Rational Design and Synthesis of New, High Efficiency, Multipotent Schiff Base-1,2,4-triazole Antioxidants Bearing Butylated Hydroxytoluene Moieties
Abstract
:1. Introduction
2. Results and Discussion
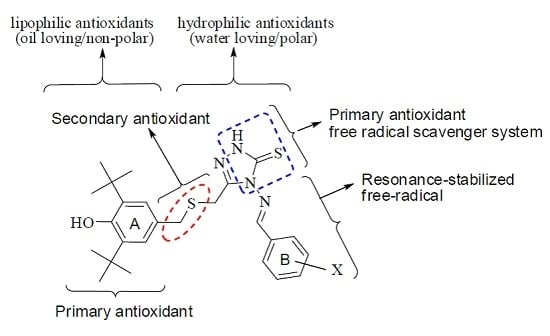
2.1. SAR and Designing of MPAO
2.2. PASS—Prediction
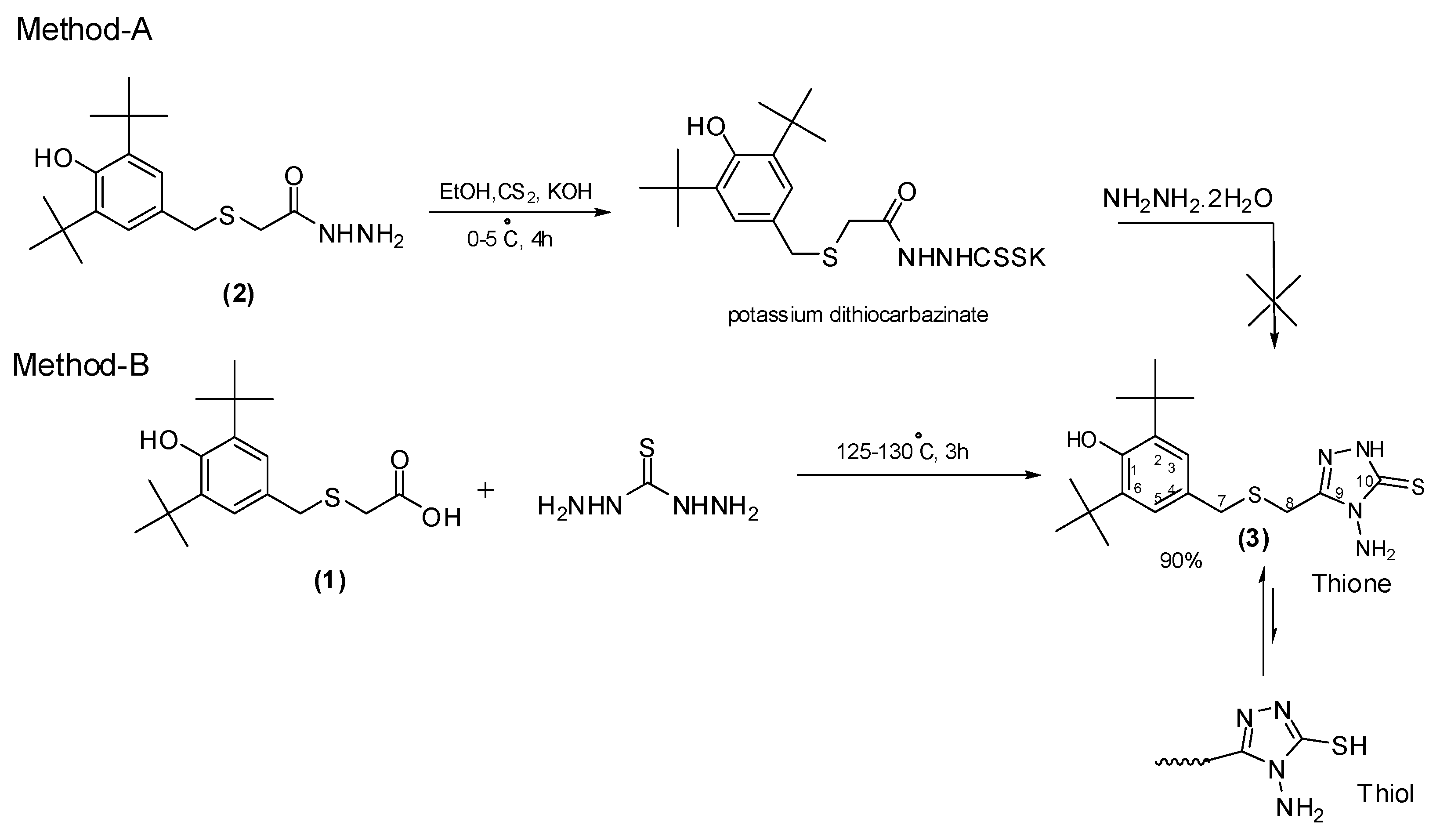
2.3. Synthesis
2.4. In Vitro DPPH Radical Scavenging Activity
2.4.1. BHT Moiety (Primary Antioxidant)
2.4.2. Thioether Function (Secondary Antioxidant)
2.4.3. Triazole-5-thione Ring
2.4.4. Effect of Ring B Substituents on Antioxidant Activity
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 4-Amino-3-((3,5-di-tert-butyl-4-hydroxybenzylthio)methyl)-1H-1,2,4-triazole-5(4H)-thione (3)
3.2.1. Method-A
Potassium Dithiocarbazinate Method
3.2.2. Method-B
Fusion Reaction Method
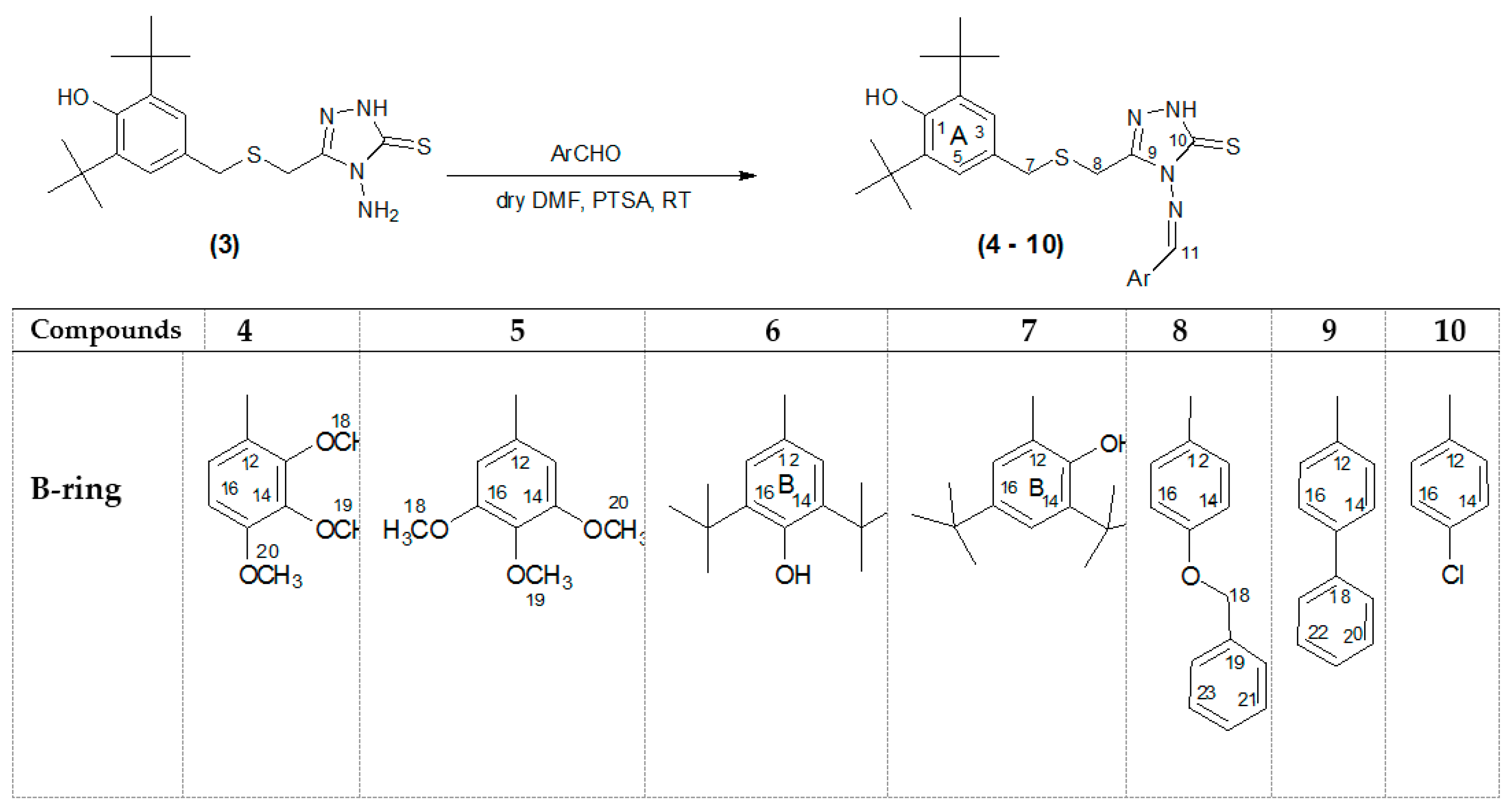
3.3. General Procedure for the Synthesis of 4-(Substituted benzylideneamino)-3-(3,5-di-tert-butyl-4-hydroxybenzyl thio) methyl)-1H-1,2,4-triazole-5(4H)-thiones 4–10
3.4. Antioxidant Assay
In Vitro DPPH Free Radical Scavenging Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Koo, N.; Min, D. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Hsieh, C.; Ho, Y.; Lai, H.; Yen, G. Inhibitory effect of carnosine and anserine on DNA oxidative damage induced by Fe2+, Cu2+ and H2O2 in lymphocytes. J. Food Drug Anal. 2002, 10, 47–54. [Google Scholar]
- Lazer, E.S.; Wong, H.C.; Possanza, G.J.; Graham, A.G.; Farina, P.R. Antiinflammatory 2,6-di-tert-butyl-4-(2-arylethenyl)phenols. J. Med. Chem. 1989, 32, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.; Swingle, K. 2,6-Di-tert-butyl-4-(2-thenoyl) phenol (r-830): A novel nonsteroidal anti-inflammatory agent with antioxidant properties. Agents Actions 1982, 12, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Grosso, P.; Vogl, O. Functional polymers. Polym. Bull. 1985, 14, 245–250. [Google Scholar]
- Dacre, J. The metabolism of 3,5-di-tert-butyl-4-hydroxytoluene and 3,5-di-tert-butyl-4-hydroxybenzoic acid in the rabbit. Biochem. J. 1961, 78, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Schöneich, C. Reactive oxygen species and biological aging: A mechanistic approach. Exp. Gerontol. 1999, 34, 19–34. [Google Scholar] [CrossRef]
