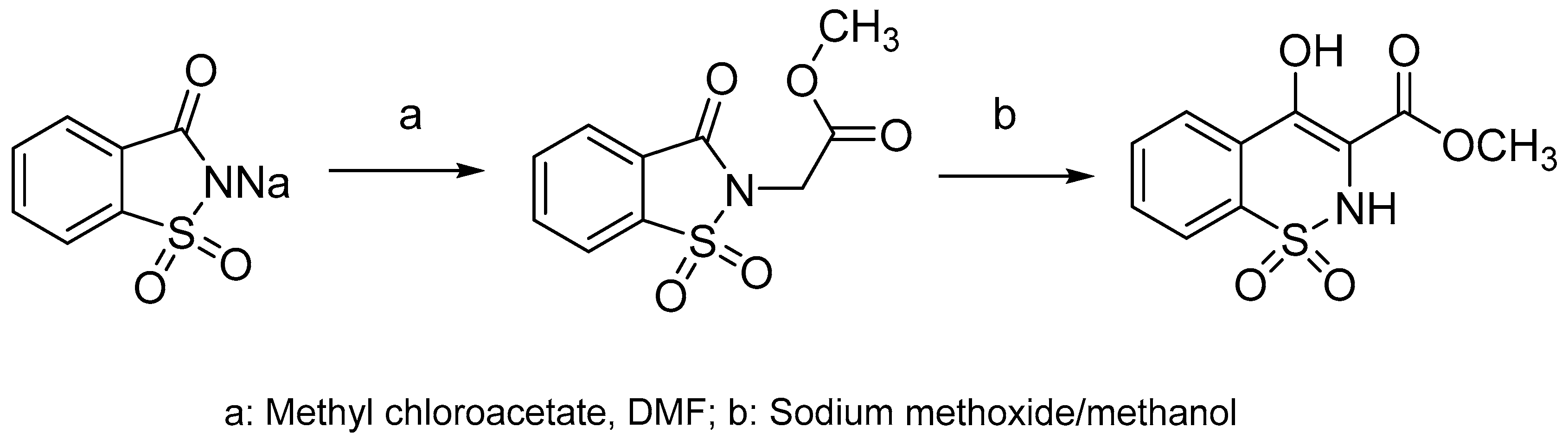
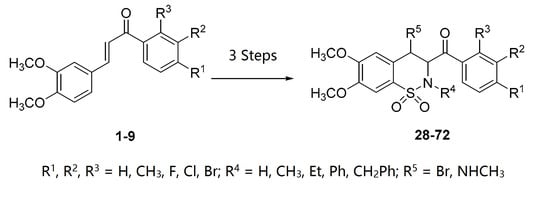
4.5. General Procedure for the Synthesis of Benzothiazines 28–72
To a stirred solution, at room temperature compounds 19–27, (1 g) in ethanol (25 mL) were added drop-wise to a primary amine (3 mol equivalent). The mixture was warmed on a water bath for 10–20 min making sure that the solvent does not evaporate. The mixture was then stirred at room temperature for a further half hour and then ice was added until a precipitate appeared. The resulting solid was filtered and allowed to dry overnight and recrystallized from the appropriate solvent.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(phenyl)methanone (28). Recrystallization solvent: ethanol; yield 68.7%; mp 203–204 °C; IR (KBr, ν cm−1) 1688.9 (C=O), 1598.8 (C-C, aromatic), 1329.6, 1146.2 (SO2). 1H-NMR (CDCl3): δ 3.87 (d, 1H), 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.51(d, 1H), 7.00–8.10 (m, 7H, Ar-H). 13C-NMR (CDCl3): δ 30.00, 46.00, 54.25, 56.51, 103.00, 107.00, 112.50, 117.00, 120.00, 128.50, 134.00, 138.00, 149.00, 151.50, 192.10. HR-MS (nES) m/z calcd [M + H]+ 346.0744: observed 346.0741.
