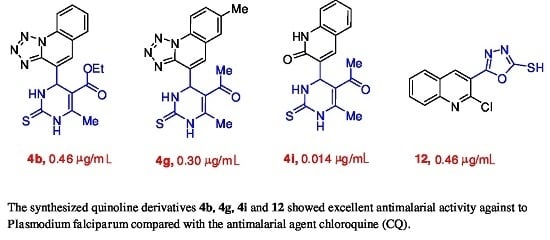
New Potential Antimalarial Agents: Design, Synthesis and Biological Evaluation of Some Novel Quinoline Derivatives as Antimalarial Agents
Abstract
:1. Introduction
2. Results
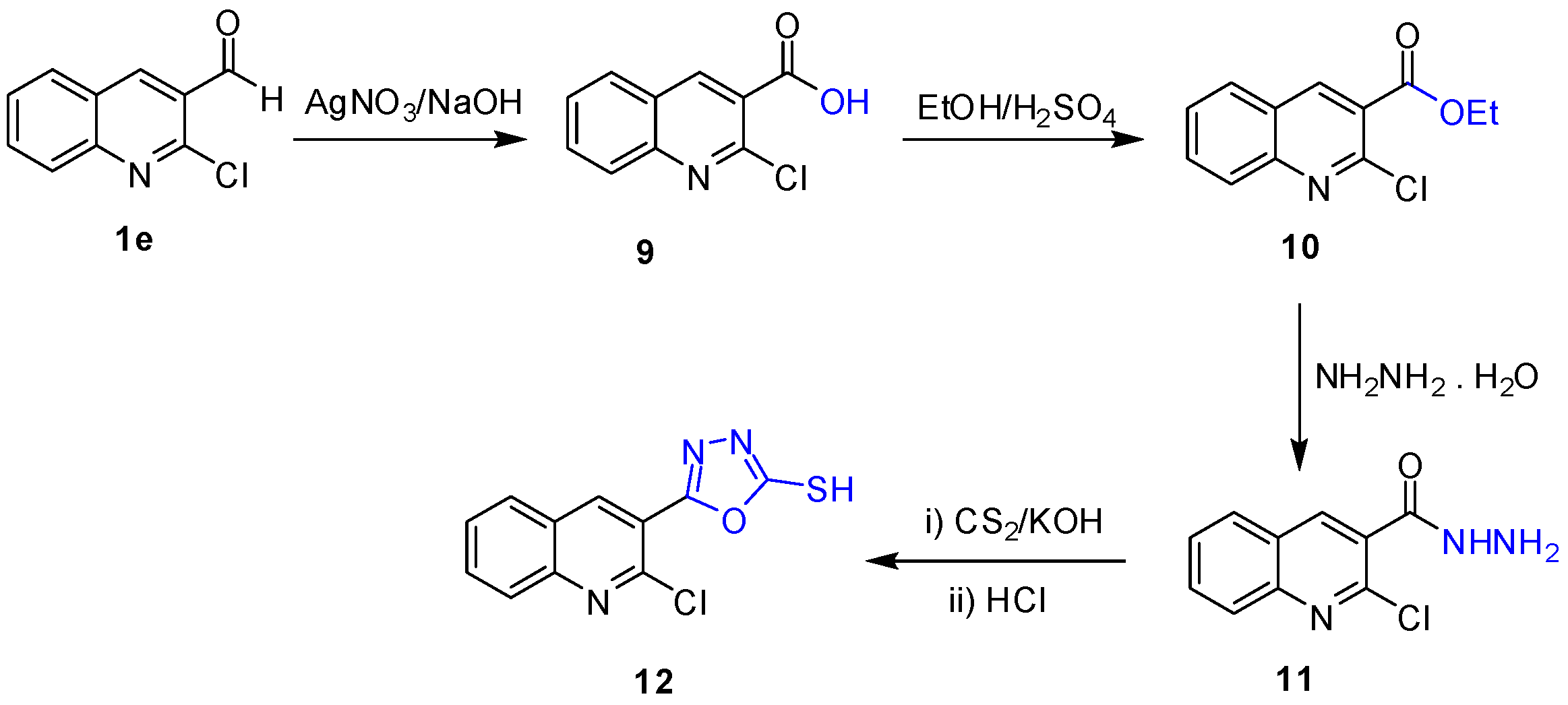
2.1. Chemistry
2.2. Antimalarial Evaluation
2.3. Structure Activity Relationship
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of Dihydropyrimidines (DHPMs) 4a–j
3.2.2. General Procedure for the Synthesis of Chalcones 6a,b
3.2.3. General Procedure for the Synthesis of 7a,b
3.3. Anti-Plasmodial Assay
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. WHO Expert Committee on Malaria: Twentieth Report, Technical Report 892. 2000. Available online: http://www.who.int/iris/handle/10665/42247#sthash.rpqlEh0j.dpuf (accessed on 12 July 2016).