- Sahinoglu, T.; Stevens, C.R.; Bhatt, B.; Blake, D.R. The role of reactive oxygen species in inflammatory disease: Evaluation of methodology. Methods 1996, 9, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Petrone, W.; English, D.; Wong, K.; McCord, J. Free radicals and inflammation: Superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc. Natl. Acad. Sci. USA 1980, 77, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Schöneich, C. Methionine oxidation by reactive oxygen species: Reaction mechanisms and relevance to alzheimer’s disease. Biochim. Biophys. Acta Proteins Proteom. 2005, 1703, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Tachibana, M.; Noda, K.; Inoue, N.; Tanaka, M.; Kuwabara, K. Relevance of anti-reactive oxygen species activity to anti-inflammatory activity of components of eviprostat, a phytotherapeutic agent for benign prostatic hyperplasia. Phytomedicine 2007, 14, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Kirkinezos, I.G.; Moraes, C.T. Reactive oxygen species and mitochondrial diseases. Semin. Cell Dev. Biol. 2001, 12, 449–457. [Google Scholar] [CrossRef] [PubMed]
- De Maria, N.; Colantonl, A.; Fagiuoli, S.; Liu, G.-J.; Rogers, B.K.; Farinati, F.; van Thiel, D.H.; Floyd, R.A. Association between reactive oxygen species and disease activity in chronic hepatitis c. Free Radic. Biol. Med. 1996, 21, 291–295. [Google Scholar] [CrossRef]
- Smith, C.; Zhang, Y.; Koboldt, C.; Muhammad, J.; Zweifel, B.; Shaffer, A.; Talley, J.; Masferrer, J.; Seibert, K.; Isakson, P. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Natl. Acad. Sci. USA 1998, 95, 13313–13318. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.; McCreath, K.; Rocha, M. Recent progress in pharmacological research of antioxidants in pathological conditions: Cardiovascular health. Recent Pat. Anti-Infect. Drug Discov. 2006, 1, 17–31. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Feskens, E.J.M.; Kromhout, D.; Hollman, P.C.H.; Katan, M.B. Dietary antioxidant flavonoids and risk of coronary heart disease: The zutphen elderly study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Braid, M. Antioxidant Lubricant Compositions. United States Patent 4198303, 4 April 1980. [Google Scholar]
- Lyons, B.J. Antioxidants of Bisphenolic Polymers. Patent US3986981A, 19 October 1976. [Google Scholar]
- Chang, D. Clear Coat/Color Coat Finish Containing an Antioxidant and an Ultraviolet Light Stabilizer. Patent US4208465A, 17 June 1980. [Google Scholar]