(6,7-Dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(phenyl)methanone (29). Recrystallization solvent: ethanol; yield 79.5%; mp 190–192 °C. IR (KBr, ν cm−1) 1598.1 (C=O), 1545.0 (C-C, aromatic), 1383.6, 1157.1 (SO2). 1H-NMR (CDCl3): δ 2.61 (d, 3H, CH3), 2.80 (s, 3H, CH3), 3.93 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.63(d, 1H), 6.80 (s, 1H), 7.24–7.79 (m, 7H, Ar-H), 11.4 (broad singlet, 1H, NH (exchangeable with D2O). 13C-NMR (CDCl3): δ 9.00, 11.00, 30.10, 30.40, 32.10, 32.20, 55.10, 54.20, 101.00, 112.00, 113.00, 126.00, 126.40, 126.81, 128.37, 128.40, 132.00, 160.00, 192.00. HR-MS (nES) m/z calcd [M + H]+ 391.1322: observed 391.1322.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(phenyl)methanone (30). Recrystallization solvent: ethanol; yield 93.0%; mp 200–201 °C. IR (KBr, ν cm−1) 1693.5(C=O), 1594.7 (C-C, aromatic), 1365.8, 1136.0 (SO2). 1H-NMR (CDCl3): δ 1.32 (t, 3H, CH3), 3.48 (q, 2H, CH2), 3.68 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 5.12 (d, 1H, CH), 5.39 (d, 1H, CH), 6.68–7.92 (7H, Ar-H). 13C-NMR (CDCl3): 13.20, 4594, 48.83, 56.20, 56.32, 62.85, 102.61, 108.00, 128.82, 128.90, 129.10, 130.00, 134.10, 134.57, 150.75, 152.83, 193.70. HR-MS (nES) m/z calcd [M + H]+ 454.0318: observed 454.0316.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(phenyl)methanone (31). Recrystallization solvent: ethanol; yield 51.5%; mp 121–124 °C. IR (KBr, ν cm−1) 1689.1 (C=O), 1597.3 (C-C, aromatic), 1382.9, 1142.8 (SO2). 1H-NMR (CDCl3): δ 3.77 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 5.759(d, 1H, CH), 7.04 (d, 1H, CH), 7.10–8.12 (m, 12H, Ar-H). 13C-NMR (CDCl3): δ 29.07, 43.56, 56.46, 56.90, 11.25, 112.45, 121.40, 122.28, 125.30, 129.00, 129.03, 129.24, 130.10, 130.70, 134.20, 136.10, 148.20, 152.25, 190.30. HR-MS (nES) m/z calcd [M + H]+ 502.0318: observed 502.0309.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(phenyl)methanone (32). Recrystallization solvent: ethanol; yield 91.8%; mp 133–134 °C. IR (KBr, ν cm−1) 1685.7 (C=O), 1589.7 (C-C, aromatic), 1359.9, 1145.8 (SO2). 1H-NMR (CDCl3): 3.90 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.38 (d, 1H, CH2), 4.63 (d, 1H, CH2), 5.00 (d, 1H, CH), 5.37 (d, 1H, CH), 7.11–7.85 (12H, Ar-H). 13C-NMR (CDCl3): δ 29.69, 48.50, 52.00, 56.38, 60.50, 98.50, 101.50, 109.00, 122.00, 123.50, 124.00, 128.12, 128.13, 128.68, 128.90. 129.00, 134.00, 135.00, 136.5, 194.00.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(p-tolyl)methanone (33). Recrystallization solvent: ethanol; yield 50.6%; mp 176–177 °C. IR (KBr, ν cm−1) 1681.6 (C=O), 1505.9 (C-C, aromatic), 1329.3, 1153.8 (SO2). 1H-NMR (CDCl3): δ 2.41 (s, 3H, CH3), 3.85 (d, 1H, CH), 3.92 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.50 (d, 1H, CH), 7.00–8.99 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 21.84, 29.68, 45.94, 55.50, 56.48, 104.18, 106.64, 125.00, 129.05, 129.21, 129.73, 146.00, 151.00, 154.00, 189.60.
(6,7-Dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(p-tolyl)methanone (34). Recrystallization solvent: ethanol; yield 79.7%; mp 183–184 °C. IR (KBr, ν cm−1) 1698.9 (C=O), 1575.0 (C-C, aromatic), 1383.0, 1161.1 (SO2). 1H-NMR (CDCl3): δ 2.34 (s, 3H, CH3), 2.59 (s, 3H, CH3), 2.80 (d, 3H, CH3), 3.93 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.62 (d, 1H, CH), 6.81 (d, 1H, CH), 7.16–7.70 (m, 6H, Ar-H), 11.30, (broad singlet, 1H, NH (exchangeable with D2O). 13C-NMR (CDCl3): δ 21.41, 29.16, 30.89, 31.66, 56.46, 56.47, 111.86, 112.71, 126.86, 129.00, 129.05, 137.50, 142.00, 148.10, 152.00, 165.00, 188.10. HR-MS (nES) m/z calcd [M + H]+ 405.1479: observed 405.1474.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(p-tolyl)methanone (35). Recrystallization solvent: ethanol; yield 86.0%; mp 157–158 °C. IR (KBr, ν cm−1) 1670.8 (C=O), 1605.5 (C-C, aromatic), 1303.6, 1150.4 (SO2). 1H-NMR (CDCl3): δ 1.03 (t, 3H, CH3), 2.41 (s, 3H, CH3), 3.34 (q, 2H, CH2), 3.94 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.05 (d, 1H, CH), 5.37 (d, 1H, CH), 7.17–7.91 (6H, Ar-H). 13C-NMR (CDCl3): δ 12.77, 21.77, 29.68, 45.30, 47.56, 56.39, 61.73, 102.27, 109.15, 129.19, 129.20, 129.74, 130.05, 132.00, 146.00, 151.80, 153.00, 192.80. HR-MS (nES) m/z calcd [M + H]+ 468.0475: observed 468.0472.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(p-tolyl)methanone (36). Recrystallization solvent: ethanol; yield 93.6%; mp 146–147 °C. IR (KBr, ν cm−1) 1670.1 (C=O), 1598.9 (C-C, aromatic), 1382.6, 1138.5 (SO2). 