Extraction and Characterization of Tamarind (Tamarind indica L.) Seed Polysaccharides (TSP) from Three Difference Sources
Abstract
:1. Introduction
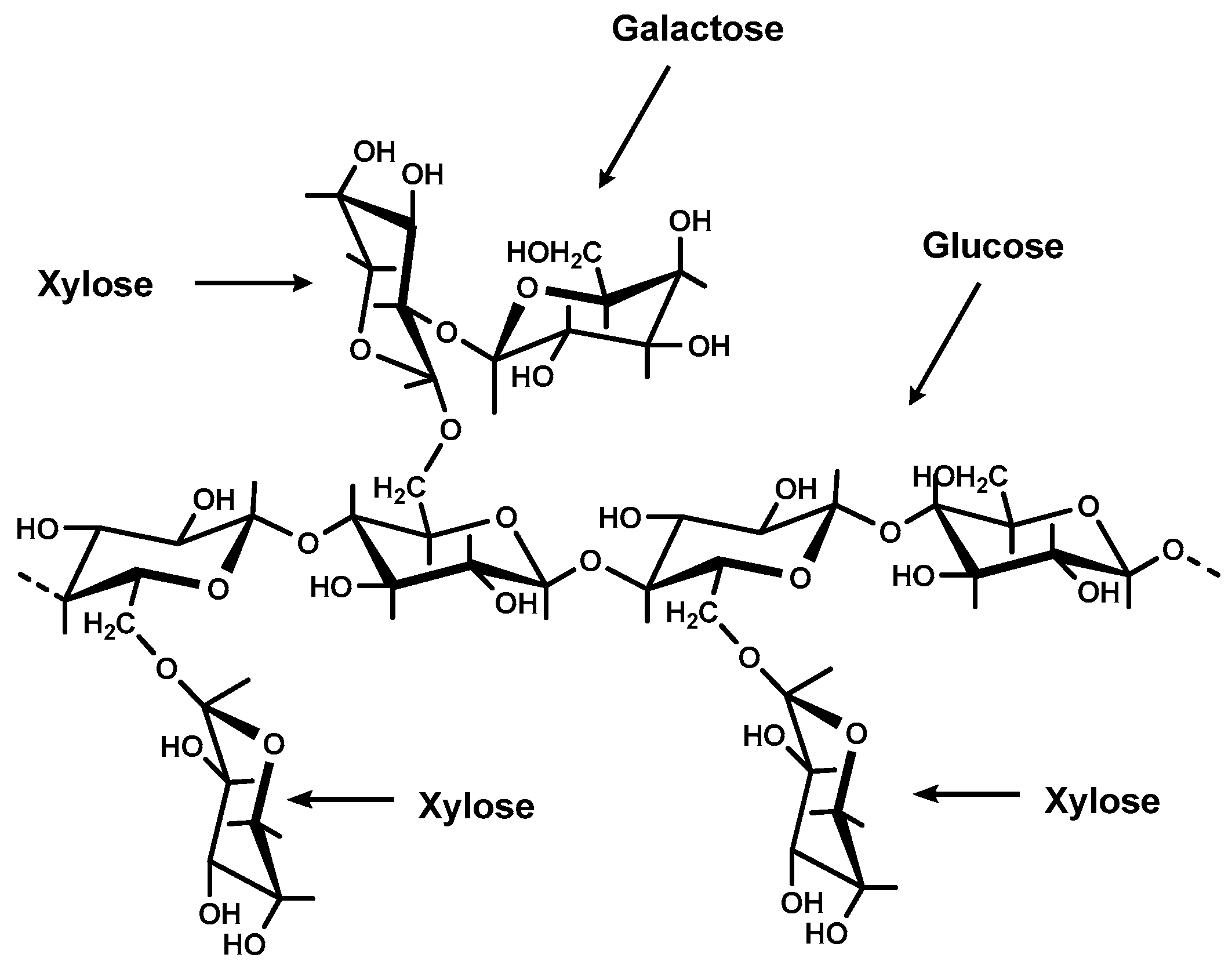
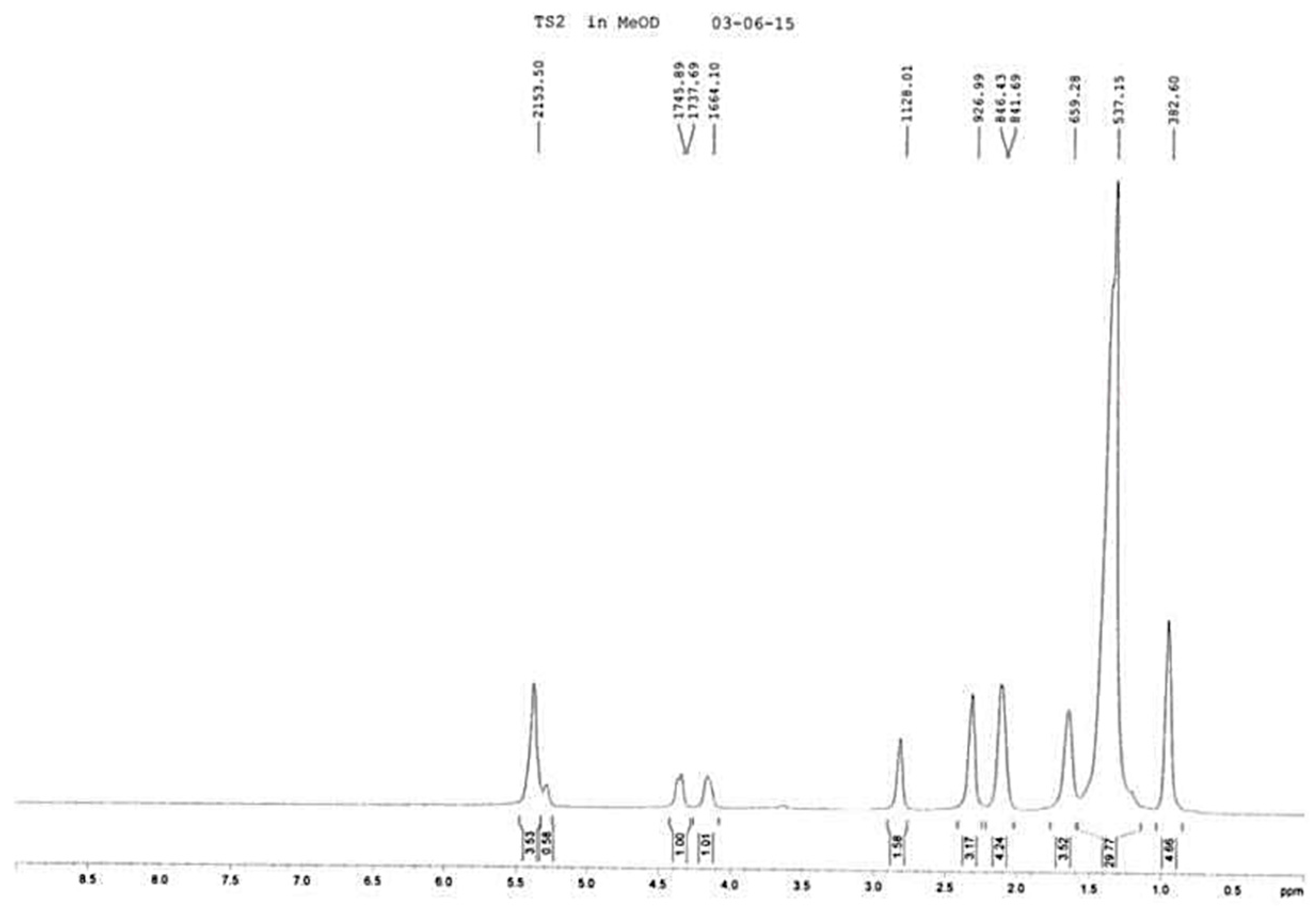