- Yamada, K.; Hung, P.; Yoshimura, K.; Taniguchi, S.; Ou Lim, B.; Sugano, M. Effect of unsaturated fatty acids and antioxidants on immunoglobulin production by mesenteric lymph node lymphocytes of sprague-dawley rats. J. Biochem. 1996, 120, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J. Antioxidants: Function, types and necessity of inclusion in pet foods. Can. Vet. J. 1989, 30, 682–684. [Google Scholar] [PubMed]
- Stecher, P. Butylated hydroxytoluene. In The Merck Index, 8th ed.; O’Neil, M.J., Ed.; Merck Research Laboratories: Rahway, NJ, USA, 1968; p. 179. [Google Scholar]
- Stuckey, B. Antioxidants as food stabilizers. Handb. Food Addit. 1972, 185, 204. [Google Scholar]
- Ariffin, A.; Rahman, N.A.; Yehye, W.A.; Alhadi, A.A.; Kadir, F.A. Pass-assisted design, synthesis and antioxidant evaluation of new butylated hydroxytoluene derivatives. Eur. J. Med. Chem. 2014, 87, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Yehye, W.A.; Abdul Rahman, N.; Alhadi, A.A.; Khaledi, H.; Weng, N.S.; Ariffin, A. Butylated hydroxytoluene analogs: Synthesis and evaluation of their multipotent antioxidant activities. Molecules 2012, 17, 7645–7665. [Google Scholar] [CrossRef] [PubMed]
- Shakir, R.; Ariffin, A.; Abdulla, M. Synthesis of new 2,5-di-substituted 1,3,4-oxadiazoles bearing 2,6-di-tert-butylphenol moieties and evaluation of their antioxidant activity. Molecules 2014, 19, 3436–3449. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Nagasawa, H. Facile synthesis of 1,2,4-triazoles via a copper-catalyzed tandem addition-oxidative cyclization. J. Am. Chem. Soc. 2009, 131, 15080–15081. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Agaiah, B.; Sarangapani, M. Antioxidant and DNA binding study 3,3′-(5,5′-methylene bis(3-mercapto-4H-1,2,4-triazole-5,4-diyl) bis (azan-1-yl-1-ylidene) diindolin-2-ones. Int. J. Pharm. Technol. 2010, 2, 366–374. [Google Scholar]
- Palaska, E.; Sahin, G.; Kelicen, P.; Durlu, N.T.; Altinok, G. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. Farmaco 2002, 57, 101–107. [Google Scholar] [CrossRef]
