Site-Specific, Covalent Immobilization of Dehalogenase ST2570 Catalyzed by Formylglycine-Generating Enzymes and Its Application in Batch and Semi-Continuous Flow Reactors
Abstract
:1. Introduction
2. Results and Discussion
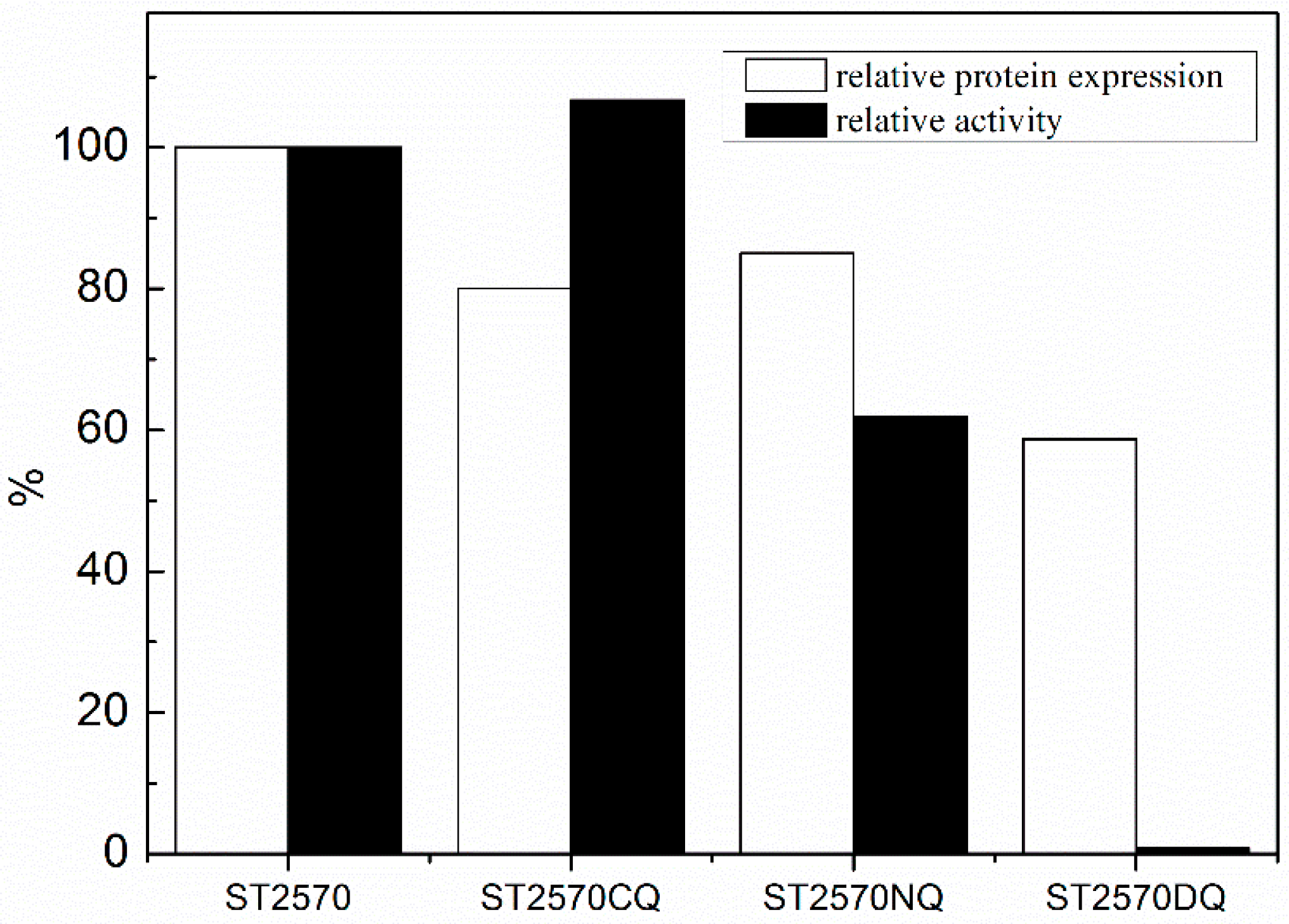
2.1. Expression and Purification
2.2. Labeling of Recombinant Proteins with Fluorescence Probe
2.3. Modification of SBA15
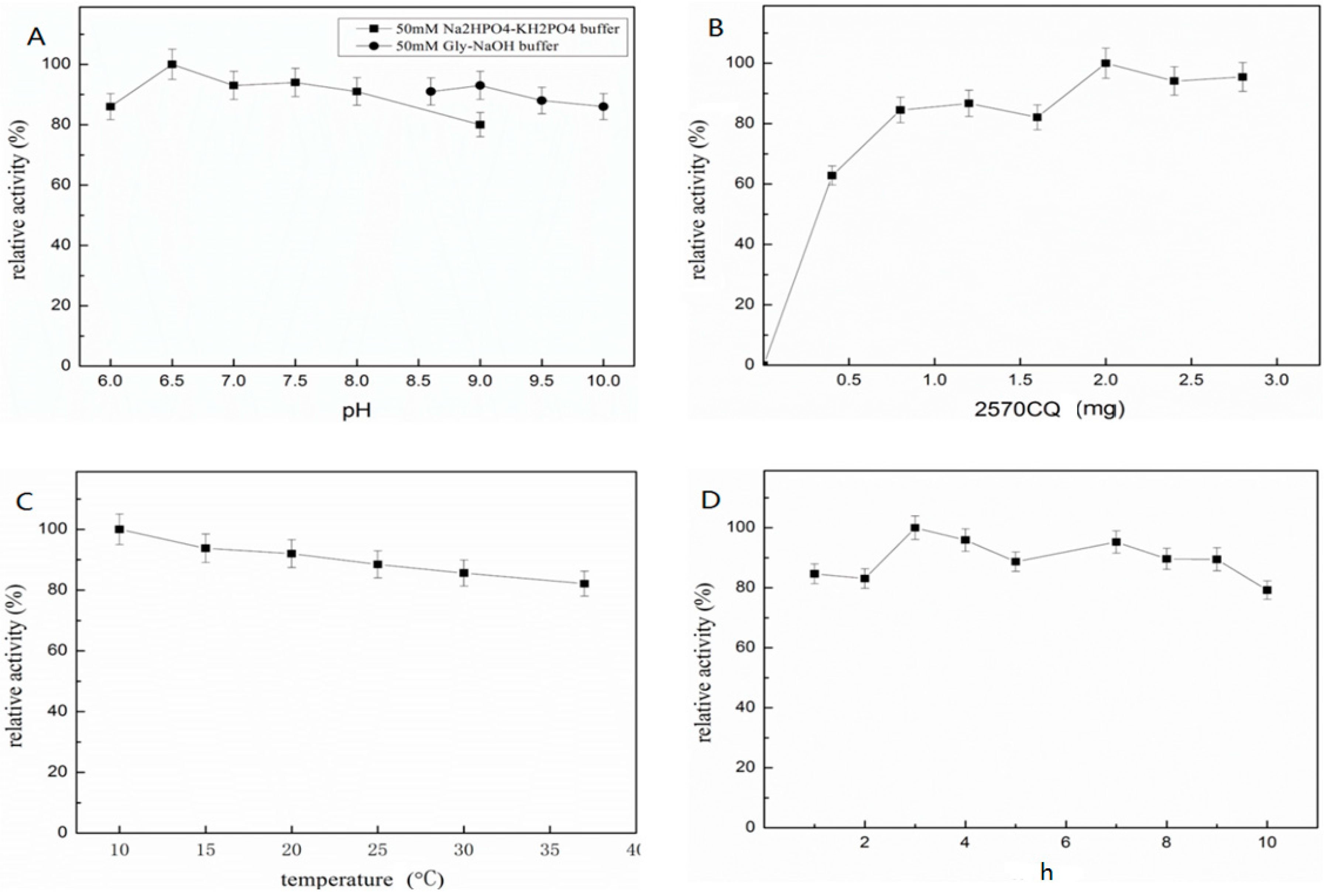
2.4. Immobilization of ST2570CQ
2.5. Characterization of Immobilized ST2570CQ
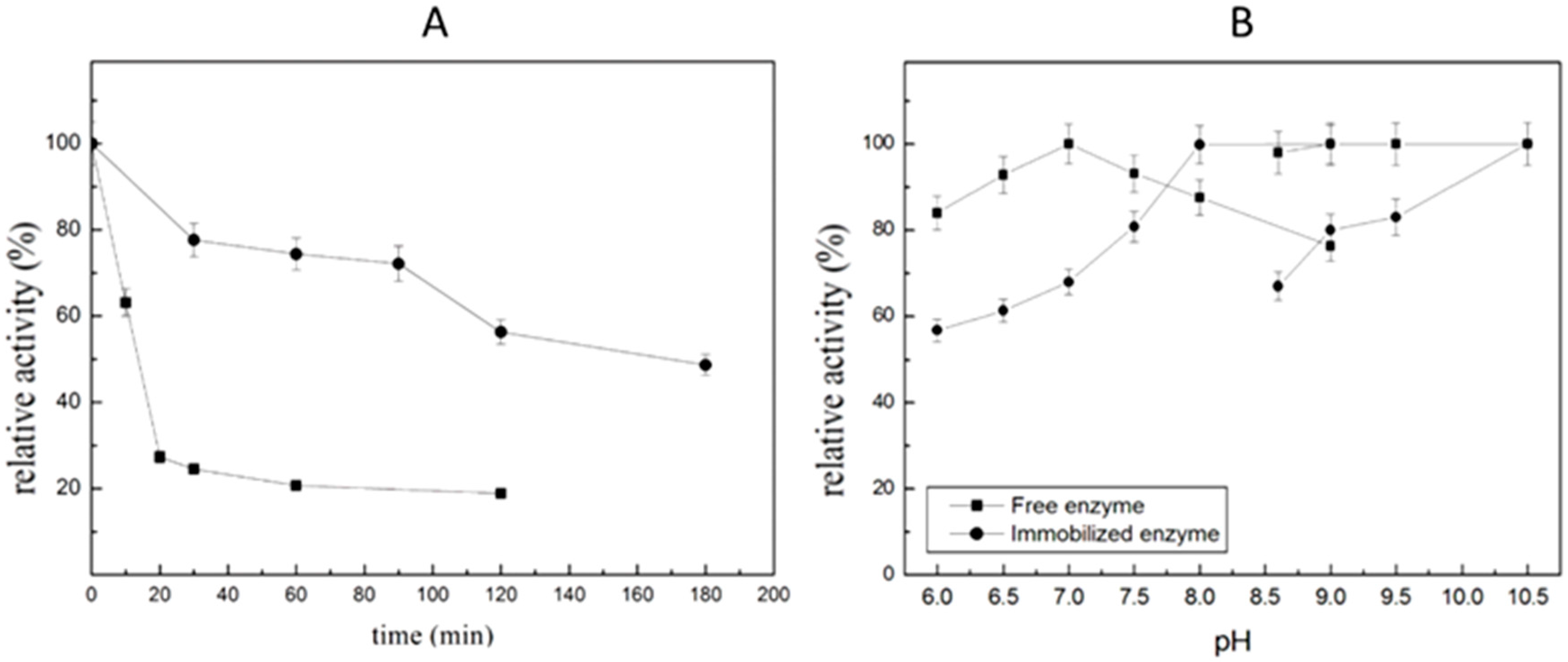
2.5.1. pH and Thermo-Stability of Immobilized ST2570CQ
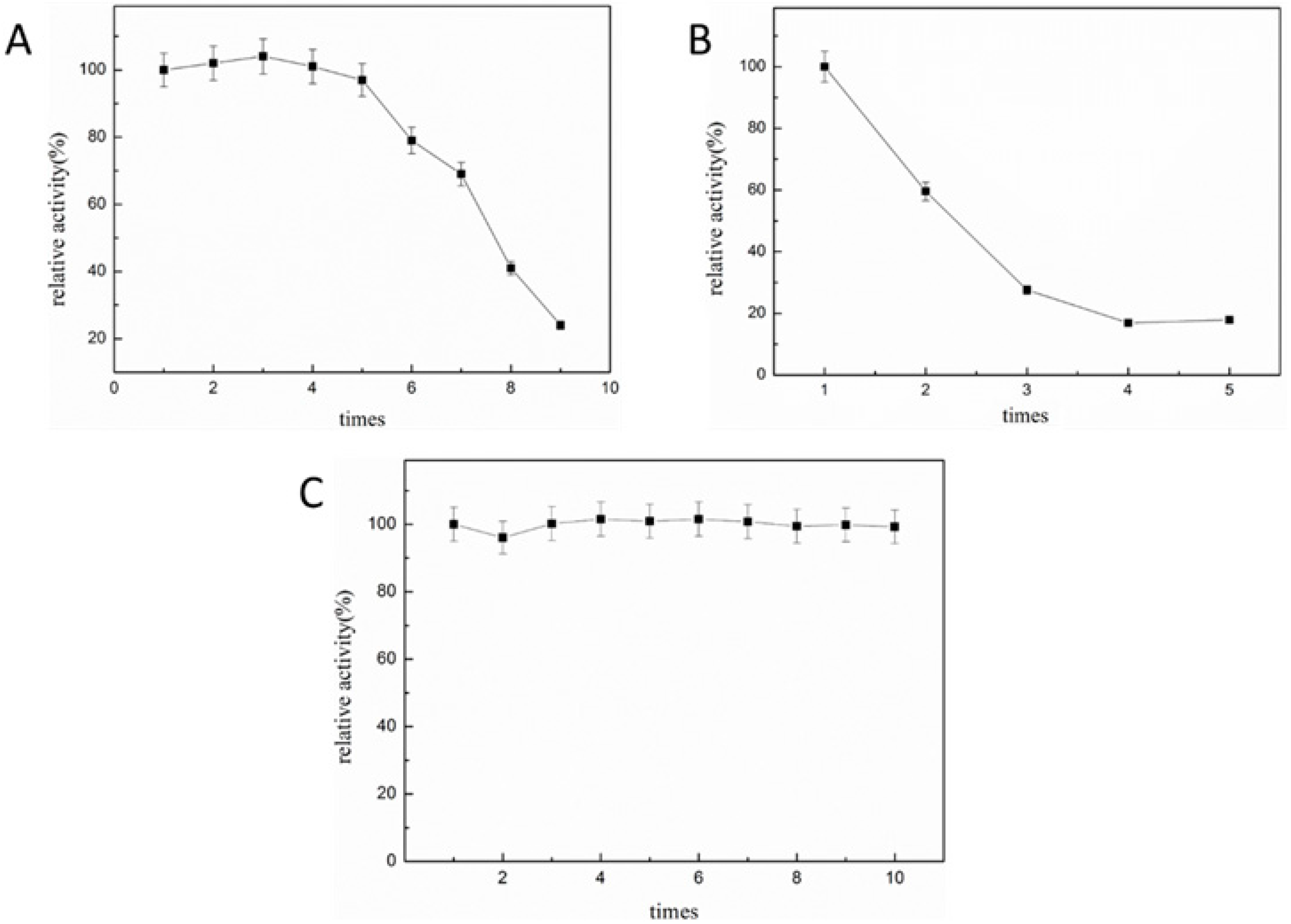
2.5.2. Operational Stabilities of Immobilized Enzyme in Batch and Semi-Continuous Reactors
2.6. Catalytic Ability of Immobilized ST2570CQ
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Expression and Purification
3.2.2. Enzyme Assay
3.2.3. Labelling of Recombinant Proteins with the Fluorescence Probe
3.2.4. Modification of SBA-15
3.2.5. Immobilization of ST2570CQ
3.2.6. Characterization of Immobilized ST2570CQ
3.2.7. Catalytic Ability of Immobilized ST2570CQ
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzym. Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzym. Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.R.; Mosisch, M.; Torda, A.E.; Hilterhaus, L. Site directed immobilization of glucose-6-phosphate dehydrogenase via thiol-disulfide interchange: Influence on catalytic activity of cysteines introduced at different positions. J. Biotechnol. 2013, 167, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Boutureira, O.; Bernardes, G.J. Advances in Chemical Protein Modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef] [PubMed]
- Popp, M.W.; Ploegh, H.L. Making and Breaking Peptide Bonds: Protein Engineering Using Sortase. Angew. Chem. Int. Ed. 2011, 50, 5024–5032. [Google Scholar] [CrossRef] [PubMed]
- Oteng-Pabi, S.K.; Pardin, C.; Stoica, M.; Keillor, J.W. Site-specific protein labelling and immobilization mediated by microbial transglutaminase. Chem. Commun. 2014, 50, 6604–6606. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, M.; Dozier, J.K.; Lenevich, S.; Distefano, M.D. Selective labeling of polypeptides using protein farnesyltransferase via rapid oxime ligation. Chem. Commun. 2010, 46, 8998–9000. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Shui, W.; Carlson, B.L.; Hu, N.; Rabuka, D.; Lee, J.; Bertozzi, C.R. Site-specific chemical modification of recombinant proteins produced in mammalian cells by using the genetically encoded aldehyde tag. Proc. Natl. Acad. Sci. USA 2009, 106, 3000–3005. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Howarth, M.; Lin, W.; Ting, A.Y. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat. Methods 2005, 2, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Suárez, M.; Baruah, H.; Martínez-Hernández, L.; Xie, K.T.; Baskin, J.M.; Bertozzi, C.R.; Ting, A.Y. Redirecting lipoic acid ligase for cell surface protein labeling with small-molecule probes. Nat. Biotechnol. 2007, 25, 1483–1487. [Google Scholar]
- Heal, W.P.; Wickramasinghe, S.R.; Bowyer, P.W.; Holder, A.A.; Smith, D.F.; Leatherbarrow, R.J.; Tate, E.W. Site-specific N-terminal labelling of proteinsin vitro and in vivo using N-myristoyl transferase and bioorthogonal ligation chemistry. Chem. Commun. 2008, 28, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Dierks, T.; Schmidt, B.; Borissenko, L.V.; Peng, J.; Preusser, A.; Mariappan, M.; von Figura, K. Multiple Sulfatase Deficiency Is Caused by Mutations in the Gene Encoding the Human Cα-Formylglycine Generating Enzyme. Cell 2003, 113, 435–444. [Google Scholar] [CrossRef]
- Carlson, B.L.; Ballister, E.R.; Skordalakes, E.; King, D.S.; Breidenbach, M.A.; Gilmore, S.A.; Berger, J.M.; Bertozzi, C.R. Function and Structure of a Prokaryotic Formylglycine-generating Enzyme. J. Biol. Chem. 2008, 283, 20117–20125. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.S.; Bertozzi, C.R. New Aldehyde Tag Sequences Identified by Screening Formylglycine Generating Enzymes in Vitro and in Vivo. J. Am. Chem. Soc. 2008, 130, 12240–12241. [Google Scholar] [CrossRef] [PubMed]