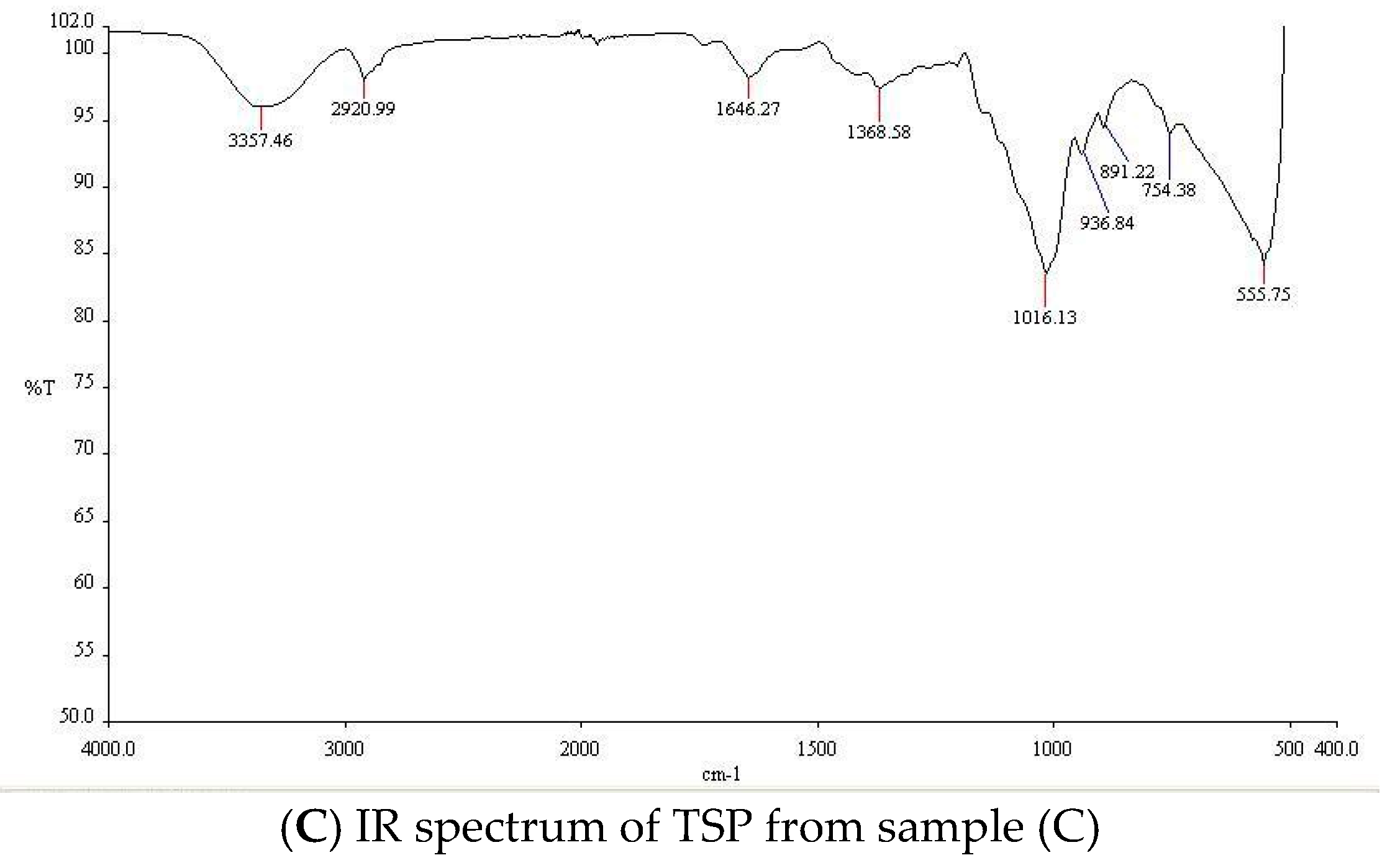
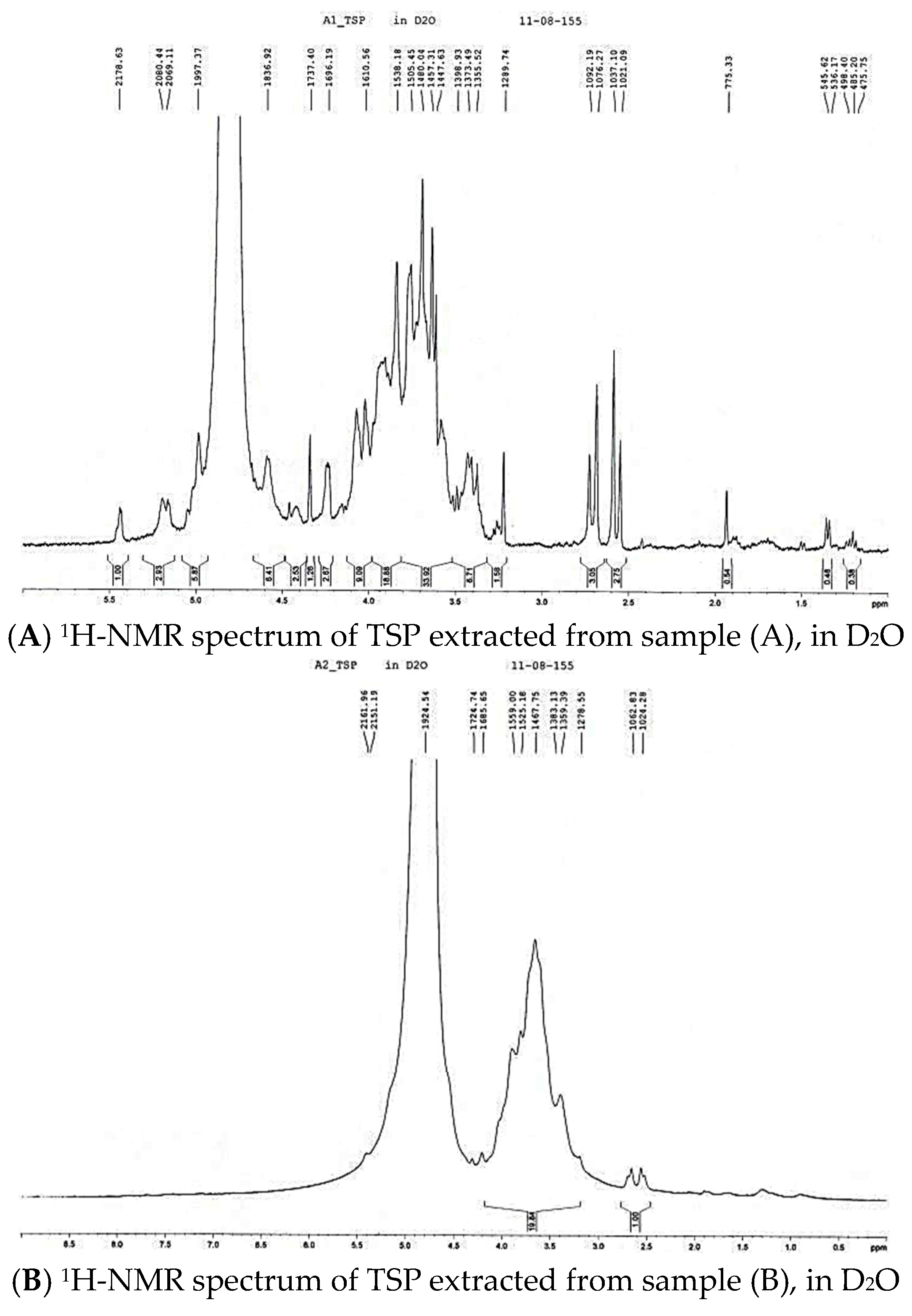
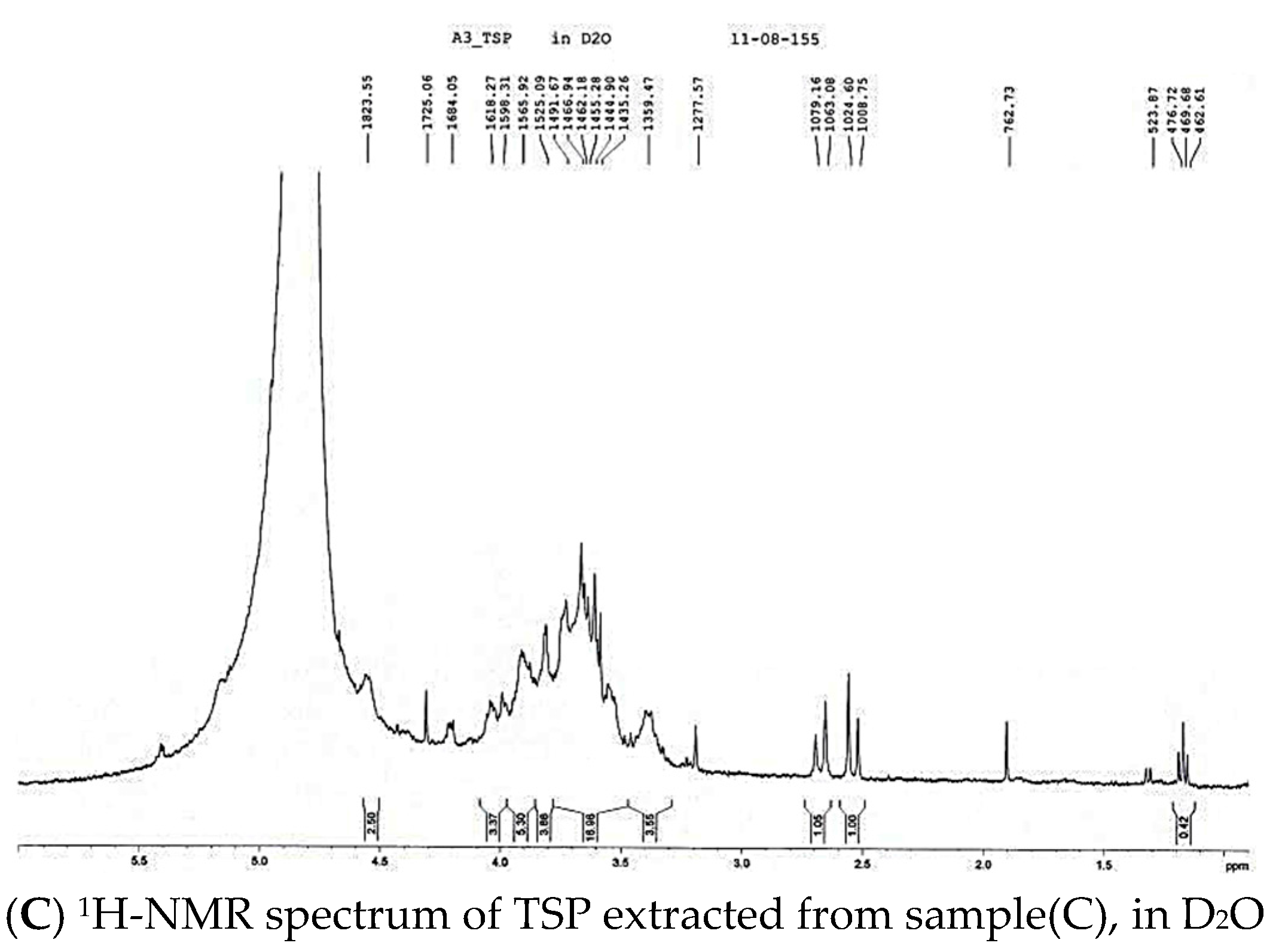