1H-NMR (CDCl3): δ 2.4 (s, 3H, CH3), 3.71 (d, 1H, CH), 3.75 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 5.75 (d, 1H, CH), 7.09–8.01 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.68, 44.00, 46.00, 56.49, 56.50, 112.00, 114.00, 122.13, 125.45, 129.14, 129.20, 129.21, 129.73, 134.05, 145.40, 148.05, 152.34, 190.10. HR-MS (nES) m/z calcd [M + H]+ 516.0475: observed 516.0469.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(p-tolyl)methanone (37). Recrystallization solvent: ethanol; yield 94.8%; mp 151–152 °C. IR (KBr, ν cm−1) 1680.3 (C=O), 1606.9 (C-C, aromatic), 1315.7, 1149.1 (SO2). 1H-NMR (CDCl3): δ 2.36 (s, 3H, CH3), 3.89 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.36 (d, 1H, CH2), 4.62 (d, 1H, CH2), 4.98 (d, 1H), 5.35 (d, 1H), 7.12–7.75 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 21.77, 29.69, 48.07, 51.18, 56.32, 56.37, 102.24, 108.91, 127.85, 128.67, 128.92, 128.96, 129.12, 129.20, 130.10, 132.00, 135.07, 146.05, 151.10, 153.20, 192.45. HR-MS (nES) m/z calcd [M + H]+ 530.0631: observed 530.0626.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-chlorophenyl)methanone (38). Recrystallization solvent: ethanol; yield 73.1%; mp 184–185 °C. IR (KBr, ν cm−1) 1686.0 (C=O), 1589.0 (C-C, aromatic), 1334.4, 1152.3 (SO2). 1H-NMR (CDCl3): δ 3.80 (s, 1H, CH), 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.50 (d, 1H, CH), 7.00–8.06 (m, 6 H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 45.85, 55.43, 56.51, 104.19, 106.64, 124.00, 128.00, 129.46, 130.49, 133.00, 141.00, 151.00, 154.00, 182.00. HR-MS (nES) m/z calcd [M − Br]+ 380.0354: observed 380.0356.
(4-Chlorophenyl)(6,7-dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)methanone (39). Recrystallization solvent: ethanol; yield 83.3%; mp 252–253 °C. IR (KBr, ν cm−1) 1668.1 (C=O), 1541.2 (C-C, aromatic), 1381.2, 1144.7 (SO2). 1H-NMR (CDCl3): δ 2.60 (s, 3H, CH3), 2.81 (d, 3H, NHCH3), 3.93 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.85 (broad singlet, 1H, NH (exchangeable with D2O), 5.57 (d, 1H, CH), 6.78 (d, 1H, CH), 7.24–7.71 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 29.60, 29.69, 32.00, 56.47, 56.49, 91.00, 111.06, 112.25, 127.00, 128.21, 128.57, 137.35, 138.56, 149.34, 152.00, 163.00, 186.25. HR-MS (nES) m/z calcd [M + H]+ 425.0932: observed 425.0931.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-chlorophenyl)methanone (40). Recrystallization solvent: ethanol; yield 64.3%; mp 185–186 °C. IR (KBr, ν cm−1) 1681.8 (C=O), 1589.9 (C-C, aromatic), 1364.5, 1136.0 (SO2). 1H-NMR (CDCl3): δ 1.26 (t, 3H, CH3), 3.58 (q, 2H, CH2), 3.58 (d, 1H, CH), 3.82 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 5.13 (d, 1H, CH), 6.78–7.90 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 13.48, 29.69, 39.54, 44.29, 56.28, 56.49, 102.47, 105.91, 127.00, 129.24, 129.67, 131.85, 134.25, 140.05, 150.00, 153.75, 197.00.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-chlorophenyl)methanone (41). Recrystallization solvent: ethanol; Yield 76.8%; mp 162–164 °C. IR (KBr, ν cm−1) 1689.4 (C=O), 1590.1 (C-C, aromatic), 1343.2, 1146.4 (SO2). 1H-NMR (CDCl3): δ 3.76 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 5.70 (d, 1H, CH), 7.10 (d, 1H, CH), 7.24–8.06 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 43.70, 46.25, 56.02, 56.20, 112.20, 113.00, 122.00, 126.05, 129.20, 129.27, 129.42, 130.41, 136.00, 141.25, 149.10, 153.70, 189.50. HR-MS (nES) m/z calcd [M + H]+ 535.9929: observed 535.9926.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-chlorophenyl)methanone (42). Recrystallization solvent: ethanol; yield 58.1%; mp 166–168 °C. IR (KBr, ν cm−1) 1682.5 (C=O), 1589.0 (C-C, aromatic), 1357.9, 1146.9 (SO2). 1H-NMR (CDCl3): δ 3.92 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.35 (d, H, CH2), 4.57 (d, 1H, CH2), 4.92 (d, 1H), 5.48 (d, 1H), 7.10–7.77 (m, 11H, Ar-H). 13C-NMR (CDCl3): 47.71, 51.71, 56.32, 56.37, 60.90, 102.28, 108.85, 128.12, 128.66, 128.90, 129.67, 129.40, 130.33, 131.25, 132.80, 135.20, 141.20, 150.72, 152.29. 191.73. HR-MS (nES) m/z calcd [M + H]+ 550.0085: observed 550.0082.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-bromophenyl)methanone (43). Recrystallization solvent: ethanol; yield 69.8%; mp 193–194 °C. IR (KBr, ν cm−1) 1686.0 (C=O), 1585.1 (C-C, aromatic), 1333.8, 1152.1 (SO2). 1H-NMR (CDCl3): δ 3.79 (d, 1H, CH), 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.50 (d, 1H, CH), 7.00–7.97 (m, 6 H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 45.00, 54.50, 56.51, 106.00, 106.05, 124.00, 127.00, 130.52, 130.60, 132.46, 133.00, 133.05, 154.00, 195.00. HR-MS (nES) m/z calcd [M − Br]+ 423.9849: observed 423.9850.