- Rye, C.A.; Isupov, M.N.; Lebedev, A.A.; Littlechild, J.A. Biochemical and structural studies of a l-haloacid dehalogenase from the thermophilic archaeon Sulfolobus tokodaii. Extremophiles 2009, 13, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Bachas-Daunert, P.G.; Sellers, Z.P.; Wei, Y. Detection of halogenated organic compounds using immobilized thermophilic dehalogenase. Anal. Bioanal. Chem. 2009, 395, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Bachas-Daunert, P.G.; Law, S.A.; Wei, Y. Characterization of a Recombinant Thermostable Dehalogenase Isolated from the Hot Spring Thermophile Sulfolobus tokodaii. Appl. Biochem. Biotechnol. 2009, 159, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Sanjust, E. Inorganic Materials as Supports for Covalent Enzyme Immobilization: Methods and Mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.V.; Anderson, K.W.; Bachas, L.G. Oriented Immobilization of Proteins. Mikrochim. Acta 1998, 128, 127–143. [Google Scholar] [CrossRef]
- Sevimli, F.; Yılmaz, A. Surface functionalization of SBA-15 particles for amoxicillin delivery. Microporous Mesoporous Mater. 2012, 158, 281–291. [Google Scholar] [CrossRef]
- Vrancken, K.C.; Possemiers, K.; Vandervoort, P.; Vansant, E.F. Surface modification of silica gels with aminoorgano-silanes. Colloids Surf. A Physicochem. Eng. Asp. 1995, 98, 235–241. [Google Scholar] [CrossRef]
