Photochemistry of 1,4-Dihydropyridine Derivatives: Diradical Formation, Delocalization and Trapping as a Route to Novel Tricyclic and Tetracyclic Nitrogen Heterocyclic Ring Systems
Abstract
:1. Introduction
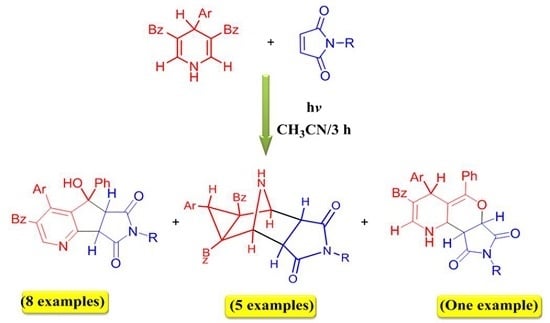
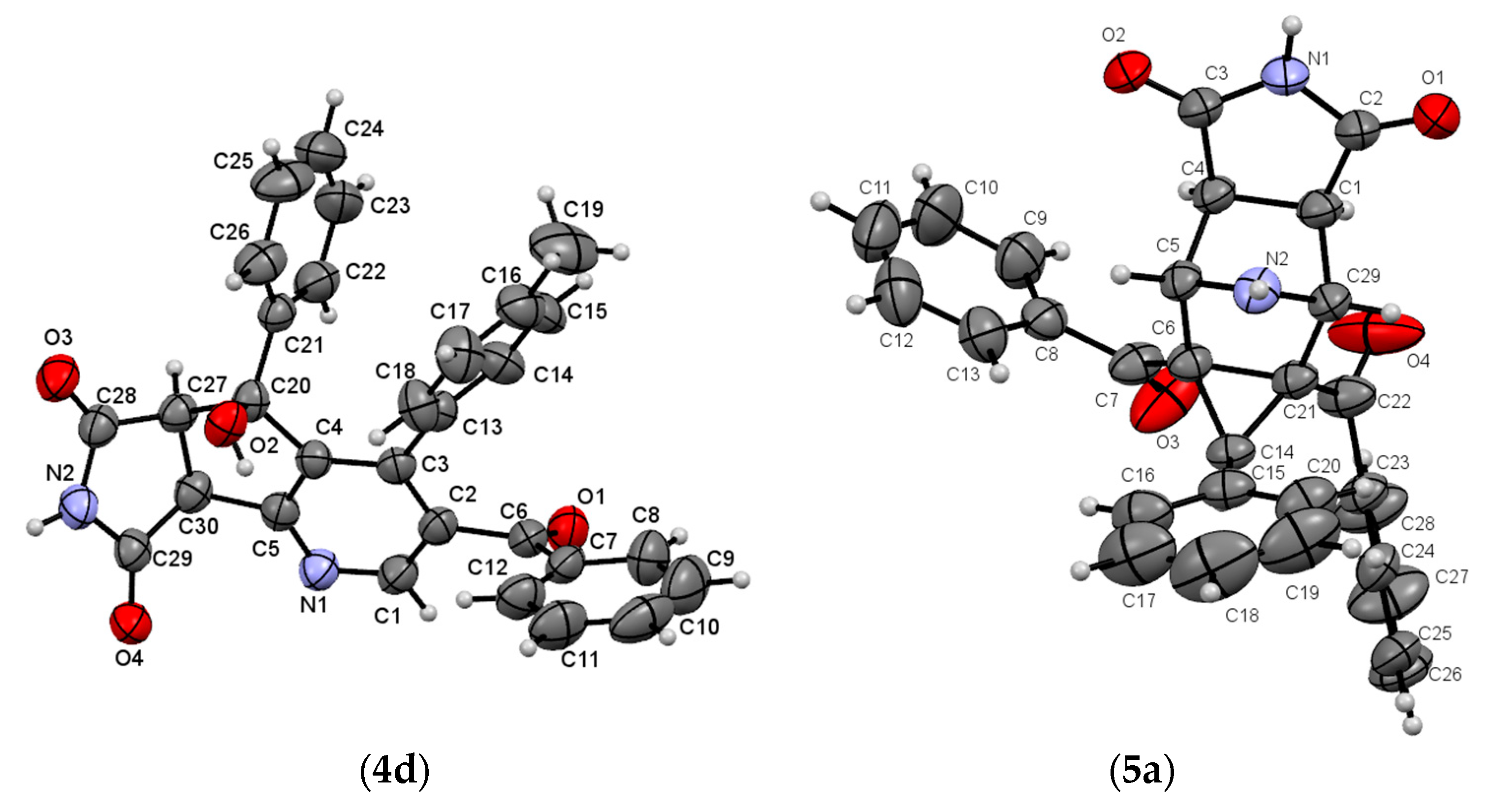
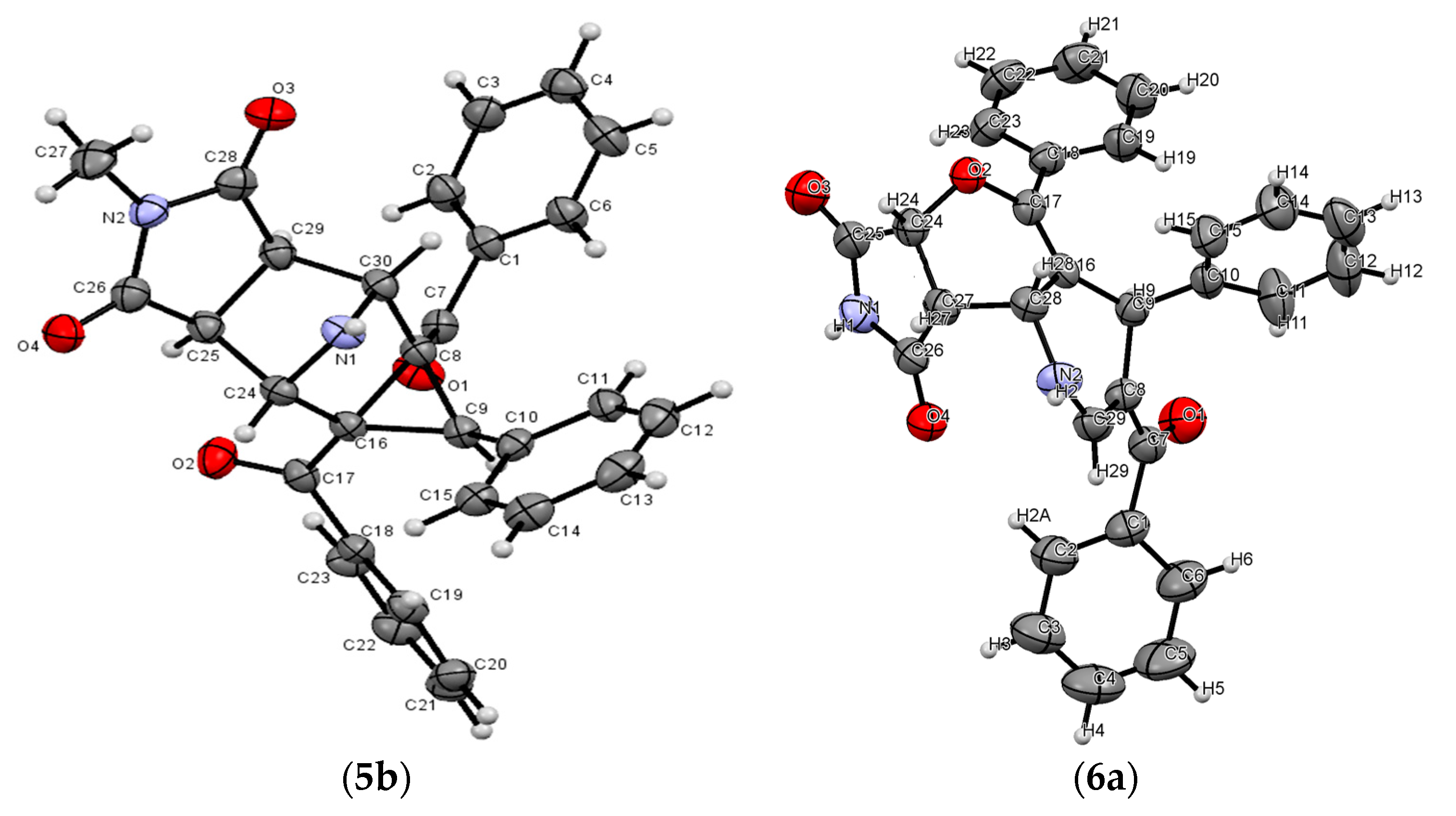
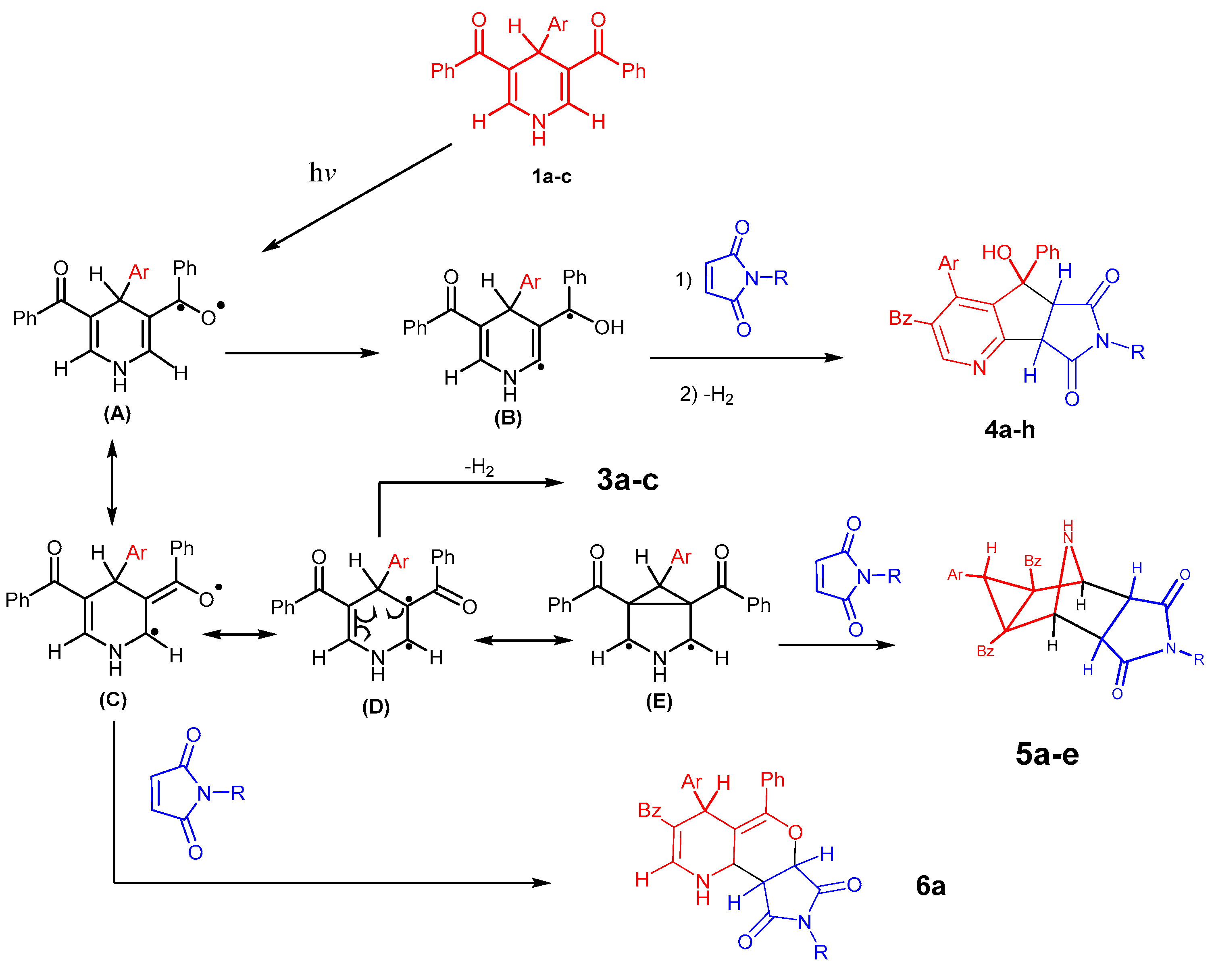
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. General procedure for the photoreaction of 1,4-dihydropyridines 1a–c with maleimides 2a–c
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kurz, J.L.; Hutton, R.; Westheimer, F.H. The Photochemical Reduction of Bromotrichloromethane by Derivatives of 1,4-Dihydropyridine. J. Am. Chem. Soc. 1961, 83, 584–588. [Google Scholar] [CrossRef]
- Eisner, U.; Williams, J.R.; Matthews, B.W.; Ziffer, H. The Photochemistry Of 3,5-Disubstituted 1,4-Dihydropyridines. Tetrahedron 1970, 26, 899–909. [Google Scholar] [CrossRef]
- Mitsunobu, O.; Matsumoto, S.; Wada, M.; Masuda, H. Photooxidation of 1,4-Dihydropyridines. Bull. Chem. Soc. Jpn. 1972, 45, 1453–1457. [Google Scholar] [CrossRef]
