Pingyangmycin and Bleomycin Share the Same Cytotoxicity Pathway
Abstract
:1. Introduction
2. Results
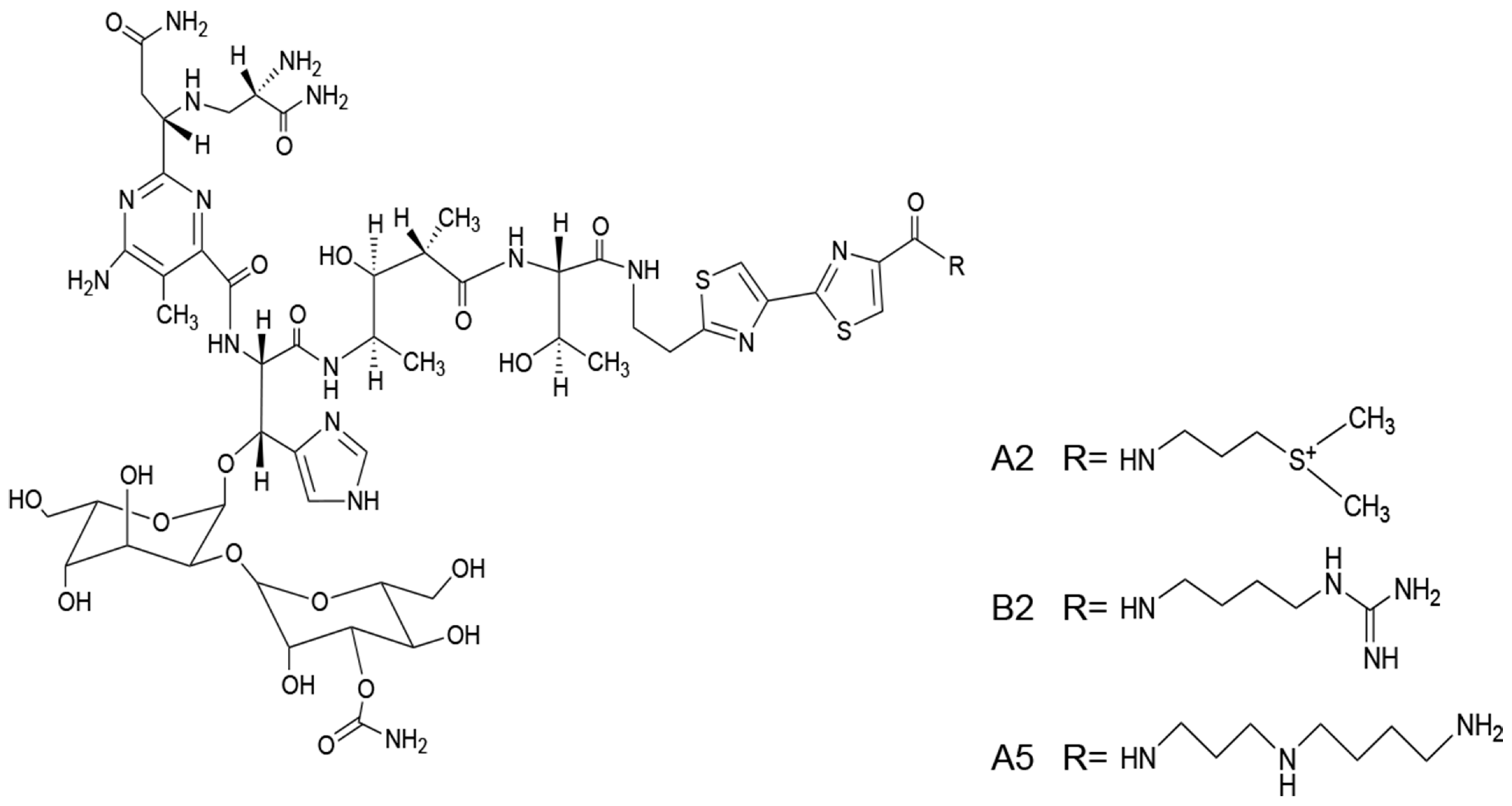
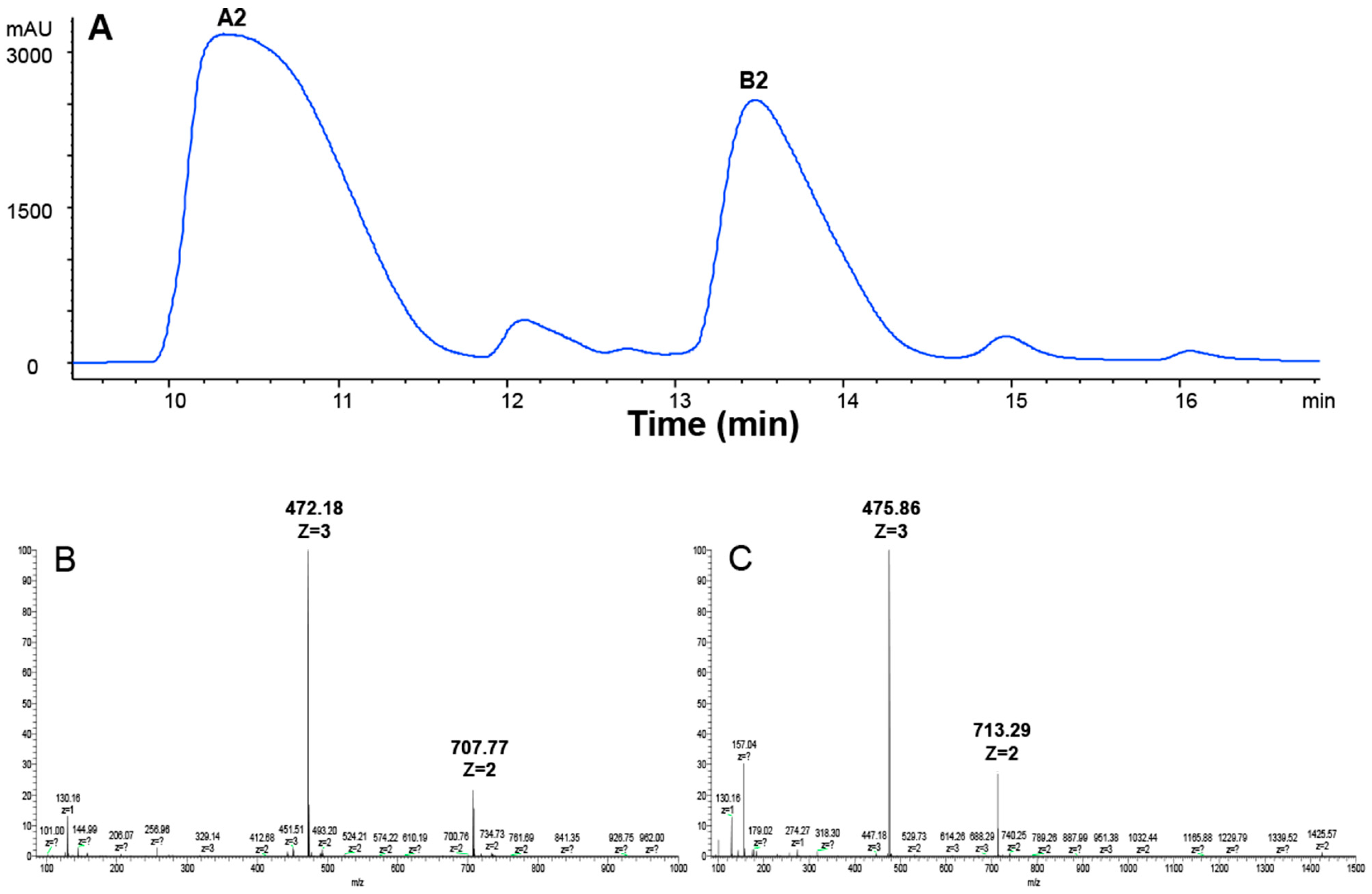
2.1. Preparation of A2 and B2 from the BLM
2.2. BLMs Were Less Toxic to a CHO Cell Mutant Cell Line Defective in Cell Surface GAG-Expression
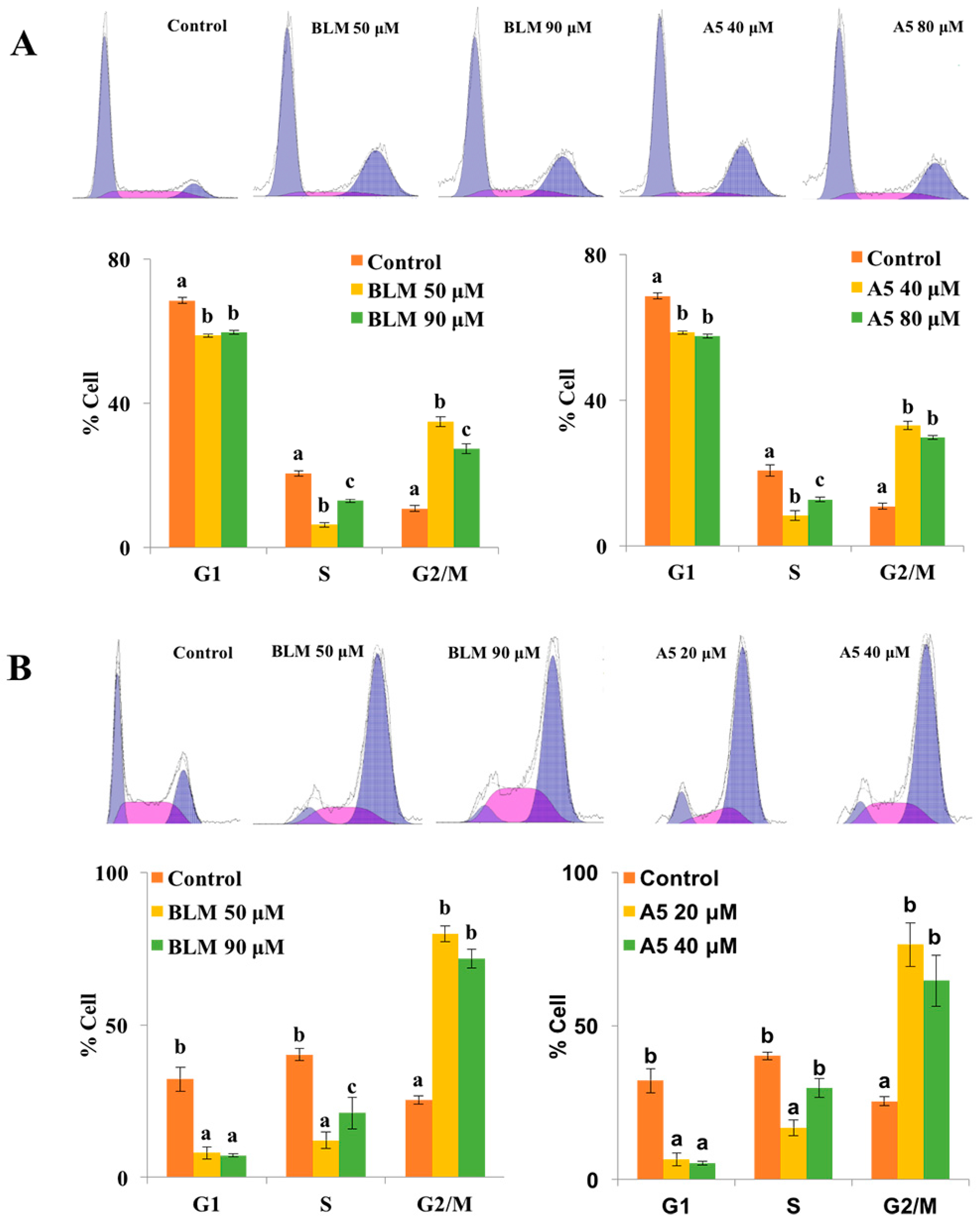
2.3. Both BLM and A5 Induced G2/M Cell Cycle Arrest in A549 and HCT116 Cells
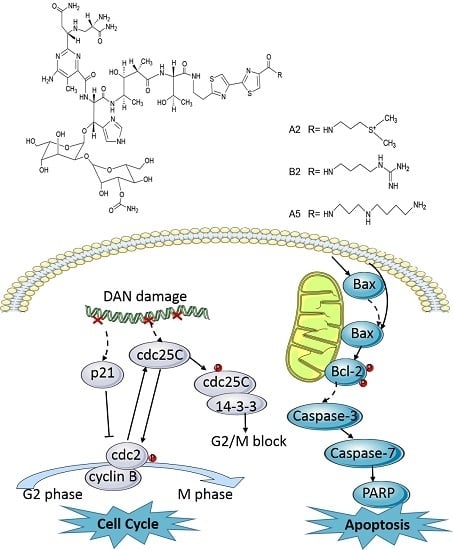
2.4. Both BLM and A5 Modified Cell Cycle and Apoptosis-Related Signaling Proteins
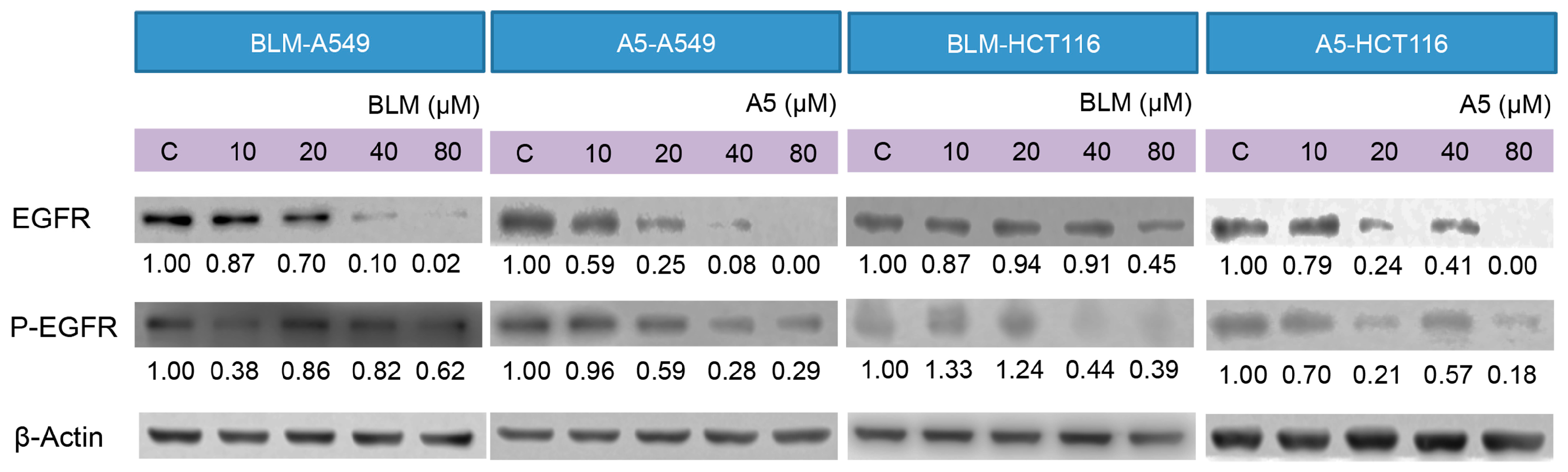
2.5. BLM and A5 Inhibited EGFR Expression and Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Materials and Instruments
4.2. Preparing A2 and B2 from BLM by HPLC
4.3. Cell Growth Inhibition Assay
4.4. Cell Cycle Analysis
4.5. Western Blot Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lazo, J. Bleomycin. Cancer Chemother. Biol. Response Modif. 1999, 18, 39–45. [Google Scholar] [PubMed]
- Chen, J.; Stubbe, J. Bleomycins: Towards better therapeutics. Nat. Rev. Cancer 2005, 5, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Li, D.; Yang, X.; Li, Q.; Xue, Y.; Zhen, Y. Antitumor activity and preclinical pharmacologic evaluation of pingyangmycin (author’s transl). Chin. J. Oncol. 1979, 1, 161–167. [Google Scholar]
- Organization, W.H. WHO Model List of Essential Medicines: 19th List; WHO: Geneva, switzerland, 2013. [Google Scholar]
- Sneader, W.E. Drug Discovery (The History); Wiley Online Library: Chichester, UK, 2005. [Google Scholar]
- Brosseau, N.; Andreev, E.; Ramotar, D. Complementation of the Yeast Model System Reveals that Caenorhabditis elegans OCT-1 Is a Functional Transporter of Anthracyclines. PLoS ONE 2015, 10, e0133182. [Google Scholar] [CrossRef] [PubMed]
- Aouida, M.; Poulin, R.; Ramotar, D. The human carnitine transporter SLC22A16 mediates high affinity uptake of the anticancer polyamine analogue bleomycin-A5. J. Biol. Chem. 2010, 285, 6275–6284. [Google Scholar] [CrossRef] [PubMed]
