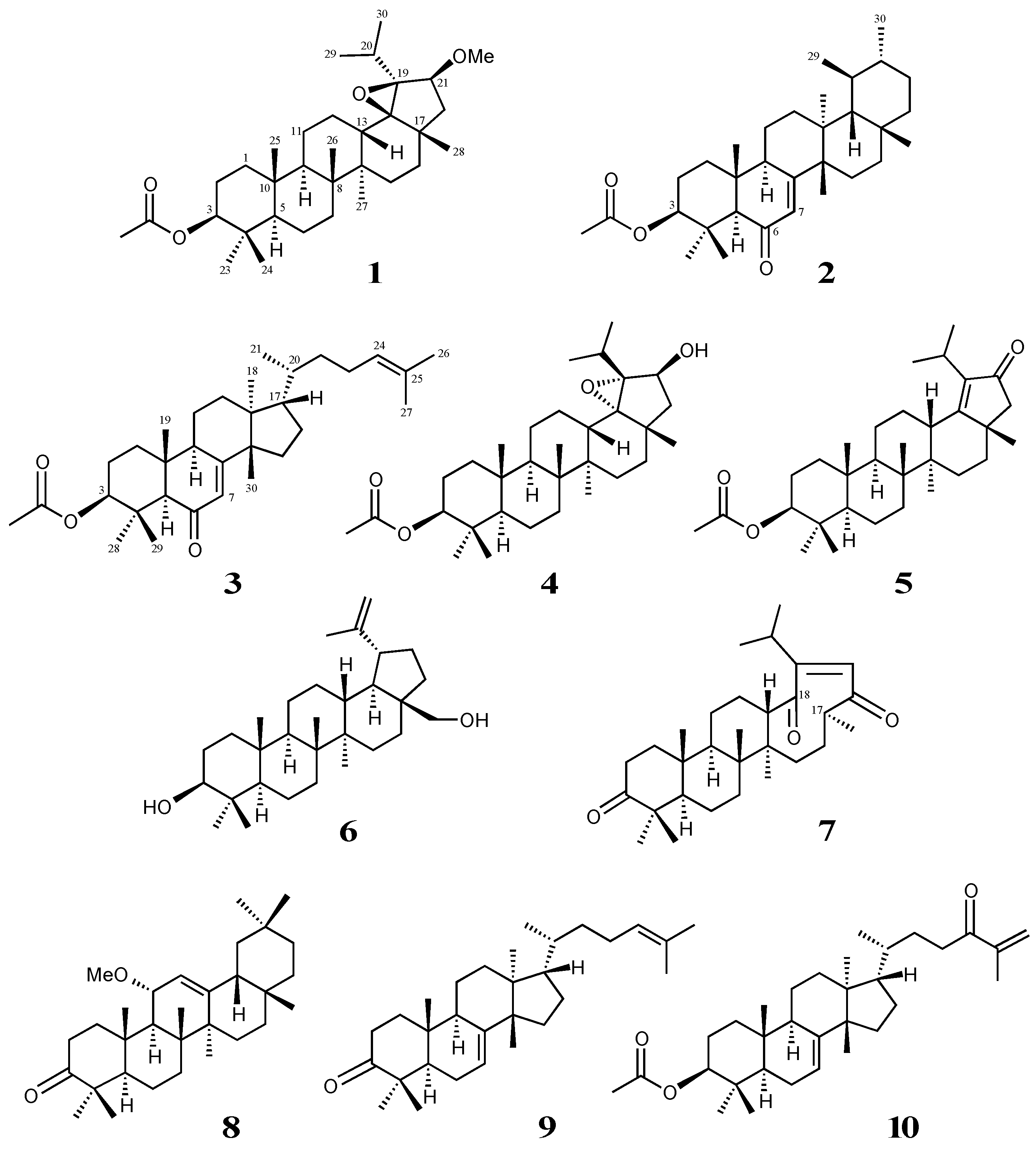
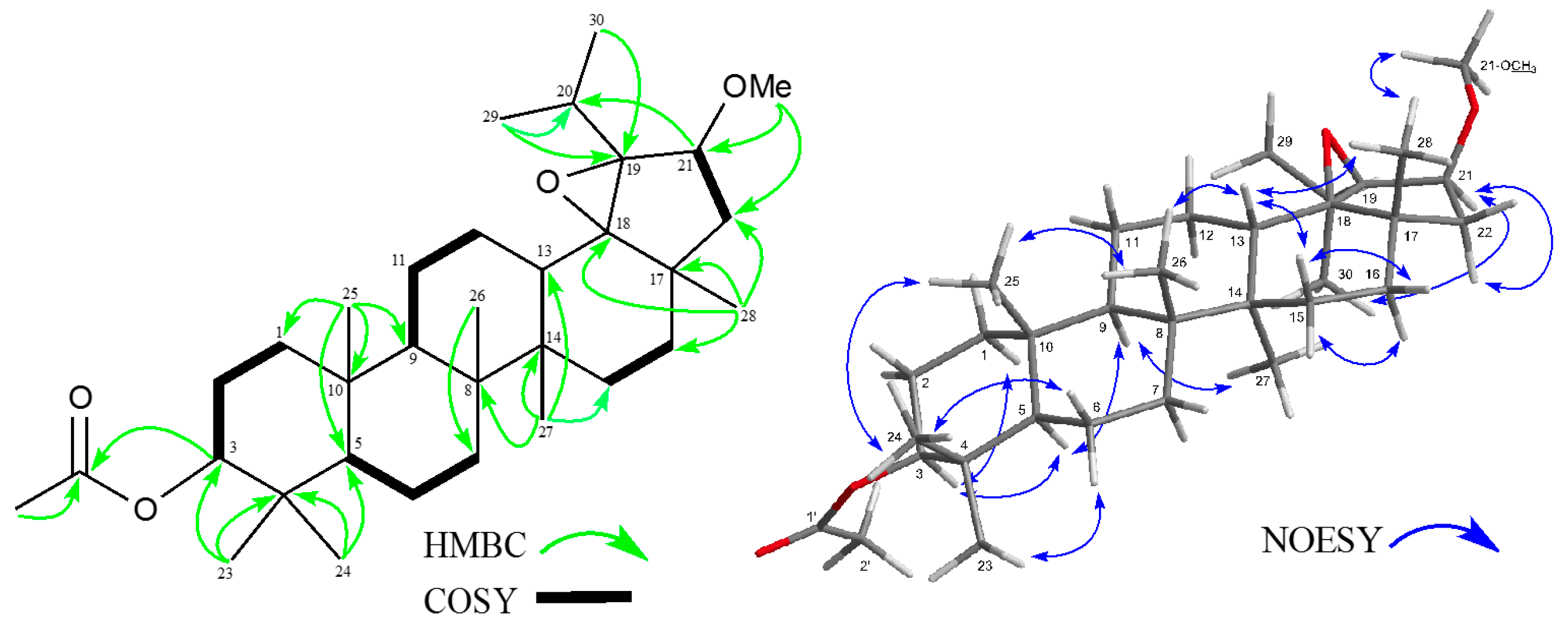
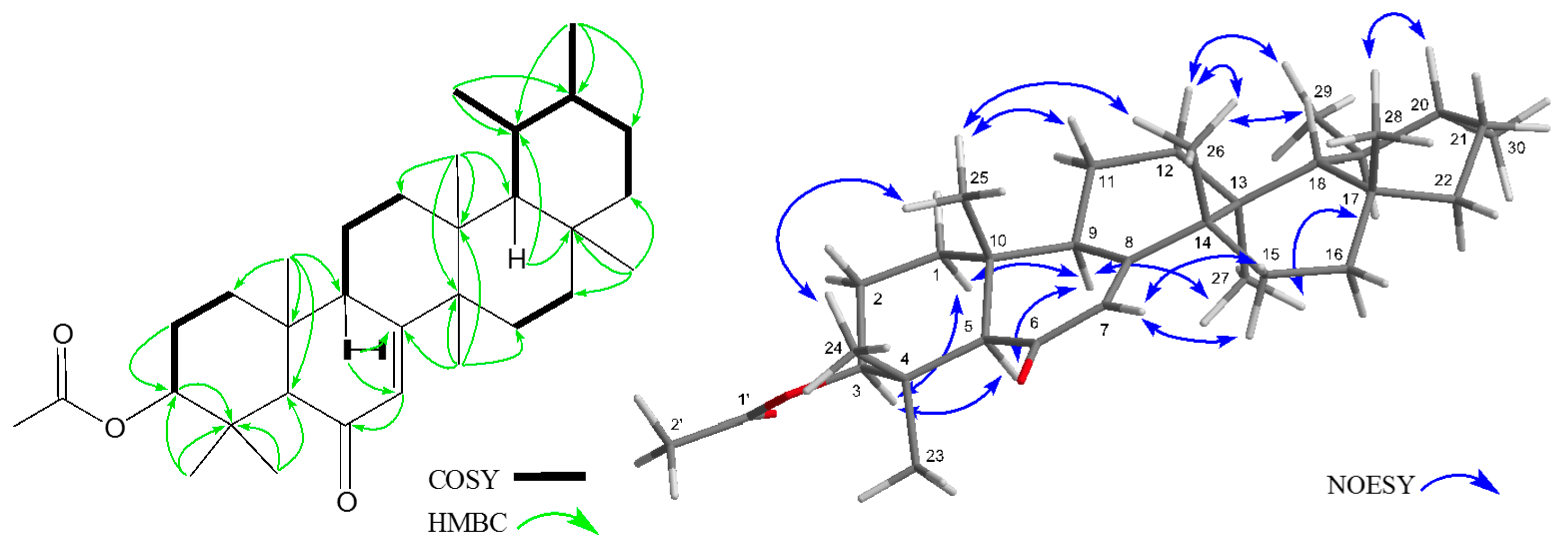
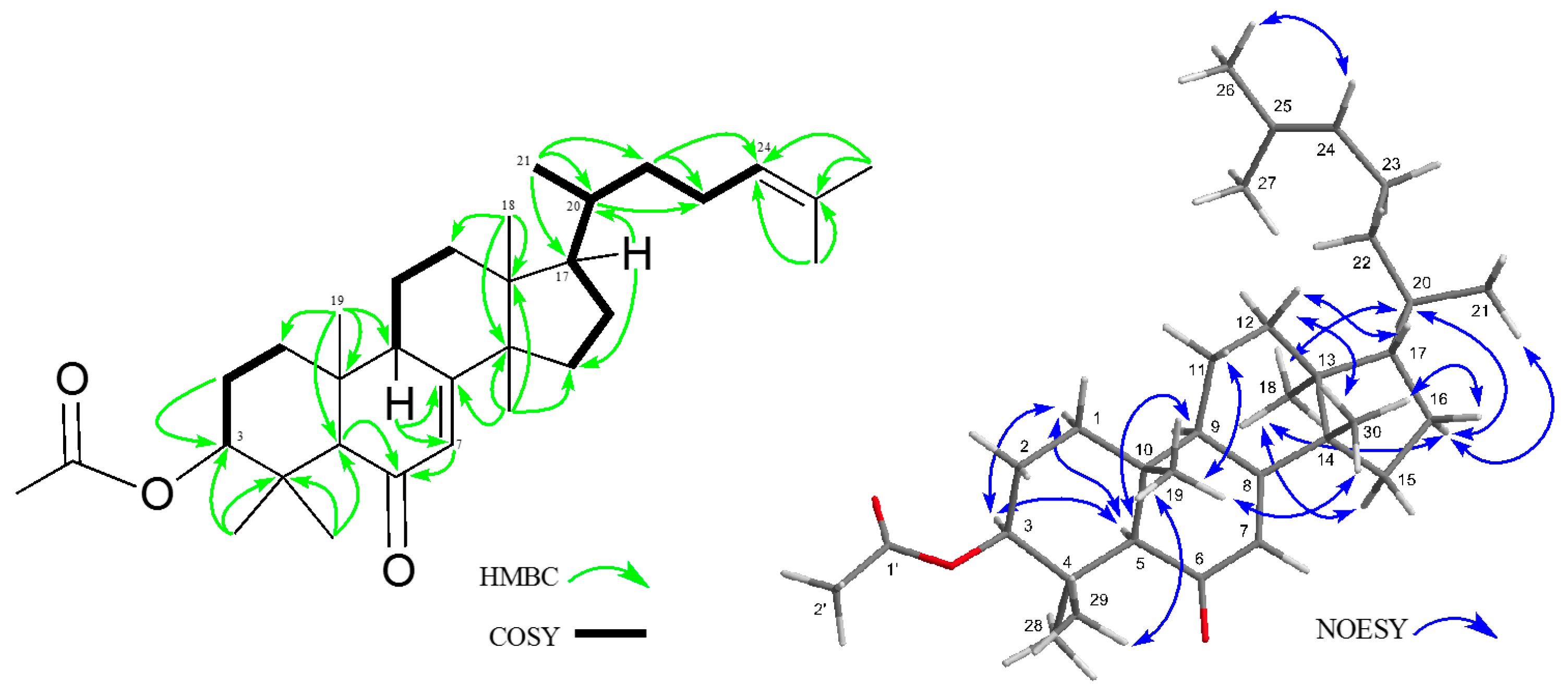
2. Results and Discussion
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Isolation of Compounds 1–3
3.4. Analytical Data
3.5. Determination of RAW264.7 Cell Proliferation
3.6. Inhibitory Assay of NO Production
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, A.; Malhotra, S.; Subban, R. Dandelion (Taraxacum officinale)-hepatoprotective herb with therapeutic potential. Pharmacogn. Rev. 2008, 2, 163–167. [Google Scholar]
- Nowak, G.; Panak, H. Forms of potassium and sodium in some species of grasses and herbage. Acta Agrobot. 1985, 35, 87–99. [Google Scholar] [CrossRef]
- Gonzalez-Castejon, M.; Garcia-Carrasco, B.; Fernandez-Dacosta, R.; Davalos, A.; Rodriguez-Casado, A. Reduction of adipogenesis and lipid accumulation by Taraxacum officinale (dandelion) extracts in 3T3L1 adipocytes: An in vivo study. Phytother. Res. 2014, 28, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y. Chinese Medicinal Capsule and Its Preparation Method. CN Patent 1660189 A 20050831, 2005. [Google Scholar]
- Saeki, D.; Yamada, T.; In, Y.; Kajimoto, T.; Tanaka, R.; Iizuka, Y.; Nakane, T.; Takano, A.; Masuda, K. Officinatrione: An unusual (17S)-17,18-seco-lupane skeleton, and four novel lupane-type triterpenoids from the roots of Taraxacum officinale. Tetrahedron 2013, 69, 1583–1589. [Google Scholar] [CrossRef]