- Zhu, X.; Ni, C.; Song, X.; Yan, H.; Zhong, R. Synthesis of 3,9-Diazatetraasteranes. Chin. J. Org. Chem. 2010, 30, 276–281. [Google Scholar]
- Hilgeroth, A.; Baumeister, U.; Heinemann, F.W. Solution-Dimerization of 4-Aryl-1,4-dihydropyridines. Eur. J. Org. Chem. 2000, 2000, 245–249. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, M.-Z.; Zhang, W.; Yang, L.; Liu, Z.-L. Photoinduced Transformation of α,β-Epoxyketones to β-Hydroxyketones by Hantzsch 1,4-Dihydropyridine. Tetrahedron Lett. 2002, 43, 9687–9689. [Google Scholar] [CrossRef]
- Memarian, H.R.; Sadeghi, M.M.; Aliyan, H. Photochemistry of Some 1,4-Dihydropyridine Derivatives: Part II. Ind. J. Chem. 1999, 38, 800–804. [Google Scholar]
- Memarian, H.R.; Bagheri, M.; Dopp, D. Synthesis and Photochemistry of Novel 3,5-Diacetyl-1,4-dihydropyridines. II. Mon. Chem. 2004, 135, 833–838. [Google Scholar] [CrossRef]
- Pavez, P.; Encinas, M.V. Photophysics and Photochemical Studies of 1,4-Dihydropyridine Derivatives. Photochem. Photobiol. 2007, 83, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Adembri, G.; Donati, D.; Fusi, S.; Ponticelli, F. Acrylonitrile Photoaddition on 1-Benzyl-1,4-dihydronicotinamide. Heterocycles 1985, 23, 2885–2589. [Google Scholar] [CrossRef]
- Adembri, G.; Camprini, A.; Donati, D.; Fusi, S.; Ponticelli, F.; Scottn, M. Photodimerization of N-benzyl-1,4-dihydronicotinamide. Tetrahedron Lett. 1983, 24, 5399–5402. [Google Scholar] [CrossRef]
- Adembri, G.; Donati, D.; Fusi, S.; Ponticelli, F. 2-Azabicyclo[4.2.0]octane Derivatives: Stereoselective Photochmical Synthesis and Chmical Reactivity. J. Chem. Soc. Perkin Trans. 1 1992, 2033–2038. [Google Scholar] [CrossRef]
- Donati, D.; Fusi, S.; Ponticelli, F. Photocycloaddition of Cyanoethylenes on to 1,4-Dihydro-and 1,4,5,6-Tetrahydro-pyridines. J. Chem. Res. Synop. 1997. [Google Scholar] [CrossRef]
- Zhu, X.; Ni, C.; Yan, H.; Zhong, R. Synthesis and X-ray Crystallographic Analysis of 1,4-Dihydropyridine Photodimerization. J. Photopolym. Sci. Technol. 2009, 22, 379–384. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Yan, H.; Zhong, R.J. Triplet Phenacylimidazoliums-Catayzed Photocycloaddition of 1,4-Dihydropyridines: An experimental and theoretical study. Photochem. Photobiol. A Chem. 2012, 241, 13–20. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, H.; Yan, H.; Song, X. Design, Synthesis and Biological Evaluation of 3,9-Diazatetraasteranes as Novel Matrilysin Inhibitors. Chem. Biol. Drug Des. 2013, 82, 567–578. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Jia, H.Z.; Yao, Q.G.; Liu, S.J.; Yue, H.K.; Liu, W.Y.; Wang, X.H.; Tian, J.C. Photodimerization of 4-Aryl-1,4-dihydropyridines in 1-Butyl-3-methylimidazolium Tetrafluoroborate. Chem. Lett. 2014, 43, 1596–1598. [Google Scholar] [CrossRef]
- Al-Awadi, N.A.; Ibrahim, M.R.; Elnagdi, M.H.; John, E.; Ibrahim, Y.A. Enaminones in a Multicomponent Synthesis of 4-Aryldihydropyridines for potential applications in Photoinduced Intramolecular electron-transfer systems. Beilstein J. Org. Chem. 2012, 8, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1a–c, 3a–c, 5a–c and 4a are available from the authors.