- Navidpour, L.; Shafaroodi, H.; Abdi, K.; Amini, M.; Ghahremani, M.H.; Dehpour, A.R.; Shafiee, A. Design, synthesis, and biological evaluation of substituted 3-alkylthio-4,5-diaryl-4H-1,2,4-triazoles as selective cox-2 inhibitors. Bioorg. Med. Chem. 2006, 14, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Wujec, M.; Pitucha, M.; Dobosz, M.; Kosikowska, U.; Malm, A. Synthesis and potential antimycotic activity of 4-substituted-3-(thiophene-2-yl-methyl)-δ2-1,2,4-triazoline-5-thiones. Acta Pharm. Short Commun. 2004, 54, 251–260. [Google Scholar]
- Valentina, P.; Ilango, K.; Deepthi, M.; Harusha, P.; Pavani, G.; Sindhura, K.; Keerthanan, C. Antioxidant activity of some substituted 1,2,4-triazo-5-thione schiff base. J. Pharm. Sci. 2009, 1, 74–77. [Google Scholar]
- Poroikov, V.; Filimonov, D.; Lagunin, A.; Gloriozova, T.; Zakharov, A. Pass: Identification of probable targets and mechanisms of toxicity. SAR QSAR Environ. Res. 2007, 18, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kadir, F.A.; Kassim, N.M.; Abdulla, M.A.; Yehye, W.A. Pass-predicted vitex negundo activity: Antioxidant and antiproliferative properties on human hepatoma cells-an in vitro study. BMC Complement. Altern. Med. 2013, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Stepanchikova, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. Prediction of biological activity spectra for substances: Evaluation on the diverse sets of drug-like structures. Curr. Med. Chem. 2003, 10, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Anzali, S.; Barnickel, G.; Cezanne, B.; Krug, M.; Filimonov, D.; Poroikov, V. Discriminating between drugs and nondrugs by prediction of activity spectra for substances (pass). J. Med. Chem. 2001, 44, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Kadir, F.; Kassim, N.B.M.; Abdulla, M.A.; Kamalidehghan, B.; Ahmadipour, F.; Yehye, W. Pass-predicted hepatoprotective activity of caesalpinia sappan in thioacetamide-induced liver fibrosis in rats. Sci. World J. 2014, in press. [Google Scholar] [CrossRef] [PubMed]
- Delmas, F.; di Giorgio, C.; Robin, M.; Azas, N.; Gasquet, M.; Detang, C.; Costa, M.; Timon-David, P.; Galy, J.-P. In vitro activities of position 2 substitution-bearing 6-nitro-and 6-amino-benzothiazoles and their corresponding anthranilic acid derivatives against leishmania infantum and trichomonas vaginalis. Antimicrob. Agents Chemother. 2002, 46, 2588–2594. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, C.; Delmas, F.; Filloux, N.; Robin, M.; Seferian, L.; Azas, N.; Gasquet, M.; Costa, M.; Timon-David, P.; Galy, J.