- Matsunaga, S.; Morita, R. Hopenol-b, a triterpene alcohol from Euphorbia supina. Phytochemistry 1983, 22, 605–606. [Google Scholar] [CrossRef]
- Chakravarty, A.K.; Das, B.; Masuda, K.; Arai, Y.; Shiojima, K. Peracid induced oxidative rearrangements of triterpenoids: Products of new skeleton from bauerenyl acetate. Tetrahedron 1998, 54, 6065–6078. [Google Scholar] [CrossRef]
- Cerda-Garcia-Rojas, C.M.; Hernandez-Vidal, H.H.; Joseph-Nathan, P. 13C-NMR assignments of D:C-friedours-7-ene derivatives. Evidence of an abnormal methyl group chemical shift. Magn. Reson. Chem. 1996, 34, 777–781. [Google Scholar] [CrossRef]
- Fukuoka, M.; Natori, S. Oxidation of bauerenol derivatives with chromium trioxide. Confirmation of the structure of bauerenol. Chem. Pharm. Bull. 1972, 20, 974–979. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Fang, S.-C.; Huang, H.-W.; Yen, G.-C. Anti-inflammatory effects of triterpenes and steroid compounds isolated from the stem bark of Hiptage benghalensis. J. Funct. Foods 2015, 12, 420–427. [Google Scholar] [CrossRef]
- Pettit, G.R.; Numata, A.; Iwamoto, C.; Morito, H.; Yamada, T.; Goswami, A.; Clewlow, P.J.; Cragg, G.M.; Schmidt, J.M. Antineoplastic agents. 489. Isolation and structures of meliastatins 1-5 and related euphane triterpenes from the tree Melia dubia. J. Nat. Prod. 2002, 65, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- Zare, S.; Ghaedi, M.; Miri, R.; Heiling, S.; Asadollahi, M.; Baldwin, I.T.; Jassbi, A.R. Phytochemical investigation on Euphorbia macrostegia (Persian wood spurge). Iran. J. Pharm. Res. 2015, 14, 243–249. [Google Scholar] [PubMed]