(4-Bromo-6,7-dimethoxy-2-methyl-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-bromophenyl)methanone (44). Recrystallization solvent: ethanol; yield 78.8%; mp 189–190 °C. IR (KBr, ν cm−1) 1677.3 (C=O), 1585.3 (C-C, aromatic), 1302.6, 1150.3 (SO2). 1H-NMR (CDCl3): δ 2.85 (s, 3H, CH3), 3.94 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.88 (d, 1H, CH), 5.31 (d, 1H, CH), 7.24–7.86 (m, 6 H, Ar-H). 13C-NMR (CDCl3): δ 29.69, 37.38, 46.81, 56.45, 56.56, 102.66, 109.42128.50, 129.00, 129.50, 130.47, 132.29, 133.10, 151.70, 152.00, 192.00.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-bromophenyl)methanone (45). Recrystallization solvent: ethanol; yield 86.3%; mp 171–172 °C. IR (KBr, ν cm−1) 1676.6 (C=O), 1583.7 (C-C, aromatic), 1306.2, 1149.4 (SO2). 1H-NMR (CDCl3): δ 1.02 (t, 3H, CH3), 3.33 (q, 2H, CH2), 3.95 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.02 (d, 1H, CH), 5.32 (d, 1H, CH), 7.11–7.87 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 29.69, 45.20, 47.75, 56.90, 57.00, 62.00, 102.10, 108.80, 110.50, 112.30, 130.20, 130.45, 132.40, 132.60, 151.80, 151.80, 152.10, 192.00. HR-MS (nES) m/z calcd [M + H]+ 517.9267: observed 517.9264.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-bromophenyl)methanone (46). Recrystallization solvent: ethanol; yield 89.5%; mp 218–219 °C. IR (KBr, ν cm−1) 1688.7 (C=O), 1585.0 (C-C, aromatic), 1382.3, 1145.6 (SO2). 1H-NMR (CDCl3): δ 3.76 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 5.70 (d, 1H, CH), 7.05 (d, 1H, CH), 7.11–7.98 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 43.45, 56.05, 56.40, 11.30, 112.10, 120.30, 120.45, 125.37, 127.35, 128.10, 128.45, 129.30, 130.10, 132.10, 132.89, 148.37, 152.35, 189.75. HR-MS (nES) m/z calcd [M + H]+ 579.9423: observed 579.9416.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-bromophenyl)methanone (47). Recrystallization solvent: ethanol; yield 80.6%; mp 176–177 °C. IR (KBr, ν cm−1) 1685.5 (C=O), 1585.3 (C-C, aromatic), 1293.6, 1146.6 (SO2). 1H-NMR (CDCl3): δ 3.89 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.33 (d, 1H, CH2), 4.58 (d, 1H, CH2), 4.91 (d, 1H, CH), 5.28 (d, 1H, CH), 7.09–7.70 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.95, 47.75, 51.45, 56.00, 60.24, 102.00, 108.50, 128.20, 128.69, 128.94, 128.98, 130.39, 132.31, 132.70, 135.10, 135.20, 136.50, 150.50, 152.34, 192.00. HR-MS (nES) m/z calcd [M + H]+ 593.9580: observed 593.9579.
(6,7-Dimethoxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-3-yl)(4-fluorophenyl)methanone (48). Recrystallization solvent: ethanol; yield 58.0%; mp 249–250 °C. IR (KBr, ν cm−1) 1638.2 (C=O), 1597.3 (C-C, aromatic), 1363.4, 1152.7 (SO2). 1H-NMR (CDCl3): δ 3.93 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 6.86–7.84 (m, 7H, Ar-H), 8.2 (broad singlet, 1H, NH (exchangeable with D2O). HR-MS (nES) m/z calcd [M + H]+ 534.0381: observed 534.0376.
