A Macrosphelide as the Unexpected Product of a Pleurotus ostreatus Strain-Mediated Biotransformation of Halolactones Containing the gem-Dimethylcyclohexane Ring. Part 1
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Procedures
3.1. General Information
3.2. Biotransformation
3.2.1. Microorganisms
3.2.2. Screening Procedure of Substrates Biotransformation
3.2.3. Preparative Biotransformations
3.2.4. The Influence of Various Factors on the Production of Macrosphelide 4
Temperature
Growth Phase of the Microorganism
Induction of Macrosphelide (4) Production
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014, 41, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Kim, Y.P.; Hiraoka, H.; Natori, M.; Takamatsu, S.; Kawakubo, T.; Masuma, R.; Komiyama, K.; Omura, S. Macrosphelide, a novel inhibitor of cell-cell adhesion molecule I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 1995, 48, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Paek, S.M. Development of advanced macrosphelides: Potent anticancer agents. Molecules 2015, 20, 4430–4449. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Iritani, M.; Minoura, K.; Numata, A.; Kobayashi, Y.; Wang, Y.G. Absolute stereostructures of cell adhesion inhibitors, macrosphelides H and L, from Periconia byssoides OUPS-N133. J. Antibiot. 2002, 55, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Kornienko, A.; Cimmino, A.; Andolfi, A.; Lefranc, F.; Mathieu, V.; Kiss, R. Fungal metabolites with anticancer activity. Nat. Prod. Rep. 2014, 31, 617–627. [Google Scholar] [CrossRef] [PubMed]
- McQuilken, M.P.; Gemmell, J.; Hill, R.A.; Whipps, J.M. Production of macrosphelide A by the mycoparasite Coniothyrium minitans. FEMS Microbiol. Lett. 2003, 219, 27–31. [Google Scholar] [CrossRef]
- Tomprefa, N.; Hill, R.; Whipps, J.; McQuilken, M. Some environmental factors affect growth and antibiotic production by the mycoparasite Coniothyrium minitans. Biocontrol Sci. Technol. 2011, 21, 721–731. [Google Scholar] [CrossRef]
- Ivanova, V.; Kolarova, M.; Aleksieva, K.; Graefe, U.; Schlegel, B. Diphenylether and macrotriolides occurring in a fungal isolate from the antarctic lichen Neuropogon. Prep. Biochem. Biotechnol. 2007, 37, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Paek, S.M. Synthetic advances in macrosphelides: Natural anticancer agents. Molecules 2014, 19, 15982–16000. [Google Scholar] [CrossRef] [PubMed]
- Omura, S. Microbial metabolites: 45 years of wandering, wondering and discovering. Tetrahedron 2011, 67, 6420–6459. [Google Scholar] [CrossRef]
- Matsuya, Y.; Kobayashi, Y.; Kawaguchi, T.; Hori, A.; Watanabe, Y.; Ishihara, K.; Ahmed, K.; Wei, Z.L.; Yu, D.Y.; Zhao, Q.L.; et al. Design, synthesis, and biological evaluation of artificial macrosphelides in the search for new apoptosis-inducing agents. Chem. Eur. J. 2009, 15, 5799–5813. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Persky, L.; Hadar, Y. Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Appl. Microbiol. Biotechnol. 2002, 58, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Maftoun, P.; Johari, H.; Soltani, M.; Malik, R.; Othman, N.Z.; El Enshasy, H.A. The edible mushroom Pleurotus spp.: I. Biodiversity and nutritional values. Int. J. Biotechnol. Wellness Ind. 2015, 4, 67–83. [Google Scholar]
- Golan-Rozen, N.; Chefetz, B.; Ben-Ari, J.; Geva, J.; Hadar, Y. Transformation of the recalcitrant pharmaceutical compound carbamazepine by Pleurotus ostreatus: Role of cytochrome P450 monooxygenase and manganese peroxidase. Environ. Sci. Technol. 2011, 45, 6800–6805. [Google Scholar] [CrossRef] [PubMed]
- Gregori, A.; Svagelj, M.; Pohleven, J. Cultivation techniques and medicinal properties of Pleurotus spp. Food Technol. Biotechnol. 2007, 45, 238–249. [Google Scholar]
- Lehnert, N.; Krings, U.; Wittig, D.S.M.; Berger, R.G. Bioconversion of car-3-ene by a dioxygenase of Pleurotus sapidus. J. Biotechnol. 2012, 159, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, V.; Schaffrath, M.; Zorn, H.; Rehbein, J.; Maison, W. Elucidation of the regio- and chemoselectivity of enzymatic allylic oxidations with Pleurotus sapidus—Conversion of selected spirocyclic terpenoids and computational analysis. Beilstein J. Org. Chem. 2013, 9, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Busmann, D.; Berger, R.G. Conversion of myrcene by submerged cultured basidiomycetes. J. Biotechnol. 1994, 37, 39–43. [Google Scholar] [CrossRef]