- Wang, L.-Y.; Wang, N.-L.; Yao, X.-S.; Miyata, S.; Kitanaka, S. Euphane and tirucallane triterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of xenopus. J. Nat. Prod. 2003, 66, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Sakano, Y.; Tanaka, H.; Lou, W.; Noguchi, H.; Shibuya, M.; Ebizuka, Y. Enzymatic cyclization of 22,23-dihydro-2,3-oxidosqualene into euph-7-en-3β-ol and bacchar-12-en-3β-ol by recombinant β-amyrin synthase. J. Am. Chem. Soc. 2004, 126, 3426–3427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Dong, B.; Ma, X.; Hou, L.; Cao, X.; Wang, C. Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages. Inflammation 2015, 38, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Kolhe, J.N.; Bhaskar, A.; Bringi, N.V. Occurrence of 3-oxo triterpenes in the unsaponifiable matter of some vegetable fats. Lipids 1982, 17, 166–168. [Google Scholar] [CrossRef]
- Warashina, T.; Umehara, K.; Miyase, T. Constituents from the roots of Taraxacum platycarpum and their effect on proliferation of human skin fibroblasts. Chem. Pharm. Bull. 2012, 60, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Rojo de Almeida, M.T.; Rios-Luci, C.; Padron, J.M.; Palermo, J.A. Antiproliferative terpenoids and alkaloids from the roots of Maytenus vitis-idaea and Maytenus spinosa. Phytochemistry 2010, 71, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.-T.; Li, F.; Chen, B.; Wang, M.-K. Euphane triterpenes from the bark of Broussonetia papyrifera. Helv. Chim. Acta 2011, 94, 2061–2065. [Google Scholar] [CrossRef]