(4-Bromo-6,7-dimethoxy-2-methyl-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-fluorophenyl)methanone (49). Recrystallization solvent: ethanol; yield 85.7%; mp 195–196 °C. IR (KBr, ν cm−1) 1685.3 (C=O), 1598.0 (C-C, aromatic), 1325.1, 1146.5 (SO2). 1H-NMR (CDCl3): δ 2.10 (s, 3H, CH3), 3.90 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 4.84 (d, 1H, CH), 5.30 (d, 1H, CH), 7.10–8.00 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 37.25, 46.25, 56.44, 56.50, 103.56, 110.65, 116.15, 117.50, 128.00, 129.67, 131.45, 132.00, 150.50, 152.00, 192.00. HR-MS (nES) m/z calcd [M + H]+ 458.0068: observed 458.0063.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-fluorophenyl)methanone (50). Recrystallization solvent: ethanol; yield 95.3%; mp 190–191 °C. IR (KBr, ν cm−1) 1693.4 (C=O), 1598.3 (C-C, aromatic), 1345.8, 1185.0 (SO2). 1H-NMR (CDCl3): δ 3.92 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.50 (d, 1H, CH), 6.50 (d, 1H, CH), 6.85–8.10 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 16.00, 25.00, 29.70, 38.00, 43.50, 57.00, 110.05, 111.00, 117.00, 117.03, 117.05, 124.00, 131.76, 131.790, 132.00, 164.00, 192.00. HR-MS (nES) m/z calcd [M + H]+ 472.0224: observed 472.0212.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-fluorophenyl)methanone (51). Recrystallization solvent: ethanol; yield 85.2%; mp 170–171 °C. IR (KBr, ν cm−1) 1688.9 (C=O), 1598.8 (C-C, aromatic), 1382.7, 1146.8 (SO2). 1H-NMR (CDCl3): δ 3.76 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 5.71 (d, 1H, CH), 7.12 (d, 1H, CH), 7.24–8.16 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.45, 43.75, 56.34, 56.78, 11.10, 112.25, 116.10, 122.00, 122.30, 125.95, 129.30, 129.46, 130.00, 130.80, 131.30, 131.57, 132.00, 152.40, 189.75. HR-MS (nES) m/z calcd [M + H]+ 520.0224: observed 520.0218.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(4-fluorophenyl)methanone (52). Recrystallization solvent: ethanol; yield 87.7%; mp 173–174 °C. IR (KBr, ν cm−1) 1683.6 (C=O), 1596.9 (C-C, aromatic), 1356.5, 1147.3 (SO2). 1H-NMR (CDCl3): δ 3.90 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.38 (d, 1H, CH2), 4.60 (d, 1H, CH2), 4.98 (d, 1H, CH), 5.31 (d, 1H, CH), 7.10–7.89 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.95, 47.96, 51.87, 56.21, 56.35, 102.35, 109.00, 116.20, 116.25, 128.00, 128.67, 128.94, 130.05, 131.00, 131.50, 132.00, 135.10, 151.58, 152.00, 192.00. HR-MS (nES) m/z calcd [M + H]+ 534.0381: observed 534.0376.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-chlorophenyl)methanone (53). Recrystallization solvent: ethanol; yield 53.5%; mp 181–182 °C. IR (KBr, ν cm−1) 1686.0 (C=O), 1594.0 (C-C, aromatic), 1328.8, 1149.9 (SO2). 1H-NMR (CDCl3): δ 3.81 (d, 1H, CH), 3.94 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.50 (d, 1H, CH), 7.01–8.04 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 45.95, 55.22, 56.51, 56.85, 104.19, 106.65, 127.33, 128.33, 128.84, 129.50, 130.41, 134.51, 135.49, 136.40, 151.44, 154.07, 188.26.