- Lobastova, T.G.; Gulevskaya, S.A.; Sukhodolskaya, G.V.; Turchin, K.F.; Donova, M.V. Screening of mycelial fungi for 7a- and 7b-hydroxylase activity towards dehydroepiandrosterone. Biocatal. Biotransform. 2007, 25, 434–442. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Białońska, A. Biotransformations of chloro-, bromo- and iodolactone with trimethylcyclohexane system using fungal strains. Biocatal. Biotransform. 2010, 28, 408–414. [Google Scholar] [CrossRef]
- Grabarczyk, M. Fungal strains as catalysts for the biotransformation of halolactones by hydrolytic dehalogenation with the dimethylcyclohexane system. Molecules 2012, 17, 9741–9753. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, M.; Mączka, W.; Wińska, K.; Żarowska, B.; Anioł, M. Antimicrobial activity of hydroxylactone obtained by biotransformation of bromo- and iodolactone with gem-dimethylcyclohexane ring. J. Braz. Chem. Soc. 2013, 24, 1913–1919. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Mączka, W.; Wińska, K.; Żarowska, B.; Anioł, M. The new halolactones and hydroxylactone with trimethylcyclohexene ring obtained through combined chemical and microbial processes. J. Mol. Catal. B. Enzym. 2014, 102, 195–203. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Wińska, K.; Mączka, W.; Żołnierczyk, A.K.; Żarowska, B.; Anioł, M. Lactones with methylcyclohexane system obtained by chemical and microbiological methods and their antimicrobial activity. Molecules 2015, 20, 3335–3353. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, M.; Wińska, K.; Mączka, W.; Żarowska, B.; Białońska, A.; Anioł, M. Hydroxylactones with the gem-dimethylcyclohexane system—Synthesis and antimicrobial activity. Arabian J. Chem. 2015. [Google Scholar] [CrossRef]
- Sunazuka, T.; Hirose, T.; Chikaraishi, N.; Harigaya, Y.; Hayashi, M.; Komiyama, K.; Sprengeler, P.A.; Smith, A.B., III; Omura, S. Absolute stereochemistries and total synthesis of (±)-macrosphelides, potent, orally bioavailable inhibitors of cell-cell adhesion. Tetrahedron 2005, 61, 3789–3803. [Google Scholar] [CrossRef]
- Ishikara, K.; Kawaguchi, T.; Matsuya, Y.; Sakurai, H.; Saiki, I.; Namoto, H. Synthesis and biological evaluation of macrosphelides cores. Eur. J. Org. Chem. 2004, 2004, 3973–3978. [Google Scholar] [CrossRef]
- Neelam, S.; Chennupati, S.; Singh, S. Comparative studies on growth parameters and physio-chemical analysis of Pleurotus ostreatus and Pleurotus florida. Asian J. Plant Sci. Res. 2013, 3, 163–169. [Google Scholar]
- Ban-Nai, T.; Muramatsu, Y.; Amachi, S. Rate of iodine volatilization and accumulation by filamentous fungi through laboratory cultures. Chemosphere 2006, 65, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.K. Small-molecule elicitation of microbial secondary metabolites. Microb. Biotechnol. 2011, 4, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Grkovic, T.; Balasubramanian, S.; Kamel, M.S.; Quinn, R.J.; Hentschel, U. Elicitation of secondary metabolism in actinomycetes. Biotechnol. Adv. 2015, 33, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, M.; Wang, Z.; Chu, J.; Zhuang, Y.; Zhang, S. Controlling the feed rate of glucose and propanol for the enhancement of erythromycin production and exploration of propanol metabolism fate by quantitative metabolic flux analysis. Bioprocess Biosyst. Eng. 2013, 36, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. Roles of trace metals in transcriptional control of microbial secondary metabolism. Biol. Met. 1990, 2, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Bajwa, R.; Shafique, U.; Anwar, J. Removal of heavy metals by adsorption on Pleurotus ostreatus. Biomass Bioenergy 2011, 35, 1675–1682. [Google Scholar] [CrossRef]
- Stihi, C.; Radulescu, C.; Busuioc, G.; Popescu, I.V.; Gheboianu, A.; Ene, A. Studies on accumulation of heavy metals from substrate to edible wild mushrooms. Rom. J. Phys. 2011, 56, 257–264. [Google Scholar]
- Almeida, S.M.; Umeo, S.H.; Marcante, R.C.; Yokota, M.E.; Valle, J.S.; Dragunski, D.C.; Colauto, N.B.; Linde, G.A. Iron bioaccumulation in mycelium of Pleurotus ostreatus. Braz. J. Microbiol. 2015, 46, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, P.; Lynch, S.; Flood, E.; Finnan, S.; Oliynyk, M. Amphotericin biosynthesis in Streptomyces nodosus: Deductions from analysis of polyketide synthase and late genes. Chem. Biol. 2001, 8, 713–723. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–4 are available from the authors.