- Siddiqui, S.; Hafeez, F.; Begum, S.; Siddiqui, B.S. Oleanderol, a new pentacyclic triterpene from the leaves of Nerium oleander. J. Nat. Prod. 1988, 51, 229–233. [Google Scholar] [CrossRef]
- Yamada, T.; Muroga, Y.; Jinno, M.; Kajimoto, T.; Usami, Y.; Numata, A.; Tanaka, R. New class azaphilone produced by a marine fish-derived Chaetomium globosum. The stereochemistry and biological activities. Bioorg. Med. Chem. 2011, 19, 4106–4113. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Kikuchi, T.; Inoue, T.; Muraoka, O.; Yamada, T.; Tanaka, R. Carapanolides J-L from the seeds of Carapa guianensis (andiroba) and their effects on LPS-activated NO production. Molecules 2014, 19, 17130–17140. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available.
| Position | 1 | 2 | 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1H (J, Hz) | 13C | 1H (J, Hz) | 13C | 1H (J, Hz) | 13C | |||||||
| 1α | 1.02 | m | 38.3 | t | 1.46 | m | 36.3 | t | 1.49 | m | 36.6 | t |
| 1β | 1.68 | m | 1.68 | m | 1.70 | m | ||||||
| 2 | 1.64 | m (2H) | 23.7 | t | 1.65 | m (2H) | 23.3 | t | 1.65 | m (2H) | 23.3 | t |
| 3 | 4.47 | dd (5.3, 11.4) | 80.9 | d | 4.47 | dd (4.1, 11.7) | 80.6 | d | 4.47 | dd (4.1, 11.7) | 80.6 | d |
| 4 | 37.8 | s | 36.9 | s | 37.0 | s | ||||||
| 5 | 0.81 | m | 55.3 | d | 2.20 | s | 65.1 | d | 2.20 | s | 65.2 | d |
| 6α | 1.54 | m | 18.2 | t | 199.8 | s | 199.4 | s | ||||
| 6β | 1.41 | m | ||||||||||