-P. In vitro activities of 7-substituted 9-chloro and 9-amino-2-methoxyacridines and their bis-and tetra-acridine complexes against leishmania infantum. Antimicrob. Agents Chemother. 2003, 47, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, C.; Delmas, F.; Ollivier, E.; Elias, R.; Balansard, G.; Timon-David, P. In vitro activity of the β-carboline alkaloids harmane, harmine, and harmaline toward parasites of the species leishmania infantum. Exp. Parasitol. 2004, 106, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mathew, V.; Keshavayya, J.; Vaidya, V.P. Heterocyclic system containing bridgehead nitrogen atom: Synthesis and pharmacological activities of some substituted 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2006, 41, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Zamani, K.; Faghiiii, K.; Sangi, M.R.; Zolgharnein, J. Synthesis of some new substituted 1,2,4-triazole and 1,3,4-thiadiazole and their derivatives. Turk. J. Chem. 2003, 27, 119–126. [Google Scholar]
- Shakir, R.M.; Ariffin, A.; Ng, S.W. Ethyl 4-[(3,5-di-tert-butyl-2-hydroxybenzylidene)amino]benzoate. Acta Crystallogr. Sect. E 2010, 66, o2915. [Google Scholar] [CrossRef] [PubMed]
- Eklund, P.C.; Langvik, O.K.; Warna, J.P.; Salmi, T.O.; Willfor, S.M.; Sjoholm, R.E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org. Biomol. Chem. 2005, 3, 3336–3347. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Ionita, P. Is DPPH stable free radical a good scavenger for oxygen active species. Chem. Papers 2005, 59, 11–16. [Google Scholar]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH. Free radical method. Lebensm. Wiss. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Dayan, N. Skin Aging Handbook—An Integrated Approach to Biochemistry and Product Development; William Andrew Publishing: Norwich, NY, USA, 2008; p. 352. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Acosta, M.; Arnao, M. A method to measure antioxidant activity in organic media: Application to lipophilic vitamins. Redox Rep. 2000, 5, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kajiyama, T.; Ohkatsu, Y. Effect of para-substituents of phenolic antioxidants. Polym. Degrad. Stab. 2001, 71, 445–452. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F.; Cipollone, M. Bond dissociation enthalpy of α-tocopherol and other phenolic antioxidants. J. Org. Chem. 1994, 59, 5063–5070. [Google Scholar] [CrossRef]