- Aouida, M.; Khodami-Pour, A.; Ramotar, D. Novel role for the Saccharomyces cerevisiae oligopeptide transporter Opt2 in drug detoxification In memory of Ghassan Belhadj. Biochem. Cell Biol. 2009, 87, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Akiyama, Y.; Xu, Z.; Konishi, K.; Hecht, S.M. Identification and cleavage site analysis of DNA sequences bound strongly by bleomycin. J. Am. Chem. Soc. 2009, 131, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, F.; Zou, G. Electrophoresis mobility shift assay and biosensor used in studying the interaction between bleomycin A5 and DNA. Anal. Chim. Acta 2007, 599, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-D.; Wang, M.; Xiao, S.-L.; Yang, M. Novel peptide derivatives of bleomycin A 5: Synthesis, antitumor activity and interaction with DNA. Bioorg. Med. Chem. Lett. 2005, 15, 3996–3999. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.M. Bleomycin: New perspectives on the mechanism of action 1. J. Nat. Prod. 2000, 63, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Du, F.; Zhou, B.R.; Zhou, H.; Zou, G.L.; Wang, C.X.; Qu, S.S. Thermodynamics and kinetics of the cleavage of DNA catalyzed by bleomycin A5. Eur. J. Biochem. 2002, 269, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cui, K.; Espin-Garcia, O.; Cheng, D.; Qiu, X.; Chen, Z.; Moore, M.; Bristow, R.G.; Xu, W.; Der, S. Resistance to bleomycin in cancer cell lines is characterized by prolonged doubling time, reduced DNA damage and evasion of G2/M arrest and apoptosis. PLoS ONE 2013, 8, e82363. [Google Scholar] [CrossRef] [PubMed]
- Aouida, M.; Ramotar, D. A new twist in cellular resistance to the anticancer drug bleomycin-A5. Curr. Drug Metab. 2010, 11, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Ramotar, D.; Wang, H. Protective mechanisms against the antitumor agent bleomycin: Lessons from Saccharomyces cerevisiae. Curr. Genet. 2003, 43, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Hrycyna, C.; Schoenlein, P.V.; Germann, U.; Pastan, I. Genetic analysis of the multidrug transporter. Annu. Rev. Genet. 1995, 29, 607–649. [Google Scholar] [CrossRef] [PubMed]