- World Health Organization. World Malaria Report; WHO: Geneva, Switzerland, 2012; Available online: http://www.who.int/malaria/publications/world_malaria_report_2012/en/ (accessed on 12 July 2016).
- Marques, M.M.; Costa, M.R.F.; Santana Filho, F.S.; Vieira, J.L.F.; Nascimento, M.T.S.; Brasil, L.W.; Nogueira, F.; Silveira, H.; Reyes-Lecca, R.B.; Monteiro, W.M.; et al. Plasmodium vivax Chloroquine Resistance and Anemia in the Western Brazilian Amazon. Antimicrob. Agents Chemother. 2013, 58, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.R.; Spangenberg, T.; Lacerda, M.V.G.; Wells, T.N.C. Malaria in South America: A drug discovery perspective. Malaria J. 2013, 12, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Gama, B.E.; Lacerda, M.V.G.; Daniel-Ribeiro, C.T.; Ferreira-da-Cruz, M.F.F. Chemoresistance of Plasmodium falciparum and Plasmodium vivax parasites in Brazil: Consequences on disease morbidity and control. Mem. Inst. Oswaldo Cruz. 2011, 106, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.D.; Diggs, C.L.; Hines, F.A.; Desjardins, R.E. Culture of human malaria parasites Plasmodium falciparum. Nature 1976, 263, 767–769. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Antimalarial drug resistance. J. Clin. Investig. 2004, 113, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Widyawaruyanti, A.; Devi, A.P.; Fatria1, N.; Tumewu, L.; Tantular, I.S.; Hafid, A.F. In vitro antimalarial activity screening of several Indonesian plants using hrp2 assay. Int. J. Pharm. Pharm. Sci. 2014, 6, 125–128. [Google Scholar]
- Wells, C.D.; Cegielski, J.P.; Nelson, L.J.; Laserson, K.F.; Holtz, T.H.; Finlay, A.; Castro, K.G.; Weyer, K. HIV infection and multidrug-resistant tuberculosis-the perfect storm. J. Infect. Dis. 2007, 196, S68–S107. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.W.; Alam, M.J.; Rashid, M.A.; Chowdhury, R. A new structural alternative in benzo[b]furans for antimicrobial activity. Bioorg. Med. Chem. 2005, 13, 4796–4805. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Khidre, R.E.; Farahat, A.A.; El-Ahl, A.A.S. 2-Chloroquinoline-3-carbaldehydes: Synthesis, reactions and applications. Arkivoc 2012, I, 211–276. [Google Scholar] [CrossRef]
- Dominguez, J.; Basante, W.; Charris, J.; Riggione, F. Synthesis and activity of some quinolone derivatives against Plasmodium falciparum in vitro. Fermaco 1996, 51, 407–412. [Google Scholar]
- Ali, M.M.; Rajanna, K.C.; Prakash, P.K.S. An Efficient and Facile Synthesis of 2-Chloro-3-formyl Quinolines from Acetanilides in Micellar Media by Vilsmeier-Haack Cyclisation. Synlett 2001, 2, 251–253. [Google Scholar]
- Pokalwar, R.U.; Hangarge, R.V.; Maske, P.V.; Shingare, M.S. Synthesis and antibacterial activities of α-hydroxyphosphonates and α-acetyloxyphosphonates derived from 2-chloroquinoline-3-carbaldehyde. Arkivoc 2006, 11, 196–204. [Google Scholar]
- Khidre, R.E.; Abu-Hashem, A.A.; El-Shazly, M. Synthesis and anti-microbial activity of some 1-substituted amino-4,6-dimethyl-2-oxo-pyridine-3-carbonitrile derivatives. Eur. J. Med. Chem. 2011, 46, 5057–5064. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Jain, M.; Reddy, R.P.; Jain, R. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010, 45, 3245–3264. [Google Scholar] [CrossRef] [PubMed]