- Magner, E. Immobilisation of Enzymes on Mesoporous Silicate Materials. Chem. Soc. Rev. 2013, 42, 6213–6222. [Google Scholar] [CrossRef] [PubMed]
- Martinek, K.; Klibanov, A.M.; Goldmacher, V.S.; Berezin, I.V. The principles of enzyme stabilization. I. Increase in thermo-stability of enzymes covalently bound to a complementary surface of a polymer support in a multipoint fashion. Biochim. Biophys. Acta 1977, 485, 1–12. [Google Scholar] [CrossRef]
- Kumari, A.; Mahapatra, P.; Kumar, G.V.; Banerjee, R. Comparative study of thermostabilty and ester synthesis ability of free and immobilized lipases on cross linked silica gel. Bioprocess Biosyst. Eng. 2008, 31, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Alptekin, O.; Tükel, S.S.; Yildirim, D.; Alagöz, D. Covalent immobilization of catalase onto spacer-arm attached modified florisil: Characterization and application to batch and plug-flow type reactor systems. Enzym. Microb. Technol. 2011, 49, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Arica, M.Y.; Yavuz, H.; Patir, S.; Denizli, A. Immobilization of glucoamylase onto spacer-arm attached magnetic ž/poly methylmethacrylate microspheres: Characterization and application to a continuous flow reactor. J. Mol. Catal. B Enzym. 2000, 11, 127–138. [Google Scholar] [CrossRef]