| 7 | 1.41 | m (2H) | 33.79 | t | 5.84 | d (3.0) | 123.9 | d | 5.69 | d (3.0) | 124.8 | d |
| 8 | 41.0 | s | 170.3 | s | 170.8 | s | ||||||
| 9 | 1.38 | dd (4.2, 12.0) | 49.8 | d | 2.73 | ddd (3.0, 6.8, 12.6) | 49.7 | d | 2.72 | ddd (3.0, 6.2, 13.2) | 50.3 | d |
| 10 | 37.0 | s | 43.5 | s | 43.6 | s | ||||||
| 11 | 1.51 | m | 20.55 | t | 1.74 | m | 16.3 | t | 1.72 | m | 17.6 | t |
| 1.55 | m | 1.56 | m | |||||||||
| 12 | 1.44 | m | 22.9 | t | 1.70 | m | 31.6 | t | 1.78 | m | 32.7 | t |
| 1.55 | m | 1.87 | m | |||||||||
| 13 | 2.48 | dd (4.1, 11.8) | 33.75 | d | 37.3 | s | 43.0 | s | ||||
| 14 | 44.4 | s | 42.8 | s | 52.4 | s | ||||||
| 15α | 1.10 | m | 26.6 | t | 1.46 | m | 28.07 | t | 1.56 | m | 32.8 | t |
| 15β | 1.79 | td (4.1, 13.5) | 1.57 | m | 1.50 | m | ||||||
| 16α | 1.59 | m | 31.5 | t | 1.54 | m | 37.2 | t | 1.35 | m | 27.9 | t |
| 16β | 1.40 | m | 1.27 | m | 1.99 | m | ||||||
| 17 | 41.2 | s | 32.0 | s | 1.56 | m | 52.7 | d | ||||
| 18 | 77.0 | s | 1.33 | brs | 54.7 | d | 0.82 | s | 22.0 | q | ||
| 19 | 71.9 | s | 1.17 | m | 35.4 | d | 0.88 | s | 14.3 | q | ||
| 20 | 1.91 | m | 29.4 | d | 1.58 | m | 31.9 | d | 1.43 | m | 35.5 | d |
| 21 | 3.77 | d (5.9) | 83.2 | d | A 1.20 | m | 29.1 | t | 0.87 | d (6.5) | 18.4 | q |
| B 1.59 | m | |||||||||||
| 22 | α 1.18 | dd (6.4, 13.5) | 39.3 | t | A 1.19 | m | 31.2 | t | A 1.01 | m | 35.1 | t |
| 22 | β 1.32 | m | B 1.59 | m | B 1.57 | m | ||||||
| 23 | 0.85 | s | 27.9 | q | 1.20 | s | 28.11 | q | A 1.88 | m | 25.3 | t |