(3-Chlorophenyl)(6,7-dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)methanone (54). Recrystallization solvent: ethanol; yield 77.0%; mp 176–177 °C. IR (KBr, ν cm−1) 1600.9 (C=O), 1505.3 (C-C, aromatic), 1381.1, 1136.4 (SO2). 1H-NMR (CDCl3): δ 2.63 (s, 3H,=3), 2.82 (s, 3H, CH3), 3.93 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.11 (d, 1H, CH), 5.55 (s, 1H, CH), 6.77–7.66 (m, 6H, Ar-H), 11.28, (broad singlet, 1H, NH (exchangeable with D2O). 13C-NMR (CDCl3): δ 29.06, 30.25, 31.84, 56.48, 56.48, 90.98, 111.71, 112.81, 124.82, 126.97, 126.97, 129.57, 130.79, 135.25, 142.00, 149.40, 153.45, 166.00, 186.00. HR-MS (nES) m/z calcd [M + H]+ 425.0932: observed 425.0927.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-chlorophenyl)methanone (55). Recrystallization solvent: ethanol; yield 67.8%; mp 141–142 °C. IR (KBr, ν cm−1) 1688.6 (C=O), 1590.7 (C-C, aromatic), 1300.1, 1143.6 (SO2). 1H-NMR (CDCl3): δ 1.04 (t, 3H, CH3), 3.35 (q, 2H, CH2), 3.94 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.02 (d, 1H, CH), 5.32 (d, 1H, CH), 7.18–7.98 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 12.65, 45.75, 47, 38, 56.43, 56.75, 61.71, 102.37, 109.07, 127.06, 129.03, 129.91, 130.33, 131.50, 132.50, 134.20, 134.70, 150.87, 152.34, 191.82. HR-MS (nES) m/z calcd [M + H]+ 487.9929: observed 487.9923.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-chlorophenyl)methanone (56). Recrystallization solvent: ethanol; yield 78.9%; mp 229–230 °C. IR (KBr, ν cm−1) 1701.3 (C=O), 1595.8 (C-C, aromatic), 1337.2, 1140.8 (SO2). 1H-NMR (CDCl3): δ 3.84 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.40 (d, 1H, CH), 5.80 (d, 1H, CH), 6.90–7.42 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 47.90, 56.37, 56.90, 63.54, 102.97, 107.62, 125.74, 126.78, 128.74, 128.90, 129.78, 130.28, 131.50, 132.00, 134.10, 134.20, 136.50, 138.00, 151.20, 153.12. HR-MS (nES) m/z calcd [M + H]+ 535.9929: observed 535.9925.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-chlorophenyl)methanone (57). Recrystallization solvent: ethanol; yield 74.4%; mp 154–155 °C. IR (KBr, ν cm−1) 1691.2 (C=O), 1591.8 (C-C, aromatic), 1335.9, 1138.2 (SO2). 1H-NMR (CDCl3): δ 3.90 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.40 (d, 1H, CH2), 4.57 (d, 1H, CH2), 4.97 (d, 1H, CH), 5.27 (d, 1H, CH), 7.10–7.85 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 47.75, 51.78, 56.35, 56.39, 60.95, 102.31, 108.85, 126.50, 127.69, 128.22, 128.70, 128.93, 130.19, 131.50, 134.17, 134.50, 134.70, 135.30, 135.50, 151.50, 152.10, 192.00. HR-MS (nES) m/z calcd [M + NH4]+ 567.0351: observed 567.0345.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-bromophenyl)methanone (58). Recrystallization solvent: ethanol; yield 44.5%; mp 177–178 °C. IR (KBr, ν cm−1) 1685.9 (C=O), 1587.6 (C-C, aromatic), 1328.8, 1149.3 (SO2). 1H-NMR (CDCl3): δ 3.80 (d, 1H, CH), 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.49 (d, 1H, CH), 7.00–8.19 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 29.95, 46.10, 55.10, 56.51, 104.10, 106.80, 123.50, 126.40, 127.50, 128.10, 131.35, 131.86, 136.40, 137.24, 151.75, 153.95, 188.10. HR-MS (nES) m/z calcd [M + HBr]+ 423.9849: observed 423.9850.
(3-Bromophenyl)(6,7-dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)methanone (59). Recrystallization solvent: ethanol; yield 76.4%; mp 178–179 °C. IR (KBr, ν cm−1) 1678.8 (C=O), 1570.4 (C-C, aromatic), 1311.2, 1136.4 (SO2). 1H-NMR (CDCl3): δ 2.63 (s, 3H, CH3), 2.82 (d, 3H, NHCH3), 3.93 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.55 (d, 1H, CH), 6.77 (d, 1H, CH), 7.21–7.84 (m, 6H, Ar-H), 11.31 (broad singlet, 1H, NH (exchangeable with D2O). 13C-NMR (CDCl3): δ 29.68, 29.80, 31.83, 56.49, 56.90, 91.20, 111.69, 112.20, 123.10, 125.32, 126.23, 128.10, 129.93, 133.74, 141.35, 149.45, 152.00, 159.56, 186.34. HR-MS (nES) m/z calcd [M + H]+ 469.0427: observed 469.0419.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-bromophenyl)methanone (60). Recrystallization solvent: ethanol; yield 85.2%; mp 130–131 °C. IR (KBr, ν cm−1) 1689.3 (C=O), 1590.2 (C-C, aromatic), 1350.4, 1142.9 (SO2). 1H-NMR (CDCl3): δ 1.02 (t, 3H, CH3), 3.36 (q, 2H, CH2), 3.94 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.03 (d, 1H, CH), 5.32 (d, 1H, CH), 7.17–8.14 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 12.64, 29.69, 45.74, 47.35, 56.43, 61.70, 101.70, 108.00, 122.10, 126.20, 128.20, 130.05, 131.15, 136.05, 137.25, 138.15, 150.25, 152.15, 192.15. HR-MS (nES) m/z calcd [M + H]+ 531.9423: observed 531.9421.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-bromophenyl)methanone (61). Recrystallization solvent: ethanol; yield 80.2%; mp 232–233 °C. IR (KBr, ν cm−1) 1701.6 (C=O), 1595.3 (C-C, aromatic), 1284.2, 1141.5 (SO2). 