- Canilho, N.; Jacoby, J.; Pasc, A.; Carteret, C.; Dupire, F.; Stébé, M.J.; Blin, J.L. Isocyanate-mediated covalent immobilization of Mucor miehei lipase onto SBA-15 for transesterification reaction. Colloids Surf. B Biointerfaces 2013, 112, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Emadi, S.; Safari, A.A.; Kermanian, M. Immobilization, stability and enzymatic activity of albumin and trypsin adsorbed onto nanostructured mesoporous SBA-15 with compatible pore sizes. R. Soc. Chem. 2013, 4, 4387–4394. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ashjari, M.; Dezvarei, S.; Yousefi, M.; Babaki, M.; Mohammadi, J. Rapid and high-density covalent immobilization of Rhizomucor miehei lipase using a multi component reaction: Application in biodiesel production. R. Soc. Chem. 2015, 5, 32698–32705. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Carrico, I.S.; Carlson, B.L.; Bertozzi, C.R. Introducing genetically encoded aldehydes into proteins. Nat. Chem. Biol. 2007, 3, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Alptekin, Ö.; Tükel, S.S.; Yıldırım, D.; Alagöz, D. Characterization and properties of catalase immobilized onto controlled pore glass and its application in batch and plug-flow type reactors. J. Mol. Catal. B Enzym. 2009, 58, 124–131. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.