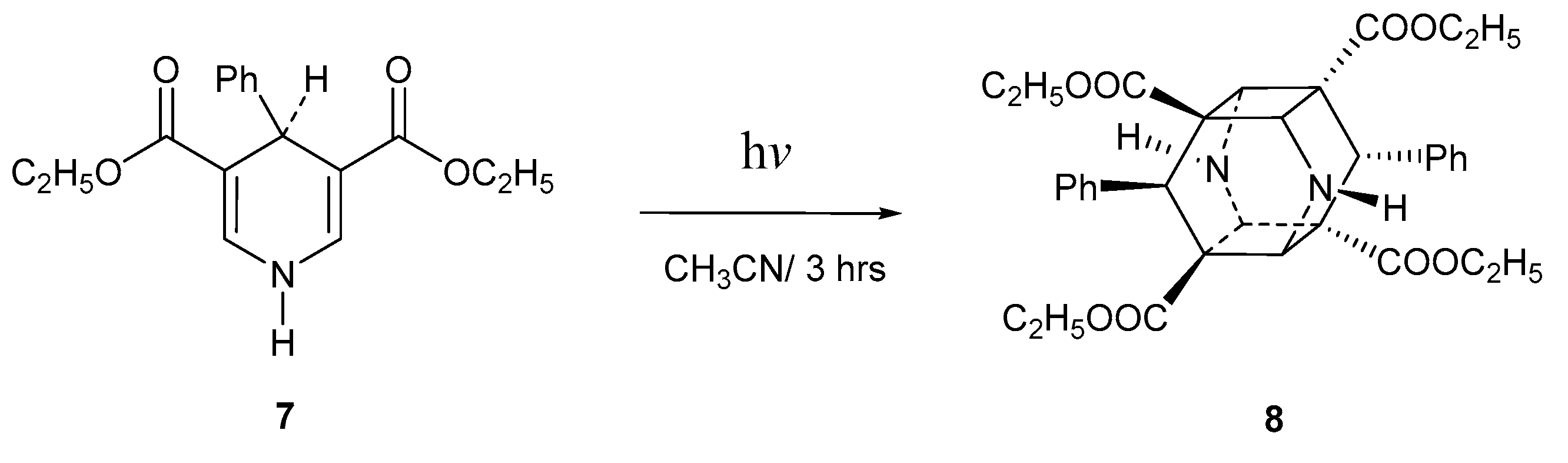
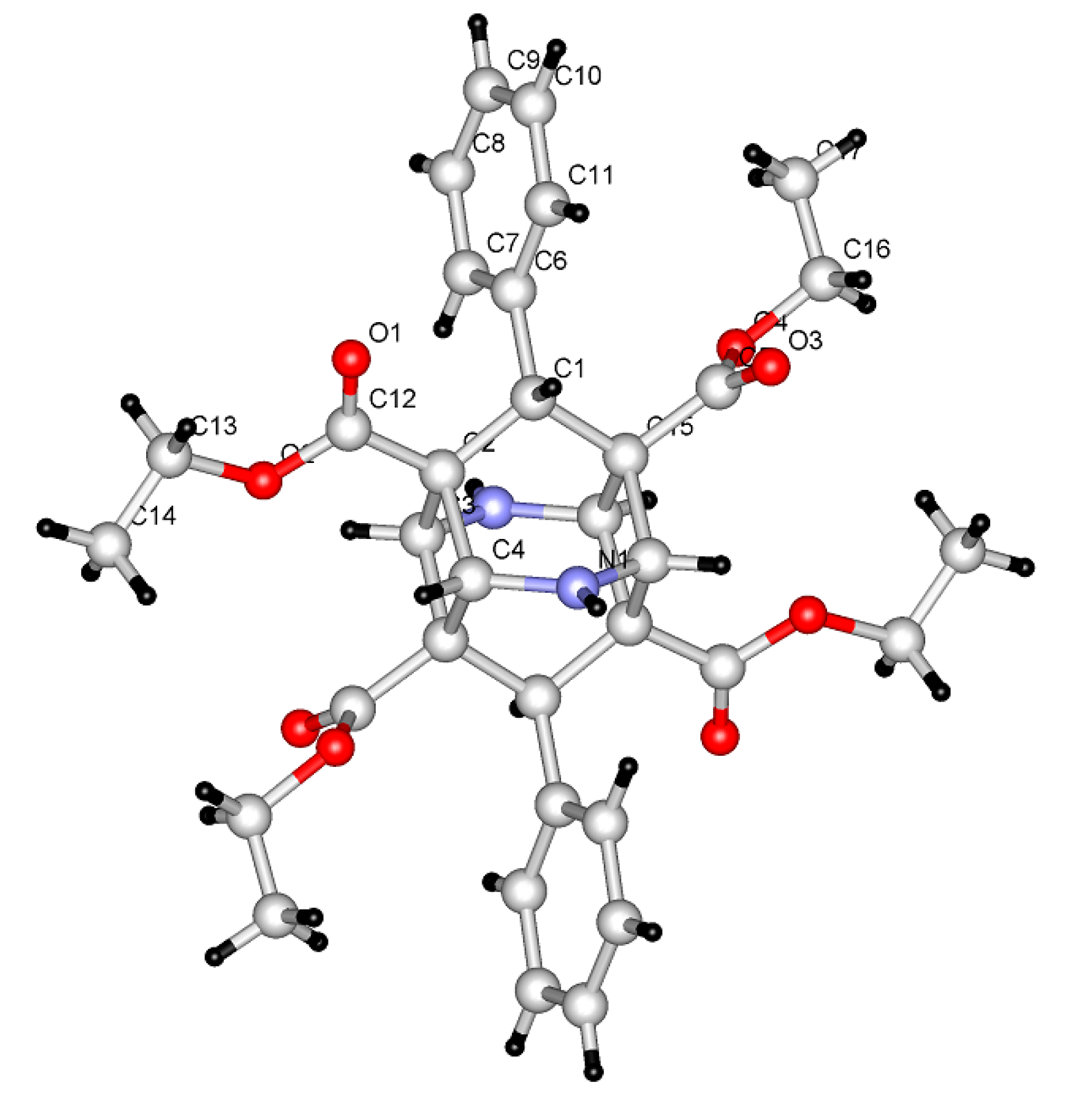
| Entry * | Reactants | Photoproducts (Yield %) | ||||
|---|---|---|---|---|---|---|
| 1a–c | 2a–c | 3a–c | 4a–h | 5a–e | 6a | |
| 1 | 1a | 2a | 3a (48) | 4a (23) | 5a (15) | 6a (13) |
| 2 | 1b | 2a | 3b (45) | 4b (28) | 5b (12) | - |
| 3 | 1c | 2a | 3c (44) | 4c (29) | - | - |
| 4 | 1a | 2b | 3a (41) | 4d (32) | - | - |
| 5 | 1b | 2b | 3b (41) | 4e (27) | 5c (13) | - |
| 6 | 1c | 2b | 3c (39) | 4f (34) | - | - |
| 7 | 1a | 2c | 3a (37) | - | 5d (23) | - |
| 8 | 1b | 2c | 3b (39) | 4g (29) | 5e (10) | - |
| 9 | 1c | 2c | 3c (42) | 4h (27) | - | - |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Jalal, N.A.; Ibrahim, Y.A.; Al-Awadi, N.A.; Ibrahim, M.R.; Sayed, O.M. Photochemistry of 1,4-Dihydropyridine Derivatives: Diradical Formation, Delocalization and Trapping as a Route to Novel Tricyclic and Tetracyclic Nitrogen Heterocyclic Ring Systems. Molecules 2016, 21, 866. https://doi.org/10.3390/molecules21070866
Al-Jalal NA, Ibrahim YA, Al-Awadi NA, Ibrahim MR, Sayed OM. Photochemistry of 1,4-Dihydropyridine Derivatives: Diradical Formation, Delocalization and Trapping as a Route to Novel Tricyclic and Tetracyclic Nitrogen Heterocyclic Ring Systems. Molecules. 2016; 21(7):866. https://doi.org/10.3390/molecules21070866
Chicago/Turabian StyleAl-Jalal, Nader A., Yehia A. Ibrahim, Nouria A. Al-Awadi, Maher R. Ibrahim, and Osama M. Sayed. 2016. "Photochemistry of 1,4-Dihydropyridine Derivatives: Diradical Formation, Delocalization and Trapping as a Route to Novel Tricyclic and Tetracyclic Nitrogen Heterocyclic Ring Systems" Molecules 21, no. 7: 866. https://doi.org/10.3390/molecules21070866