1H-NMR (CDCl3): δ 3.85 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 5.45 (d, 1H, CH), 5.80 (d, 1H, CH), 6.80–7.94 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 51.93, 56.24, 56.38, 56.48, 62.06, 102.53, 108.04, 127.33, 127.43, 128.17, 129.18, 129.40, 130.03, 132.67, 133.84, 134.86, 138.10, 150.96, 152.97, 196.11. HR-MS (nES) m/z calcd [M + NH4]+ 596.9689: observed 596.9685.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(3-bromophenyl)methanone (62). Recrystallization solvent: ethanol; Yield 96.9%; mp 167–168 °C. IR (KBr, ν cm−1) 1690.8 (C=O), 1590.8 (C-C, aromatic), 1334.6, 1137.9 (SO2). 1H-NMR (CDCl3): δ 3.90 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 4.40 (d, 1H, CH2), 4.56 (d, 1H, CH2), 4.97 (d, 1H, CH), 5.26 (d, 1H, CH), 7.10–7.96 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.69, 47.72, 56.35, 56.39, 60.97, 102.31, 108.85, 122.80, 127.40, 127.75, 128.24, 128.60, 128.71, 128.93, 130.41, 131.89, 135.10, 136.20, 137.25, 151.45, 152.10, 192.23. HR-MS (nES) m/z calcd [M + NH4]+ 610.9845: observed 610.9842.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-chlorophenyl)methanone (63). Recrystallization solvent: ethanol; yield 72.8%; mp 185–186 °C. IR (KBr, ν cm−1) 1696.5 (C=O), 1677.0 (C-C, aromatic), 1337.3, 1153.7 (SO2). 1H-NMR (CDCl3): δ 3.80 (d, 1H, CH), 3.91 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.41 (d, 1H, CH), 6.97–7.53 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 47.50, 56.49, 56.51, 104.00, 106.00, 122.00, 125.00, 126.50, 130.79, 133.90, 147.50, 153.50, 160.0, 161.90, 192.00.
(2-Chlorophenyl)(6,7-dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4dihydro-2H-benzo[e][1,2]thiazin-3-yl)methanone (64). Recrystallization solvent: ethanol; yield 65.4%; mp 118–119 °C. IR (KBr, ν cm−1) 1602.0 (C=O), 1568.8 (C-C, aromatic), 1309.7, 1147.6 (SO2). 1H-NMR (CDCl3): δ 2.56 (s, 3H, CH3), 2.81 (s, 3H, CH3), 3.92 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.91 (d, 1H, CH), 5.26 (s, 1H, CH), 7.24–7.52 (m, 6H, Ar-H), 6.76, (broad singlet, 1H, NH. 13C-NMR (CDCl3): δ 27.80, 32.00, 56.50, 57.50, 95.00, 101.00, 101.90, 111.00, 112.00, 126.00, 127.00, 129.92, 130.00, 131.00, 132.00, 164.00, 190.00. HR-MS (nES) m/z calcd [M + H]+ 425.0932: observed 425.0928.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-chlorophenyl)methanone (65). Recrystallization solvent: ethanol; yield 48.2%; mp 129–131 °C. IR (KBr, ν cm−1) 1701.3 (C=O), 1589.9 (C-C, aromatic), 1383.4, 1141.1 (SO2). 1H-NMR (CDCl3): δ 1.31 (t, 3H, CH3), 3.44 (q, 2H, CH2), 3.82 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 5.29 (d, 1H, CH), 5.44 (d, 1H, CH), 6.86–7.74 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 45.04, 51.63, 52.98, 56.01, 61.49, 62.44, 102.36, 10258, 108.08, 108.72, 127.04, 127.09, 127.68, 130.93, 132.95, 150.85, 152.44, 152.92, 193.34. HR-MS (nES) m/z calcd [M + H]+ 487.9927: observed 487.9918.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-chlorophenyl)methanone (66). Recrystallization solvent: ethanol; yield 84.2%; mp 209–210 °C. IR (KBr, ν cm−1) 1710.0 (C=O), 1587.7 (C-C, aromatic), 1304.6, 1144.7 (SO2). 1H-NMR (CDCl3): δ 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 5.52 (d, 1H, CH), 5.80 (d, 1H, CH), 6.80–7.94 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 52.05, 56.42, 56.46, 102.45, 108.20, 126.36, 126.78, 127.14, 127.56, 127.85, 128.45, 129.56, 130.04, 132.05, 133.56, 137.34, 136.00, 150.78, 153.45, 196.67. HR-MS (nES) m/z calcd [M + H]+ 535.9929 535: observed 535.9924.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-chlorophenyl)methanone (67). Recrystallization solvent: ethanol; yield 90.8%; mp 156–157 °C. IR (KBr, ν cm−1) 1692.5 (C=O), 1592.0 (C-C, aromatic), 1342.2, 1140.2 (SO2). 1H-NMR (CDCl3): δ 3.89 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.45 (d, 1H, CH2), 4.70 (d, 1H, CH2), 5.05 (d, 1H, CH), 5.28 (d, 1H, CH), 7.01–7.42 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 50.75, 52.52, 56.38, 60.45, 102.34, 108.25, 126.50, 127.34, 128.50, 128.78, 129.04, 129.42, 130.87, 131.50, 132.23, 135.60, 135.80, 136.00, 150.70, 152.10, 194.00. HR-MS (nES) m/z calcd [M + NH4]+ 567.0351: observed 567.0342.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-bromophenyl)methanone (68). Recrystallization solvent: ethanol; yield 56.6%; mp 183–184 °C. IR (KBr, ν cm−1) 1696.7 (C=O), 1676.3 (C-C, aromatic), 1336.3, 1153.2 (SO2). 1H-NMR (CDCl3): δ 3.85 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 5.45 (d, 1H, CH), 5.78 (d, 1H, CH) 6.98–7.93 (m, 6H, Ar-H). 13C-NMR (CDCl3): δ 29.70, 47.96, 56.41, 63.53, 102.97, 107.62, 124.10, 125.74, 127.21, 128.00, 129.79, 130.50, 131.65, 137.26. 138.05, 150.12, 190.45. HR-MS (nES) m/z calcd [M − Br]+ 423.9849: observed 423.9849.