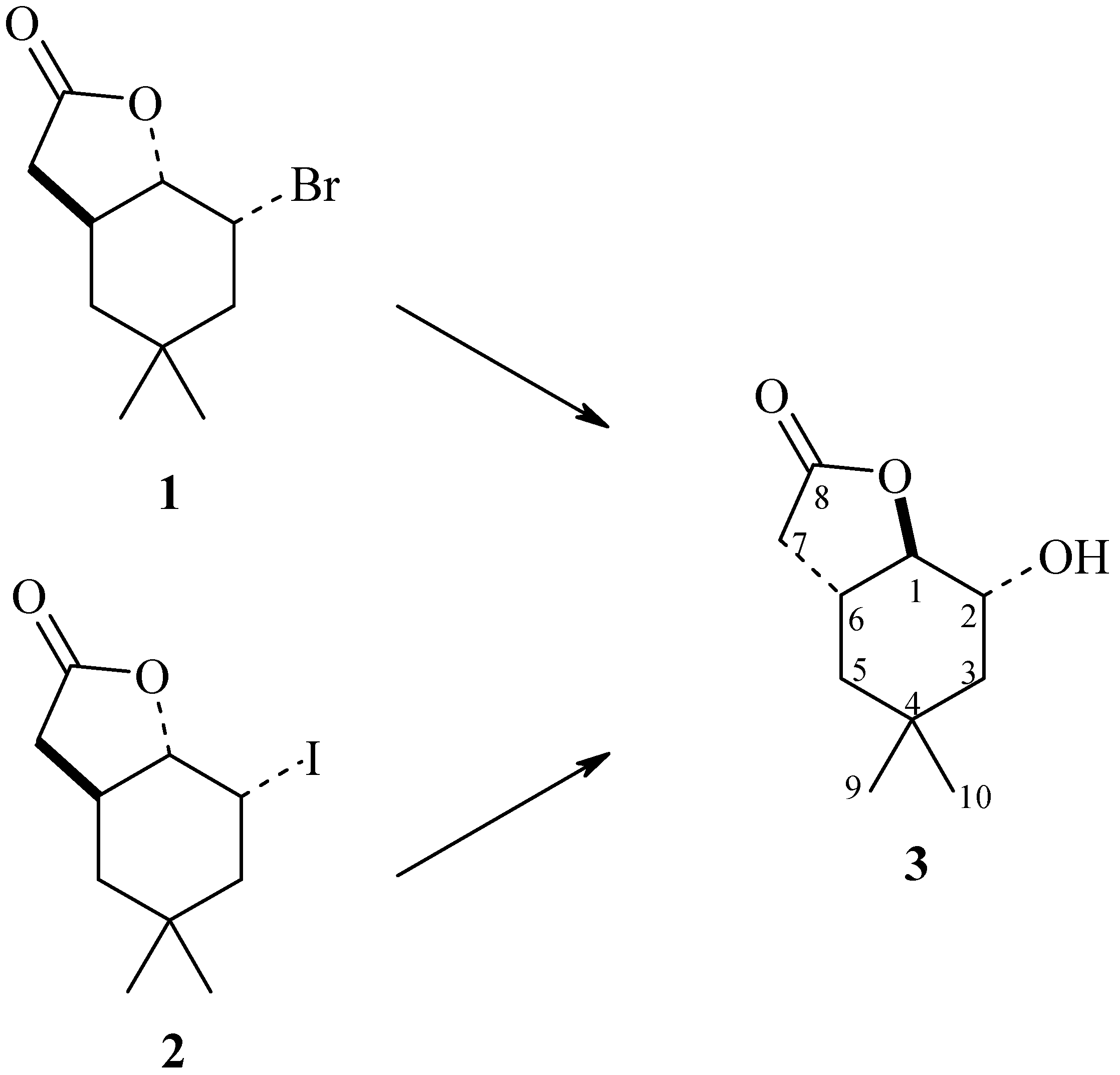
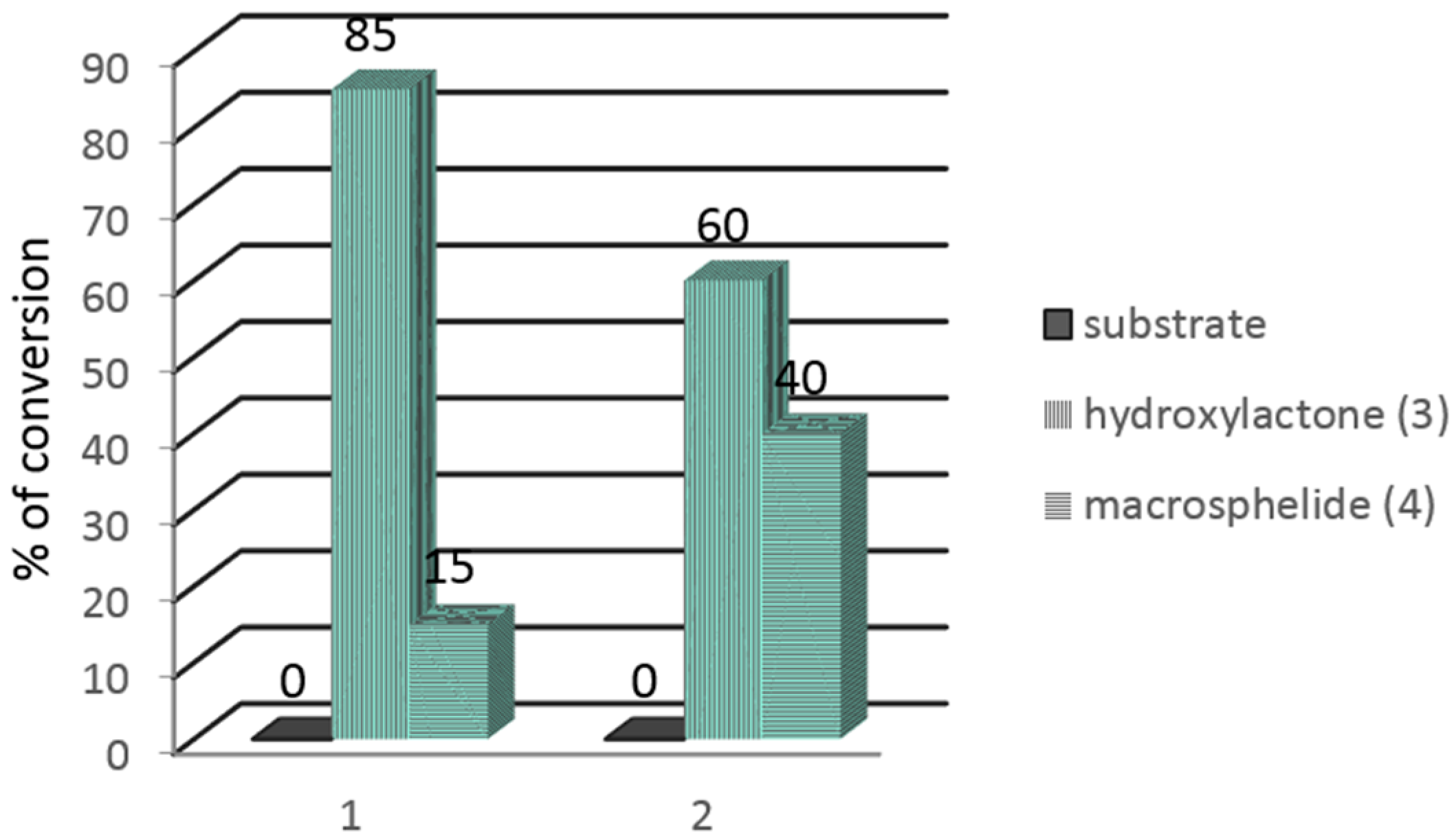
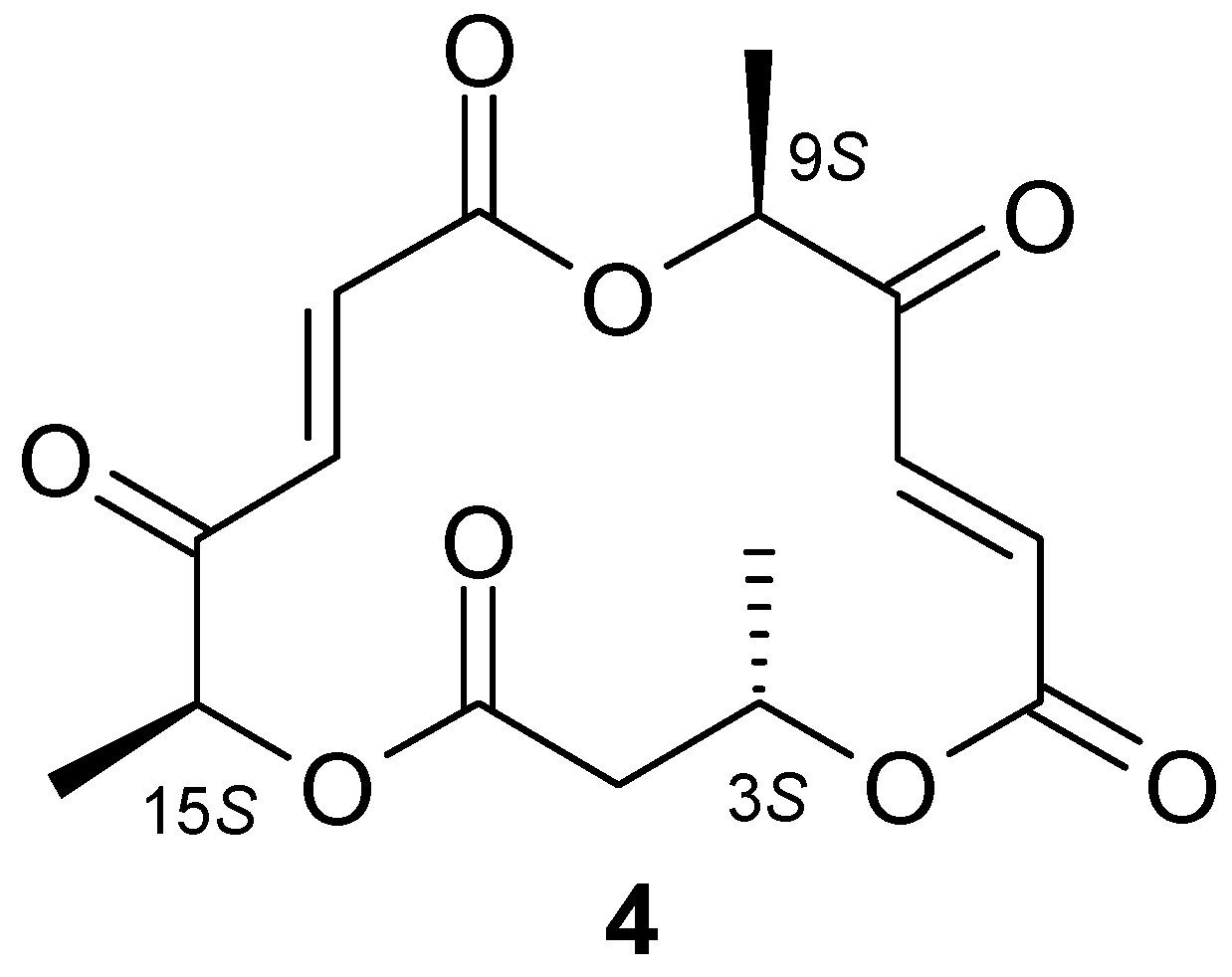
| No. | Strain | Substrate (1) (%) | Product (3) (%) | Substrate (2) (%) | Product (3) (%) |
|---|---|---|---|---|---|
| 1 | Y. lipolytica AM71 | 100 | n.d. | 100 | n.d. |
| 2 | R. marina AM77 | 100 | n.d. | 100 | n.d. |
| 3 | R. rubra AM82 | 100 | n.d. | 100 | n.d. |
| 4 | P. vermiculatum AM30 | 58.8 | 41.2 | 69.0 | 31.0 |
| 5 | A. glauca AM254 | 54.4 | 45.6 | 57.7 | 42.3 |
| 6 | A. cylindrospora AM336 | 100 | n.d. | 73.9 | 26.1 |
| 7 | P. frequentans AM351 | 57.8 | 42.2 | 64.7 | 35.3 |
| 8 | A. ochraceus AM456 | 100 | n.d. | 100 | n.d. |
| 9 | P. ostreatus AM482 | n.d. | 100 | n.d. | 100 |