- Decottignies, A.; Goffeau, A. Complete inventory of the yeast ABC proteins. Nat. Genet. 1997, 15, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ramotar, D. Cellular resistance to bleomycin in Saccharomyces cerevisiae is not affected by changes in bleomycin hydrolase levels. Biochem. Cell Biol. 2002, 80, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Zimny, J.; Sikora, M.; Guranowski, A.; Jakubowski, H. Protective mechanisms against homocysteine toxicity the role of bleomycin hydrolase. J. Biol. Chem. 2006, 281, 22485–22492. [Google Scholar] [CrossRef] [PubMed]
- Poddevin, B.; Orlowski, S.; Belehradek, J., Jr.; Mir, L.M. Very high cytotoxicity of bleomycin introduced into the cytosol of cells in culture. Biochem. Pharmacol. 1991, 42, S67–S75. [Google Scholar] [CrossRef]
- Aouida, M.; Tounekti, O.; Belhadj, O.; Mir, L.M. Comparative roles of the cell wall and cell membrane in limiting uptake of xenobiotic molecules by Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2003, 47, 2012–2014. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, S.; Belehradek, J.; Paoletti, C.; Mir, L.M. Transient electropermeabilization of cells in culture: Increase of the cytotoxicity of anticancer drugs. Biochem. Pharmacol. 1988, 37, 4727–4733. [Google Scholar] [CrossRef]
- Tounekti, O.; Pron, G.; Belehradek, J.; Mir, L.M. Bleomycin, an apoptosis-mimetic drug that induces two types of cell death depending on the number of molecules internalized. Cancer Res. 1993, 53, 5462–5469. [Google Scholar] [PubMed]
- Aouida, M.; Pagé, N.; Leduc, A.; Peter, M.; Ramotar, D. A genome-wide screen in Saccharomyces cerevisiae reveals altered transport as a mechanism of resistance to the anticancer drug bleomycin. Cancer Res. 2004, 64, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Belting, M.; Borsig, L.; Fuster, M.M.; Brown, J.R.; Persson, L.; Fransson, L.