(2-Bromophenyl)(6,7-dimethoxy-2-methyl-4-(methylamino)-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)methanone (69). Recrystallization solvent: ethanol; yield 75.2%; mp 122–123 °C. IR (KBr, ν cm−1) 1737.9 (C=O), 1655.7 (C-C, aromatic), 1383.0, 1147.1 (SO2). 1H-NMR (CDCl3): δ 2.55 (s, 3H, CH3), 2.83 (s, 3H, CH3), 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.80 (d, 1H, CH), 5.16 (s, 1H, CH), 6.77–7.53 (m, 6H, Ar-H), 11.35, (broad singlet, 1H, NH (exchangeable with D2O). 13C-NMR (CDCl3): δ 29.31, 29.78, 31.82, 56.51, 56.58, 94.73, 11.70, 112.76, 118.00, 127.44, 128.79, 129.40, 130.21, 130.75, 133.01, 143.45, 148.12, 151.75, 164.91, 192.30. HR-MS (nES) m/z calcd [M + H]+ 469.0427: observed 469.0418.
(4-Bromo-2-ethyl-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-bromophenyl)methanone (70). Recrystallization solvent: ethanol; yield 69.3%; mp 129–130 °C. IR (KBr, ν cm−1) 1689.5 (C=O), 1590.1 (C-C, aromatic), 1350.4, 1142.8 (SO2). 1H-NMR (CDCl3): δ 1.05 (t, 3H, CH3), 3.35 (q, 2H, CH2), 3.89 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 5.03 (d, 1H, CH), 5.29 (d, 1H, CH), 7.19–8.15 (m, 6H, Ar-H). 13C-NMR (CDCl3): 12.64, 45.73, 47.34, 56.35, 56.41, 61.69, 102.35, 109.05, 123.37, 127.48, 129.89, 130.53, 131.94, 132.20, 136.00, 137.08, 150.85, 152.32, 191.72.
(4-Bromo-6,7-dimethoxy-1,1-dioxido-2-phenyl-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-bromophenyl)methanone (71). Recrystallization solvent: ethanol; yield 96.8%; mp 210–211 °C. IR (KBr, ν cm−1) 1709.5 (C=O), 1588.1 (C-C, aromatic), 1305.1, 1144.7 (SO2). 1H-NMR (CDCl3): δ 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 5.52 (d, 1H, CH), 5.79 (d, 1H, CH), 7.24–7.62 (m, 11H, Ar-H). 13C-NMR (CDCl3): δ 29.69, 51.96, 56.42, 56.51, 102.45, 108.23, 119.50, 126.60, 126.85, 127.25, 127.45, 128.85, 128.95, 130.06, 132.32, 132.85, 134.23, 138.45, 151.50, 151.80, 196.10. HR-MS (nES) m/z calcd [M + H]+ 579.9423: observed 579.9423.
(2-Benzyl-4-bromo-6,7-dimethoxy-1,1-dioxido-3,4-dihydro-2H-benzo[e][1,2]thiazin-3-yl)(2-bromophenyl)methanone (72). Recrystallization solvent: ethanol; yield 81.6%; mp 123–125 °C. IR (KBr, ν cm−1) 1690.6 (C=O), 1590.4 (C-C, aromatic), 1334.3, 1137.9 (SO2). 1H-NMR (CDCl3): δ 3.89 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.39 (d, 1H, CH2), 4.55 (d, 1H, CH2), 4.97 (d, 1H, CH), 5.27 (d, 1H, CH), 7.10–7.96 (m, 11H, Ar-H). 13C-NMR (CDCl3): 47.72, 51.74, 56.34, 56.38, 60.97, 102.30, 108.83, 123.80, 127.37, 128.21, 128.68, 128.89, 130.40, 131.85, 135.00, 135.60, 140.10, 150.74, 152.32, 191.62. HR-MS (nES) m/z calcd [M + H]+ 593.9580: observed 593.9578.