| Time of Biotransformation (Days) | Iodolactone (2) (%) | Hydroxylactone (3) (%) | Macrosphelide (4) (%) |
|---|---|---|---|
| 3 | 55.7 | 10.3 | 34.0 |
| 5 | 30.2 | 17.9 | 51.9 |
| 7 | 17.2 | 25.5 | 57.3 |
| 9 | n.d. | 65.0 | 35.0 |
| Time of Biotransformation (Days) | Iodolactone (2) (%) | Hydroxylactone (3) (%) | Macrosphelide (4) (%) |
|---|---|---|---|
| 3 | 56.7 | n.d. | 43.3 |
| 5 | 11.6 | 45.4 | 43.0 |
| 7 | n.d. | 51.9 | 48.1 |
| 9 | n.d. | 100 | n.d. |
| No | Inducer | Amount | Result * |
|---|---|---|---|
| 1 | Bromolactone 1 | 10 mg | + |
| 2 | Iodolactone 2 | 10 mg | +++ |
| 3 | Acetone | 1 mL | - |
| 4 | DMSO | 1 mL ** | - |
| 5 | H2O2 | 2 mL ** | - |
| 6 | Ethanol | 1.2 mL ** | - |
| 7 | Propanol | 0.75 mL ** | + |
| 8 | Propionic acid | 0.38 mL ** | - |
| 9 | NaCl | 3 mg–585 mg | - |
| 10 | KBr | 5 mg | + |
| 11 | KI | 5 mg | +++ |
| 12 | Fe2+ | 5 mg | +++ |
| 13 | Fe3+ | 5 mg | ++ |
| 14 | Cu2+ | 5 mg | +++ |
| 15 | Cu+ | 5 mg | + |
| 16 | Co2+ | 5 mg | ++ |
| 17 | Zn2+ | 5 mg | - |
| 18 | Mn2+ | 5 mg | ++ |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wińska, K.; Mączka, W.; Grabarczyk, M.; Sugimoto, K.; Matsuya, Y.; Szumny, A.; Anioł, M. A Macrosphelide as the Unexpected Product of a Pleurotus ostreatus Strain-Mediated Biotransformation of Halolactones Containing the gem-Dimethylcyclohexane Ring. Part 1. Molecules 2016, 21, 859. https://doi.org/10.3390/molecules21070859
Wińska K, Mączka W, Grabarczyk M, Sugimoto K, Matsuya Y, Szumny A, Anioł M. A Macrosphelide as the Unexpected Product of a Pleurotus ostreatus Strain-Mediated Biotransformation of Halolactones Containing the gem-Dimethylcyclohexane Ring. Part 1. Molecules. 2016; 21(7):859. https://doi.org/10.3390/molecules21070859
Chicago/Turabian StyleWińska, Katarzyna, Wanda Mączka, Małgorzata Grabarczyk, Kenji Sugimoto, Yuji Matsuya, Antoni Szumny, and Mirosław Anioł. 2016. "A Macrosphelide as the Unexpected Product of a Pleurotus ostreatus Strain-Mediated Biotransformation of Halolactones Containing the gem-Dimethylcyclohexane Ring. Part 1" Molecules 21, no. 7: 859. https://doi.org/10.3390/molecules21070859