- Scott, G. Antioxidants. Bull. Chem. Soc. Jpn. 1988, 61, 165–170. [Google Scholar] [CrossRef]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (bht): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Barclay, L.R.C.; Vinqvist, M.R. Phenols as Antioxidants; John Wiley and Sons, Ltd.: Chichester, UK, 2003; pp. 839–908. [Google Scholar]
- Klein, E.; Lukeš, V.; Cibulková, Z. On the energetics of phenol antioxidants activity. Pet. Coal 2005, 47, 33–39. [Google Scholar]
- Pizzi, A.; Mittal, K.L. Handbook of Adhesive Technology, 2nd ed.; Pizz, A., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press: Boca Raton, FL, USA, 2005; p. 1024. [Google Scholar]
- Luedtke, A.E.; Timberlake, J.W. Effect of oxidized states of heteroatoms and of orthogonal Pi. Systems on radical stabilities. J. Org. Chem. 1985, 50, 268–270. [Google Scholar] [CrossRef]
- Escobar-Valderrama, J.; Garcia-Tapia, J.; Ramirez-Ortiz, J.; Rosales, M.; Toscano, R.; Valdes-Martinez, J. Crystal, molecular and electronic structure of 1-H-3-methyl-4-amine-5-thione-1,2,4-triazol. Can. J. Chem. 1989, 67, 198–201. [Google Scholar] [CrossRef]
- Khan, I.; Ali, S.; Hameed, S.; Rama, N.; Hussain, M.; Wadood, A.; Uddin, R.; Ul-Haq, Z.; Khan, A. Synthesis, antioxidant activities and urease inhibition of some new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5200–5207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Structure-activity relationships and rational design strategies for radical-scavenging antioxidants. Curr. Comput. Aided Drug Des. 2005, 1, 257–273. [Google Scholar] [CrossRef]
- Nazarbahjat, N.; Nordin, N.; Abdullah, Z.; Abdulla, M.; Yehye, W.; Halim, S.; Kee, C.; Ariffin, A. New thiosemicarbazides and 1,2,4-triazolethiones derived from 2-(ethylsulfanyl) benzohydrazide as potent antioxidants. Molecules 2014, 19, 11520–11537. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Lucarini, M.; Mugnaini, V.; Pedulli, G. Antioxidant activity of o-bisphenols: The role of intramolecular hydrogen bonding. J. Org. Chem. 2003, 68, 5198–5204. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Wu, D. How many peroxyl radicals can be scavenged by hydroxyl-substituted schiff bases in the oxidation of linoleic acid? J. Phys. Org. Chem. 2009, 22, 308–312. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available.