-Å.; Esko, J.D. Tumor attenuation by combined heparan sulfate and polyamine depletion. Proc. Natl. Acad. Sci. USA 2002, 99, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Christianson, H.C.; Belting, M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014, 35, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.J.; Sharma, R.; Nisakar, D.; Purohit, G.; Ganguli, M. Exogenous chondroitin sulfate glycosaminoglycan associate with arginine-rich peptide–DNA complexes to alter their intracellular processing and gene delivery efficiency. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zaia, J. Organ-specific heparan sulfate structural phenotypes. J. Biol. Chem. 2009, 284, 11806–11814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Glycosaminoglycan (GAG) biosynthesis and GAG-binding proteins. Prog. Mol. Biol. Transl. 2010, 93. [Google Scholar] [CrossRef]
- Favretto, M.E.; Wallbrecher, R.; Schmidt, S.; Putte, R.V.D.; Brock, R. Glycosaminoglycans in the cellular uptake of drug delivery vectors—Bystanders or active players? J. Control. Release 2014, 180, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Intern. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dane, M.J.; van den Berg, B.M.; Lee, D.H.; Boels, M.G.; Tiemeier, G.L.; Avramut, M.C.; van Zonneveld, A.J.; van der Vlag, J.; Vink, H.; Rabelink, T.J. A microscopic view on the renal endothelial glycocalyx. Am. J. Physiol. Renal Physiol. 2015, 308, F956–F966. [Google Scholar] [CrossRef] [PubMed]
- Piva, M.B.; Suarez, E.R.; Melo, C.M.; Cavalheiro, R.P.; Nader, H.B.; Pinhal, M.A. Glycosaminoglycans affect heparanase location in CHO cell lines. Glycobiology 2015, 25, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Della Latta, V.; Cecchettini, A.; Del Ry, S.; Morales, M. Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions. Pharmacol. Res. 2015, 97, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.M. CHO quasispecies—Implications for manufacturing processes. Processes 2013, 1, 296–311. [Google Scholar] [CrossRef]