| B 2.05 | m | |||||||||||
| 24 | 0.84 | s | 16.5 | q | 1.19 | s | 15.9 | q | 5.09 | tt (1.5, 14.1) | 124.8 | d |
| 25 | 0.88 | s | 16.2 | q | 0.88 | s | 14.2 | q | 131.2 | s | ||
| 26 | 1.075 | s | 16.0 | q | 1.06 | s | 21.3 | q | 1.69 | s | 25.7 | q |
| 27 | 1.05 | s | 14.7 | q | 0.96 | s | 22.7 | q | 1.61 | s | 17.7 | q |
| 28 | 1.083 | s | 23.4 | q | 1.06 | s | 37.8 | q | 1.20 | s | 16.0 | q |
| 29 | 1.11 | d (7.4) | 20.63 | q | 1.07 | d (8.0) | 25.6 | q | 1.20 | s | 28.2 | q |
| 30 | 1.11 | d (7.4) | 18.4 | q | 0.92 | d (5.9) | 22.5 | q | 1.05 | s | 24.9 | q |
| 21-OMe | 3.22 | s | 56.5 | q | ||||||||
| 1′ | 171.0 | s | 171.0 | s | 171.0 | s | ||||||
| 2′ | 2.04 | s | 21.3 | q | 2.07 | s | 21.2 | q | 2.07 | s | 21.2 | q |
| Produced NO % (Cell Viavility %) a | |||||
|---|---|---|---|---|---|
| 1 μM | 3 μM | 10 μM | 30 μM | IC50 (μM) | |
| 1 | 94.8 ± 1.2 | 101.4 ± 0.7 | 90.5 ± 2.4 | 95.7 ± 4.6 | >30 |
| (95.0 ± 0.7) | (104.2 ± 1.3) | (102.6 ± 0.7) | (107.4 ± 2.0) | ||
| 2 | 95.6 ± 2.7 | 100.7 ± 2.6 | 86.5 ± 1.4 ** | 71.4 ± 1.8 ** | >30 |
| (100.0 ± 1.3) | (102.2 ± 0.3) | (105.1 ± 0.3) | (105.7 ± 2.0) | ||
| 3 | 100.6 ± 2.6 | 93.7 ± 1.4 | 93.3 ± 1.6 | 93.1 ± 0.9 | >30 |
| (100.0 ± 1.4) | (94.6 ± 8.2) | (104.2 ± 0.7) | (103.8 ± 1.0) | ||
| 4 | 83.6 ± 0.9 ** | 76.8 ± 2.5 ** | 66.0 ± 0.8 ** | 35.4 ± 2.2 ** | 16.9 |
| (98.4 ± 0.9) | (97.9 ± 0.3) | (92.7 ± 1.0) | (49.5 ± 0.7) | ||
| 5 | 110.3 ± 5.2 | 106.1 ± 3.2 | 94.9 ± 4.3 | 92.7 ± 4.7 | >30 |
| (98.4 ± 3.1) | (94.8 ± 3.9) | (90.7 ± 1.8) | (88.9 ± 2.3) | ||