- Phenolic group
- Two tert-butyl substituents flanking the OH group
- Primary antioxidant, acting as chain-breaking antioxidant
- –CH2– that stabilizes and decreases the BDE value of OH
- Secondary antioxidant, hydroperoxide decomposers
- N–H exchangeable proton
- Triazole, inhibitor ring
- Substituted phenyl to increase the free radical stabilization
- Electron donating group
- Thiourea (free radical scavenger system)
- Imino group to extend free radical delocalization




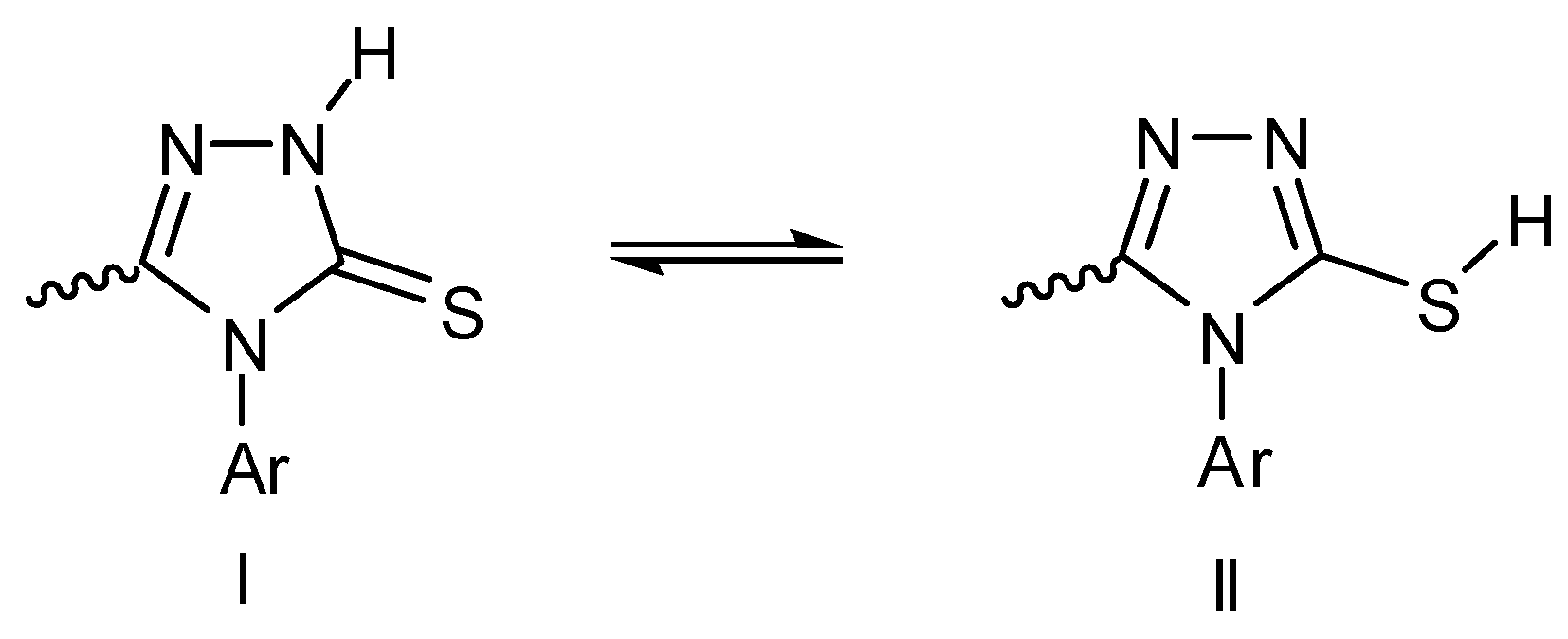
| Mode of Biological Activity | 1 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | BHT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | |
| Lipid peroxidase inhibitor | 0.44 | 0.02 | 0.71 | 0.00 | 0.52 | 0.01 | 0.46 | 0.02 | 0.38 | 0.03 | 0.40 | 0.03 | 0.36 | 0.04 | 0.34 | 0.05 | 0.24 | 0.09 | 0.72 | 0.005 |
| Antioxidant | 0.41 | 0.01 | 0.41 | 0.01 | 0.29 | 0.02 | 0.31 | 0.02 | 0.30 | 0.02 | 0.29 | 0.02 | 0.26 | 0.03 | 0.27 | 0.02 | 0.25 | 0.03 | 0.58 | 0.005 |
| Free radical scavenger | 0.51 | 0.00 | - | - | 0.14 | 0.13 | 0.17 | 0.09 | - | - | - | - | - | - | - | - | - | 0.51 | 0.010 | |
| Lipoxygenase inhibitor | 0.37 | 0.00 | 0.21 | 0.01 | 0.17 | 0.02 | 0.18 | 0.02 | 0.18 | 0.02 | 0.17 | 0.02 | 0.22 | 0.01 | 0.18 | 0.02 | 0.16 | 0.02 | 0.56 | 0.004 |
| 5-Lipoxygenase inhibitor | 0.28 | 0.00 | 0.13 | 0.01 | 0.11 | 0.02 | 0.11 | 0.02 | 0.18 | 0.02 | 0.10 | 0.02 | 0.14 | 0.01 | 0.11 | 0.02 | 0.10 | 0.02 | 0.47 | 0.004 |
| Entry | Compound 1 Mole | Thiocarbohydrazide Mole | Time h | Temperature °C | Yield (%) |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 3 | 90–120 | 24 |
| 2 | 1 | 1.25 | 3 | 125–130 | 70 |