- Abdou, W.M.; Kamel, A.A.; Khidre, R.E.; Geronikaki, A.; Ekonomopoulou, M.T. Synthesis of 5- and 6-N-heterocyclic methylenebisphosphonate derivatives of cytogenetic activity in normal human lymphocyte cultures. Chem. Biol. Drug. Des. 2012, 79, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Abdou, W.M.; Shaddy, A.A.; Khidre, R.E.; Awad, G.E.A. Synthesis and Antimicrobial Evaluation of Newly Synthesized N, S-Bisphosphonate Derivatives. J. Heterocycl. Chem. 2015. [Google Scholar] [CrossRef]
- Tayebee, R.; Amini, M.M.; Ghadamgahi, M.; Armaghan, M. H5PW10V2O40/Pip-SBA-15: A novel reusable organic-inorganic hybrid material as potent Lewis acid catalyst for one-pot solvent-free synthesis of 3,4-dihydropyrimidinones. J. Mol. Catal. Chem. 2013, 366, 266–274. [Google Scholar]
- Biginelli, P. Aldehyde-urea derivatives of aceto-and oxaloacetic acids. Gazz. Chim. Ital. 1893, 23, 360–369. [Google Scholar]
- Hu, E.H.; Sidler, D.R.; Dolling, U.-H. Unprecedented catalytic three component one-pot condensation reaction: An efficient synthesis of 5-alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones. J. Org. Chem. 1998, 63, 3454–3457. [Google Scholar] [CrossRef]
- Abdou, W.M.; Khidre, R.E.; Kamel, A.A. Elaborating on Efficient Anti-Proliferation Agents of Cancer Cells and Anti-Inflammatory-Based N-Bisphosphonic Acids. Arch. Pharm. Chem. Life Sci. 2012, 345, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Meth-Cohn, O.; Narine, B.; Tarnowski, B. A versatile new synthesis of quinolines and related fused pyridines, Part 5. The synthesis of 2-chloroquinoline-3-carbaldehydes. J. Chem. Soc. Perkin Trans. 1 1981, 9, 1520–1530. [Google Scholar]
- Liu, Z.C.; Wang, B.D.; Li, B.; Wang, Q.; Yang, Z.Y.; Li, T.R.; Li, Y. Crystal structures, DNA-binding and cytotoxic activities studies of Cu(II) complexes with 2-oxo-quinoline-3-carbaldehyde Schiff-bases. Eur. J. Med. Chem. 2010, 45, 5353–5361. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kaur, R. Synthesis of fused quinazolinethiones and their S-alkyl/aryl derivatives. Arkivoc 2007, 15, 315–323. [Google Scholar]
- Abelman, M.M.; Smith, S.C.; James, D.R. Cyclic ketones and substituted α-keto acids as alternative substrates for novel Biginelli-like scaffold syntheses. Tetrahedron Lett. 2003, 44, 4559–4562. [Google Scholar] [CrossRef]
- Obaid, A.; Sandhya, B.; Suresh, K.; Rajiv, K.; Md Quamrul, H. Design, Synthesis Chalcones/Amines and Evaluation of Novel 2-piperidinyl Quinoline as Potential Antidepressant Agents Lett. Drug Des. Discov. 2013, 10, 75–85. [Google Scholar]
- Rao, K.R.; Bhanumathi, N.; Sattur, P.B. Synthesis of novel quino[2,3-b][1,5]benzodiazepin-12-ones. J. Heterocycl. Chem. 1991, 28, 1339–1340. [Google Scholar] [CrossRef]
- Silva, L.F.R.; Montoia, A.; Amorim, R.C.N.; Melo, M.R.; Henrique, M.C.; Nunomura, S.M.; Costa, M.R.F.; Andrade Neto, V.F.; Costa, D.S.; Dantas, G.; et al. Comparative in vitro and in vivo antimalarial activity of the indole alkaloids ellipticine, olivacine, cryptolepine and a synthetic cryptolepine analog. Phytomedicine 2012, 20, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Lentner, C.; Lentner, C.; Wink, A. Student’s t-distribution tables. In Geigy Scientific Tables Vol. 2. International Medical and Pharmaceutical Information; Ciba-Geigy Limited: Basel, Switzerland, 1982. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Sample Availability: Samples of the compounds 4, 7, 8, and 12 are available from the authors.