- Esko, J.D.; Stewart, T.E.; Taylor, W.H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 1985, 82, 3197–3201. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Qian, Y.; Zhou, X.; Lu, H.; Ramacciotti, E.; Zhang, L. Chemically oversulfated glycosaminoglycans are potent modulators of contact system activation and different cell signaling pathways. J. Biol. Chem. 2010, 285, 22966–22975. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Matsuda, D.; Ohtawa, M.; Fukuda, T.; Nagamitsu, T.; Yamori, T.; Tomoda, H. Potentiation of bleomycin in jurkat cells by fungal pycnidione. Biol. Pharm. Bull. 2012, 35, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-C.; Li, S.-H.; Majima, H.J.; Huang, Y.-H.; Chen, C.-P.; Liu, C.-C.; Tu, Y.-C.; Chen, C.-W. Upregulation of antioxidant enzymes and coenzyme Q10 in a human oral cancer cell line with acquired bleomycin resistance. Free Radic. Res. 2011, 45, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Hagimori, K.; Fukuda, T.; Hasegawa, Y.; Ōmura, S.; Tomoda, H. Fungal malformins inhibit bleomycin-induced G2 checkpoint in Jurkat cells. Biol. Pharm. Bull. 2007, 30, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-T.; Wolter, K.G.; Youle, R.J. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 3668–3672. [Google Scholar] [CrossRef] [PubMed]
- Oliver, F.J.; de la Rubia, G.; Rolli, V.; Ruiz-Ruiz, M.C.; de Murcia, G.; Ménissier-de Murcia, J. Importance of poly (ADP-ribose) polymerase and its cleavage in apoptosis Lesson from an uncleavable mutant. J. Biol. Chem. 1998, 273, 33533–33539. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y. The EGFR family and its ligands in human cancer: Signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 2001, 37, 3–8. [Google Scholar] [CrossRef]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Downward, J.; Parker, P.; Waterfield, M. Autophosphorylation sites on the epidermal growth factor receptor. Nature 1984, 311, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-H.; Liu, X.-J.; Li, Y.; Zhen, Y.-S. Pingyangmycin downregulates the expression of EGFR and enhances the effects of cetuximab on esophageal cancer cells and the xenograft in athymic mice. Cancer Chemother. Pharm. 2012, 69, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Belehradek, J.J.; Orlowski, S.; Ramirez, L.H.; Pron, G.; Poddevin, B.; Mir, L.M. Electropermeabilization of cells in tissues assessed by the qualitative and quantitative electroloading of bleomycin. Biochim. Biophys. Acta Biomembr. 1994, 1190, 155–163. [Google Scholar] [CrossRef]