| 3 | 1 | 1.25 | 3 | 155–160 | 45 |
| 4 | 1 | 1.5 | 3 | 125–130 | 90 |
| 5 | 1 | 2 | 3 | 125–130 | 90 |
| 6 | 1 | 1.5 | 3 | 145–150 | 65 |
| Compound | DPPH IC50 a Values (µM) ± S.E.M b | Radical Scavenging Max. Inhibition% ± S.E.M |
|---|---|---|
| 4 | 64.04 ± 0.32 | 68.13 ± 0.27 |
| 5 | 58.26 ± 0.63 | 74.32 ± 0.14 |
| 6 | 46.13 ± 0.31 | 89.52 ± 0.09 |
| 7 | 52.80 ± 0.58 | 79.61 ± 0.23 |
| 8 | 67.59 ± 0.27 | 66.72 ± 0.15 |
| 9 | 68.04 ± 0.25 | 63.37 ± 0.13 |
| 10 | 72.16 ± 0.46 | 57.18 ± 0.21 |
| BHT | >100 c | 25.23 ± 0.17 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yehye, W.A.; Abdul Rahman, N.; Saad, O.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M.; Matlob, A.A. Rational Design and Synthesis of New, High Efficiency, Multipotent Schiff Base-1,2,4-triazole Antioxidants Bearing Butylated Hydroxytoluene Moieties. Molecules 2016, 21, 847. https://doi.org/10.3390/molecules21070847
Yehye WA, Abdul Rahman N, Saad O, Ariffin A, Abd Hamid SB, Alhadi AA, Kadir FA, Yaeghoobi M, Matlob AA. Rational Design and Synthesis of New, High Efficiency, Multipotent Schiff Base-1,2,4-triazole Antioxidants Bearing Butylated Hydroxytoluene Moieties. Molecules. 2016; 21(7):847. https://doi.org/10.3390/molecules21070847
Chicago/Turabian StyleYehye, Wageeh A, Noorsaadah Abdul Rahman, Omar Saad, Azhar Ariffin, Sharifah Bee Abd Hamid, Abeer A. Alhadi, Farkaad A. Kadir, Marzieh Yaeghoobi, and Abdulsalam A. Matlob. 2016. "Rational Design and Synthesis of New, High Efficiency, Multipotent Schiff Base-1,2,4-triazole Antioxidants Bearing Butylated Hydroxytoluene Moieties" Molecules 21, no. 7: 847. https://doi.org/10.3390/molecules21070847