| Compound | R1 | R2 | X | Yield % | Compound | R1 | R2 | X | Yield % |
|---|---|---|---|---|---|---|---|---|---|
| 4a |  | OEt | O | 89 | 4f |  | OEt | S | 88 |
| 4b |  | OEt | S | 86 | 4g |  | Me | S | 86 |
| 4c |  | Me | O | 87 | 4h |  | Me | O | 85 |
| 4d |  | Me | S | 85 | 4i |  | Me | S | 89 |
| 4e |  | OEt | O | 88 | 4j |  | OEt | S | 85 |

| Compound | % Mean Parasite Inhibition Per 1500 RBC | |||||||
|---|---|---|---|---|---|---|---|---|
| Concentrations (μg/mL) | ||||||||
| 0.625 | 1.25 | 2.5 | 5 | |||||
| Range | Mean ± SE | Range | Mean ± SE | Range | Mean ± SE | Range | Mean ± SE | |
| 4a | 2.0–7.8 | 5.7 ± 1.85 | 8.2–18.2 | 14.1 ± 3.02 | 31.3–41.8 | 36.6 ± 3.03 | 47.1–53.1 | 51.0 ± 1.93 |
| 4c | 14.2–15.6 | 14.7 ± 0.42 | 26.5–36.3 | 35.3 ± 3.09 | 54.5–62.7 | 57.4 ± 2.63 | 85.7–92.1 | 89.5 ± 1.96 |
| 4d | 2.0–5.4 | 3.7 ± 0.98 | 0.0–3.6 | 1.8 ± 1.03 | 6.1–7.8 | 7.1 ± 0.49 | 14.3–18.1 | 16.1 ± 1.11 |
| 4e | 0.0–2.0 | 1.3 ± 0.63 | 2.0–7.2 | 4.4 ± 1.51 | 13.7–14.2 | 14.1 ± 0.23 | 24.4–35.2 | 29.5 ± 3.12 |
| 4f | 9.8–12.2 | 10.9 ± 0.69 | 27.4–29.1 | 28.3 ± 0.49 | 32.7–43.1 | 37.5 ± 3.02 | 69.1–78.4 | 72.9 ± 2.79 |
| 4h | 20.4–23.6 | 21.8 ± 0.93 | 24.5–35.2 | 31.4 ± 3.05 | 69.1–75.5 | 72.3 ± 1.84 | 80–83.7 | 82.0 ± 1.07 |
| 4j | 1.9–7.2 | 3.7 ± 1.75 | 0.0–5.4 | 2.5 ± 1.57 | 19.6–20.4 | 20.0 ± 0.23 | 49–56.8 | 51.6 ± 2.58 |
| 6b | 42.8–43.6 | 43.2 ± 0.23 | 52.7–62.7 | 56.2 ± 3.26 | 55.1–69.1 | 61.0 ± 4.18 | 100–100 | 100 ± 00 |
| 7a | 27.4–29.1 | 29.1 ± 0.92 | 37.3–45.5 | 42.6 ± 2.63 | 64.7–67.3 | 66.4 ± 0.86 | 66.7–73.5 | 71.0 ± 2.14 |
| 7b | 34.5–35.3 | 34.8 ± 0.25 | 54.5–55.1 | 54.8 ± 0.17 | 78.4–89.1 | 83.0 ± 3.17 | 100–100 | 100 ± 00 |
| 8 | 35.3–50.1 | 42.3 ± 4.3 | 63.3–76.4 | 70.1 ± 3.8 | 100–100 | 100 ± 00 | - | - |
| 10 | 11.8–25.5 | 17.2 ± 4.21 | 43.1–60 | 51.4 ± 4.88 | 67.3–74.5 | 70.3 ± 2.16 | 84.3–84.3 | 84.3 ± 00 |
| 11 | 5.9–18.2 | 12.8 ± 3.62 | 39.2–49 | 44.6 ± 2.86 | 63.2–74.5 | 70.1 ± 350 | 75.5–83.6 | 80.0 ± 2.51 |
| 12 | 35.3–45.4 | 41.8 ± 3.27 | 59.2–70.9 | 64.3 ± 3.46 | 74.5–78.2 | 76.7 ± 1.13 | 86.3–91.8 | 89.7 ± 1.70 |
| CQ | 59.9–63.8 | 61.8 ± 1.13 | 75.9–78.1 | 76.7 ± 0.67 | 100–100 | 100 ± 0.0 | - | - |
| Concentrations (μg/mL) | Compound | ||||
|---|---|---|---|---|---|
| 4b | 4g | 4i | CQ | ||
| 0.078 | Range | NP | NP | 32.7–36.7 | NP |
| Mean ± SE | NP | NP | 34.8 ± 1.16 | NP | |
| 0.156 | Range | NP | 23.5–25.4 | NP | 28.9–33.7 |