| 6 | 83.4 ± 1.5 ** | 75.0 ± 3.2 ** | 65.4 ± 0.5 ** | 36.3 ± 0.6 ** | 17.0 |
| (99.8 ± 0.1) | (93.7 ± 1.1) | (76.7 ± 0.7) | (66.7 ± 1.2) | ||
| 7 | 101.0 ± 1.0 | 101.0 ± 2.5 | 89.8 ± 1.1 ** | 66.8 ± 0.8 ** | >30 |
| (98.3 ± 2.6) | (103.0 ± 2.4) | (99.7 ± 3.8) | (99.8 ± 3.0) | ||
| 8 | 102.9 ± 0.7 | 105.0 ± 3.1 | 90.2 ± 7.1 | 64.1 ± 3.3 ** | >30 |
| (99.9 ± 0.1) | (100.4 ± 0.1) | (100.5 ± 0.2) | (103.0 ± 1.6) | ||
| 9 | 98.4 ± 3.9 | 100.4 ± 1.6 | 109.1 ± 2.0 ** | 108.2 ± 4.7 * | >30 |
| (99.3 ± 0.2) | (98.8 ± 0.2) | (99.1 ± 0.4) | (99.9 ± 0.6) | ||
| 10 | 93.8 ± 1.8 | 81.6 ± 3.7 ** | 67.5 ± 2.4 ** | 37.0 ± 1.8 ** | 18.4 |
| (108.3 ± 4.7) | (100.4 ± 0.2) | (100.2 ± 0.1) | (2.6 ± 0.4) | ||
| l-NMMA b | 93.3 ± 2.2 | 91.4 ± 0.8 | 68.9 ± 4.5 ** | 43.1 ± 1.1 ** | 23.9 |
| (101.5 ± 0.9) | (101.9 ± 0.4) | (98.5 ± 0.9) | (109.4 ± 0.5) | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikuchi, T.; Tanaka, A.; Uriuda, M.; Yamada, T.; Tanaka, R. Three Novel Triterpenoids from Taraxacum officinale Roots. Molecules 2016, 21, 1121. https://doi.org/10.3390/molecules21091121
Kikuchi T, Tanaka A, Uriuda M, Yamada T, Tanaka R. Three Novel Triterpenoids from Taraxacum officinale Roots. Molecules. 2016; 21(9):1121. https://doi.org/10.3390/molecules21091121
Chicago/Turabian StyleKikuchi, Takashi, Ayaka Tanaka, Mayu Uriuda, Takeshi Yamada, and Reiko Tanaka. 2016. "Three Novel Triterpenoids from Taraxacum officinale Roots" Molecules 21, no. 9: 1121. https://doi.org/10.3390/molecules21091121