- Pron, G.; Belehradek, J.; Orlowski, S.; Mir, L.M. Involvement of membrane bleomycin-binding sites in bleomycin cytotoxicity. Biochem. Pharmacol. 1994, 48, 301–310. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.; Westrick, L.; Esko, J.; Mosher, D. Altered metabolism of thrombospondin by Chinese hamster ovary cells defective in glycosaminoglycan synthesis. J. Biol. Chem. 1988, 263, 6400–6406. [Google Scholar] [PubMed]
- Weyers, A. A structural analysis of glycosaminoglycans from lethal and nonlethal breast cancer tissues: Toward a novel class of theragnostics for personalized medicine in oncology? Omics J. Integr. Biol. 2012, 16, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Schwartz, J.J.; Miller, J.; Liu, J.; Fritze, L.M.; Shworak, N.W.; Rosenberg, R.D. The retinoic acid and cAMP-dependent upregulation of 3-O-sulfotransferase-1 leads to a dramatic augmentation of anticoagulantly active heparan sulfate biosynthesis in F9 embryonal carcinoma cells. J. Biol. Chem. 1998, 273, 27998–28003. [Google Scholar] [CrossRef] [PubMed]
- Shworak, N.W. High-Specific-Activity 35 S-Labeled Heparan Sulfate Prepared from Cultured Cells. Proteoglycan Protocols 2001, 171, 79–89. [Google Scholar]
- McDowell, L.M.; Frazier, B.A.; Studelska, D.R.; Giljum, K.; Chen, J.; Liu, J.; Yu, K.; Ornitz, D.M.; Zhang, L. Inhibition or activation of Apert syndrome FGFR2 (S252W) signaling by specific glycosaminoglycans. J. Biol. Chem. 2006, 281, 6924–6930. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.I.; Lidholt, K.; Flay, N.W.; Liang, J.; Vertel, B.; Lindahl, U.; Esko, J.D. Stable heparin-producing cell lines derived from the Furth murine mastocytoma. Proc. Natl. Acad. Sci. USA 1992, 89, 11327–11331. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J.H.; Caputo, C.; Hascall, V. The effect of cycloheximide on synthesis of proteoglycans by cultured chondrocytes from the Swarm rat chondrosarcoma. J. Biol. Chem. 1981, 256, 4368–4376. [Google Scholar] [PubMed]