| Mean ± SE | NP | 24.4 ± 0.54 | NP | 31.8 ± 1.44 | |
| 0.312 | Range | 41.8–44.9 | 56.3–61.2 | 63.6–71.4 | 43.3–46.4 |
| Mean ± SE | 43.3 ± 0.89 | 58.7 ± 1.41 | 67.8 ± 2.28 | 44.9 ± 0.90 | |
| 0.625 | Range | 55.1–70.1 | 82.3–89.7 | 74.5–86.2 | 59.9–63.8 |
| Mean ± SE | 63.3 ± 4.38 | 87.0 ± 2.35 | 79.4 ± 3.5 | 61.8 ± 1.13 | |
| 1.25 | Range | 73.5–82.3 | 100–100 | 85.7–94.1 | 75.9–78.1 |
| Mean ± SE | 76.7 ± 2.78 | 100 ± 0.0 | 89.9 ± 2.59 | 76.7 ± 0.67 | |
| 2.5 | Range | 89.1–95.9 | - | 100–100 | 100–100 |
| Mean ± SE | 91.7 ± 2.11 | - | 100 ± 0.0 | 100 ± 0.0 | |
| 5 | Range | 100–100 | - | - | - |
| Mean ± SE | 100 ± 0.0 | - | - | - | |
| Compound | IC50 (μg/mL) | Slope | R2 |
|---|---|---|---|
| 4a | 4.59 | 10.32 | 0.929 |
| 4b | 0.46 | 10.43 | 0.772 |
| 4c | 2.39 | 16.28 | 0.968 |
| 4d | 15.87 | 3.17 | 0.932 |
| 4e | 8.10 | 6.55 | 0.997 |
| 4f | 3.29 | 13.34 | 0.977 |
| 4g | 0.30 | 61.62 | 0.791 |
| 4h | 2.21 | 14.0 | 0.831 |
| 4i | 0.014 | 23.48 | 0.733 |
| 4j | 4.96 | 11.67 | 0.975 |
| 6a | 1.13 | 12.41 | 0.965 |
| 7a | 2.09 | 9.11 | 0.790 |
| 7b | 1.06 | 14.14 | 0.889 |
| 8 | 0.76 | 29.79 | 0.971 |
| 10 | 1.91 | 13.27 | 0.782 |
| 11 | 2.21 | 13.59 | 0.767 |
| 12 | 0.46 | 9.62 | 0.834 |
| CQ | 0.49 | 27.01 | 0.927 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radini, I.A.M.; Elsheikh, T.M.Y.; El-Telbani, E.M.; Khidre, R.E. New Potential Antimalarial Agents: Design, Synthesis and Biological Evaluation of Some Novel Quinoline Derivatives as Antimalarial Agents. Molecules 2016, 21, 909. https://doi.org/10.3390/molecules21070909
Radini IAM, Elsheikh TMY, El-Telbani EM, Khidre RE. New Potential Antimalarial Agents: Design, Synthesis and Biological Evaluation of Some Novel Quinoline Derivatives as Antimalarial Agents. Molecules. 2016; 21(7):909. https://doi.org/10.3390/molecules21070909
Chicago/Turabian StyleRadini, Ibrahim Ali M., Tarek M. Y. Elsheikh, Emad M. El-Telbani, and Rizk E. Khidre. 2016. "New Potential Antimalarial Agents: Design, Synthesis and Biological Evaluation of Some Novel Quinoline Derivatives as Antimalarial Agents" Molecules 21, no. 7: 909. https://doi.org/10.3390/molecules21070909
APA StyleRadini, I. A. M., Elsheikh, T. M. Y., El-Telbani, E. M., & Khidre, R. E. (2016). New Potential Antimalarial Agents: Design, Synthesis and Biological Evaluation of Some Novel Quinoline Derivatives as Antimalarial Agents. Molecules, 21(7), 909. https://doi.org/10.3390/molecules21070909