| Enzymes | Recognition Motif | Mechanism of Enzyme-Mediated System | Ref(s) |
|---|---|---|---|
| sortase A | LPXTG | A cysteine residue in sortase catalyzes the cleavage of the peptide bond between the threonine and glycine residues within the motif. Sortase will accept the N-terminus of an oligoglycine nucleophile. | [7] |
| microbial transglutaminase (mTG) | WALQRPH | TG2 mediates the incorporation of functionalized amines into a high-affinity Gln-substrate peptide tag. | [8] |
| farnesyltransferase (PFTase) | CAAX | PFTase catalyzes the transfer of a farnesyl isoprenoid group from farnesyl pyrophosphate to a sulfur atom present in the cysteine residue of the CAAX box. | [9] |
| formylglycine generating enzyme (FGE) | LCXPXR | FGE converts cysteine to formylglycine (FGly) within the LCTPSR motif. Proteins bearing a unique aldehyde group were chemically modified by selective reaction with hydrazide- or aminooxy-functionalized reagents. | [10] |
| biotin ligase (BirA) | GLNDIFEAQKIEWHE | BirA catalyzes the sequence-specific ligation of the biotin or the ketone isostere of biotin to the lysine side-chain of a 15-amino-acid acceptor peptide (AP). | [11] |
| microbial lipoic acid ligase (LplA) | DEVLVEIETDKAVLEVPGGEEE | LplA catalyzes the specific attachment of octanoic acid, 6-thio-octanoic acid, selenolipoic acid and alkyl azide to an engineered LplA acceptor peptide (LAP). The alkyl azide was selectively derivatized with cyclooctyne conjugates to various probes. | [12] |
| N-myristoyl transferase (NMT) | GXXXS | NMT specifically attaches myristate or modified myristate to peptides with amino-terminal Gly residues. Peptides with Asn, Gln, Ser, Val or Leu penultimate to the amino terminal Gly are substrates. | [13] |
| Ratio of APTES and Ethanol % (v/v) | Loading Capacity mg/gcarrier | Specific Activity U/mg | Specific Activity U/gcarrier | Immobilization Efficiency % | Retention of Activity % |
|---|---|---|---|---|---|
| 0 | 4.31 | 103 | 443.93 | 90.79 | 57.17 |
| 2 | 3.17 | 141.63 | 448.97 | 66.31 | 78.61 |
| 6 | 4.37 | 108.68 | 474.93 | 91.42 | 60.32 |
| 10 | 4.23 | 109.56 | 478.78 | 88.49 | 60.81 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, H.; Wang, Y.; Bai, Y.; Li, R.; Gao, R. Site-Specific, Covalent Immobilization of Dehalogenase ST2570 Catalyzed by Formylglycine-Generating Enzymes and Its Application in Batch and Semi-Continuous Flow Reactors. Molecules 2016, 21, 895. https://doi.org/10.3390/molecules21070895
Jian H, Wang Y, Bai Y, Li R, Gao R. Site-Specific, Covalent Immobilization of Dehalogenase ST2570 Catalyzed by Formylglycine-Generating Enzymes and Its Application in Batch and Semi-Continuous Flow Reactors. Molecules. 2016; 21(7):895. https://doi.org/10.3390/molecules21070895
Chicago/Turabian StyleJian, Hui, Yingwu Wang, Yan Bai, Rong Li, and Renjun Gao. 2016. "Site-Specific, Covalent Immobilization of Dehalogenase ST2570 Catalyzed by Formylglycine-Generating Enzymes and Its Application in Batch and Semi-Continuous Flow Reactors" Molecules 21, no. 7: 895. https://doi.org/10.3390/molecules21070895
APA StyleJian, H., Wang, Y., Bai, Y., Li, R., & Gao, R. (2016). Site-Specific, Covalent Immobilization of Dehalogenase ST2570 Catalyzed by Formylglycine-Generating Enzymes and Its Application in Batch and Semi-Continuous Flow Reactors. Molecules, 21(7), 895. https://doi.org/10.3390/molecules21070895