- Dechecchi, M.; Melotti, P.; Bonizzato, A.; Santacatterina, M.; Chilosi, M.; Cabrini, G. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J. Virol. 2001, 75, 8772–8780. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Alexander, R.E.; MacLennan, G.T.; Cummings, O.W.; Montironi, R.; Lopez-Beltran, A.; Cramer, H.M.; Davidson, D.D.; Zhang, S. Molecular pathology of lung cancer: Key to personalized medicine. Mod. Pathol. 2012, 25, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Pao, W.; Miller, V.A.; Politi, K.A.; Ricly, G.; Somwar, R.; Zakowski, M.F.; Kris, M.G.; Varmus, H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005, 2, e73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Wei, S.; Song, Y. T790M and acquired resistance of EGFR TKI: A literature review of clinical reports. J. Thorac. Dis. 2011, 3, 10–18. [Google Scholar] [PubMed]
- Wu, K.; House, L.; Liu, W.; Cho, W. Personalized targeted therapy for lung cancer. Int. J. Mol. Sci. 2012, 13, 11471–11496. [Google Scholar] [CrossRef] [PubMed]
- Avirutnan, P.; Zhang, L.; Punyadee, N.; Manuyakorn, A.; Puttikhunt, C.; Kasinrerk, W.; Malasit, P.; Atkinson, J.P.; Diamond, M.S. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathog. 2007, 3, e183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lawrence, R.; Frazier, B.A.; Esko, J.D. CHO glycosylation mutants: Proteoglycans. Methods Enzymol. 2006, 416, 205–221. [Google Scholar] [PubMed]
- Zhang, L.; Beeler, D.L.; Lawrence, R.; Lech, M.; Liu, J.; Davis, J.C.; Shriver, Z.; Sasisekharan, R.; Rosenberg, R.D. 6-O-sulfotransferase-1 represents a critical enzyme in the anticoagulant heparan sulfate biosynthetic pathway. J. Biol. Chem. 2001, 276, 42311–42321. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Zhang, M.; Hao, C.; Wang, W.; Qiu, P.; Wan, S.; Zhang, L.; Jiang, T. Different cytotoxicities and cellular localizations of novel quindoline derivatives with or without boronic acid modifications in cancer cells. Chem. Commun. 2013, 49, 8516–8518. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Yang, C.S.; Li, S.; Jin, H.; Ho, C.T.; Patel, T. Monodemethylated polymethoxyflavones from sweet orange (Citrus sinensis) peel Inhibit growth of human lung cancer cells by apoptosis. Mol. Nutr. Food Res. 2009, 53, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cui, Y.; Xu, L.; Wang, S.; Zhang, M. The Anticancer Mechanism of an Approved Disease Modifying Herb Medicine: Total Glucosides of Paeony Target both EGFR and HER-2 in Lung Cancer Cell Lines. Transl. Med. 2014, 15, 129. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds, Bleomycin and Bleomycin A5 are available from the authors.


| BLMs | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| A 549 | H 1299 | HCT 116 | HT 29 | CHO745 | CHOK1 | |
| BLM | 17.47 ± 2.4 | 233.4 ± 5.2 | 13.35 ± 1.7 | 71.20 ± 0.2 | >500 | 446.6 ± 3.4 |
| A2 | 44.8 ± 2.2 | >500 | 28.1 ± 1.7 | 138.4 ± 3.5 | >500 | >500 |
| B2 | 118.5 ± 0.8 | >500 | 77.3 ± 1.7 | 218.3 ± 7.8 | >500 | >500 |
| A5 | 11.6 ± 1.5 | 71.7 ± 4.1 | 9.6 ± 1.7 | 55.4 ± 1.3 | 327.7 ± 4.3 | 126.4 ± 2.7 |
| A2A5 | 9.0 ± 2.1 | 159.5 ± 4.9 | 5.7 ± 0.1 | 66.0 ± 2.0 | >500 | 125.9 ± 2.9 |
| B2A5 | 13.9 ± 1.9 | 147.2 ± 2.4 | 8.9 ± 1.0 | 79.7 ± 2.7 | >500 | 227.8 ± 3.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Lan, Y.; Liu, Y.; Yu, H.; Han, Z.; Li, X.; Zhang, L. Pingyangmycin and Bleomycin Share the Same Cytotoxicity Pathway. Molecules 2016, 21, 862. https://doi.org/10.3390/molecules21070862
He Y, Lan Y, Liu Y, Yu H, Han Z, Li X, Zhang L. Pingyangmycin and Bleomycin Share the Same Cytotoxicity Pathway. Molecules. 2016; 21(7):862. https://doi.org/10.3390/molecules21070862
Chicago/Turabian StyleHe, Yanli, Ying Lan, Yong Liu, Haibo Yu, Zhangrun Han, Xiulian Li, and Lijuan Zhang. 2016. "Pingyangmycin and Bleomycin Share the Same Cytotoxicity Pathway" Molecules 21, no. 7: 862. https://doi.org/10.3390/molecules21070862
APA StyleHe, Y., Lan, Y., Liu, Y., Yu, H., Han, Z., Li, X., & Zhang, L. (2016). Pingyangmycin and Bleomycin Share the Same Cytotoxicity Pathway. Molecules, 21(7), 862. https://doi.org/10.3390/molecules21070862