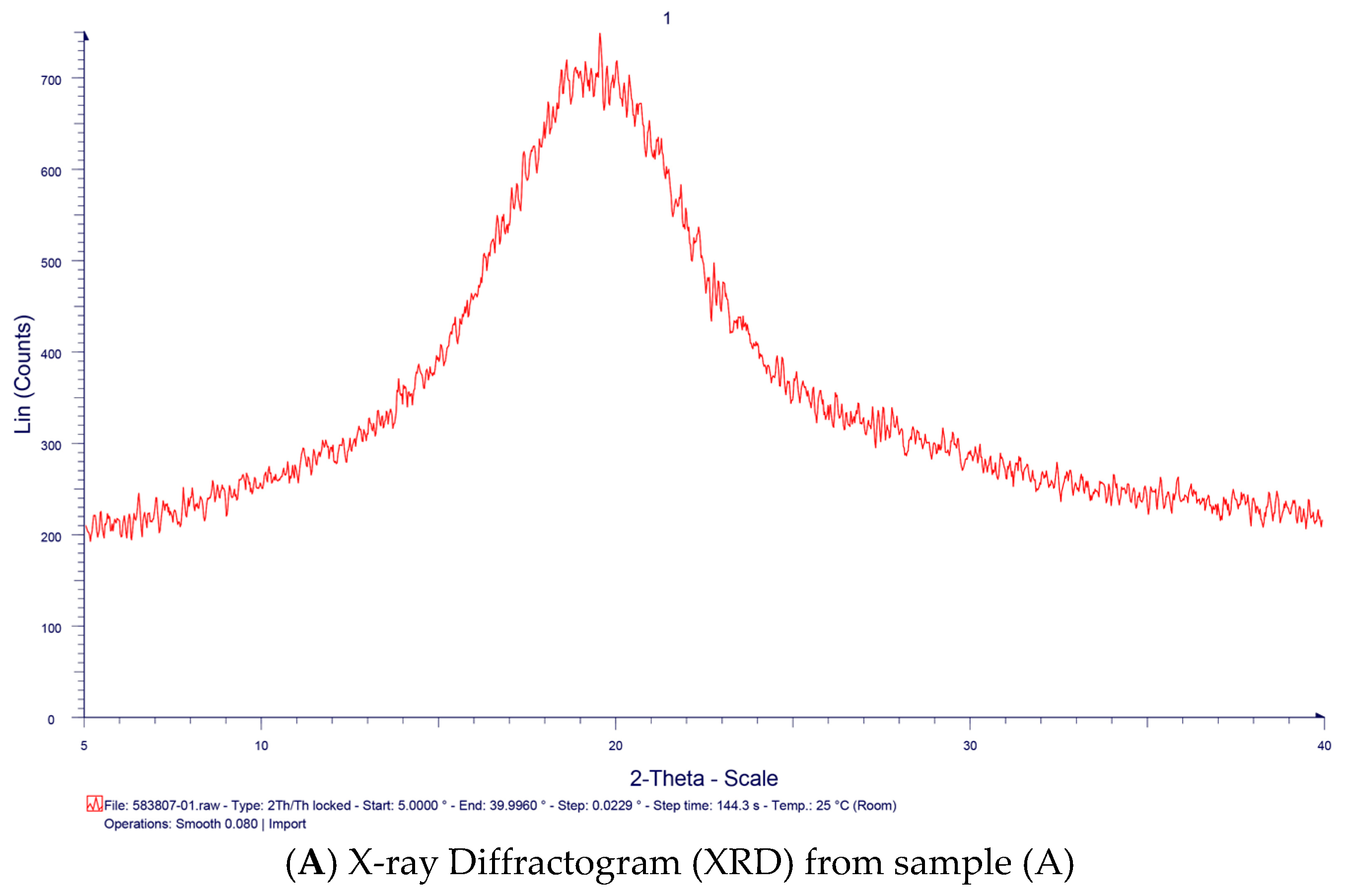
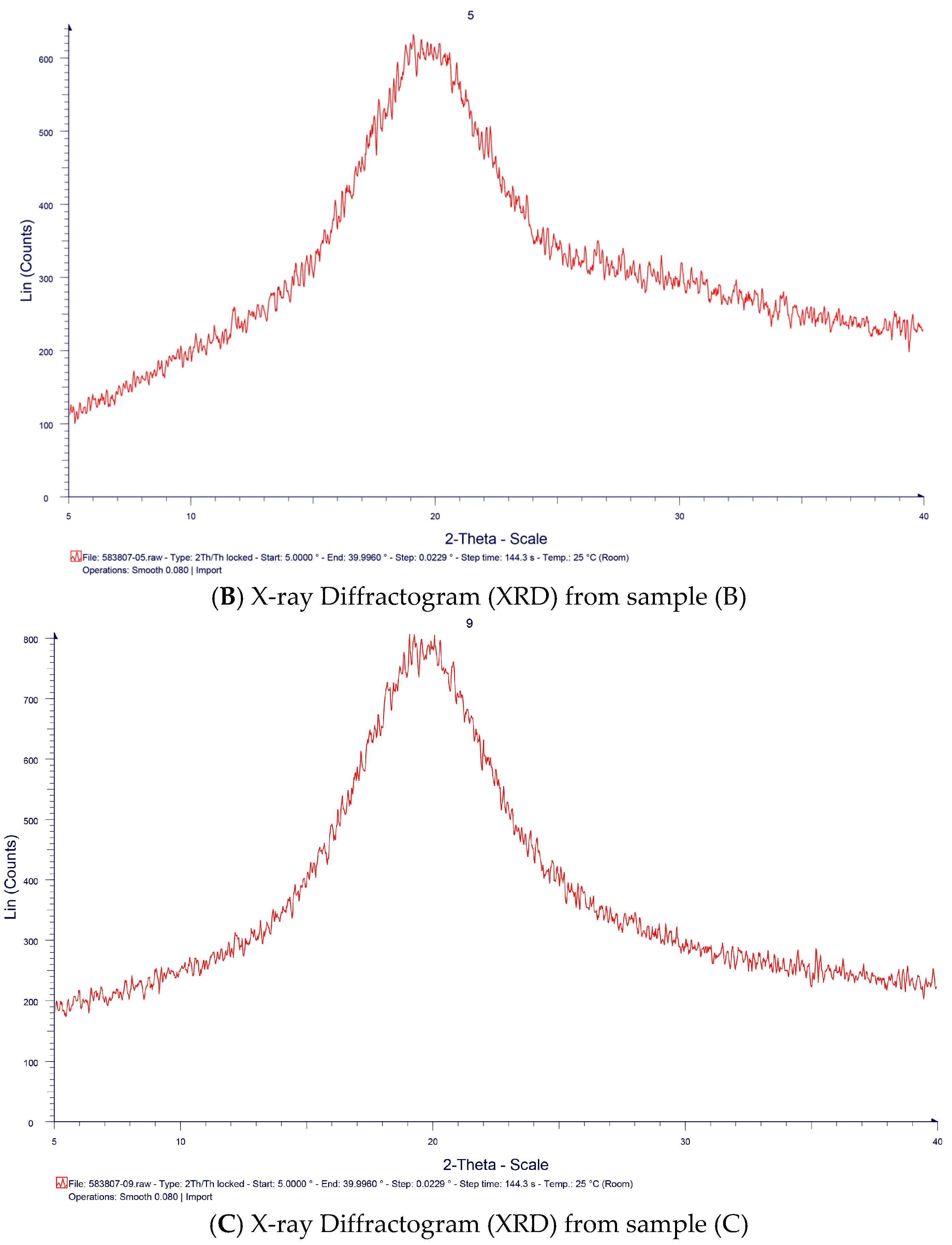
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. TSP Extraction Procedure
3.2.1. Tamarind Seed Preparation
3.2.2. TSP Extraction
3.3. Methods
3.4 Characterization of TSP
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaur, H.; Ahuja, M.; Kumar, S.; Dilbaghi, N. Carboxymethyl tamarind kernel polysaccharide nanoparticles for ophthalmic drug delivery. Int. J. Biol. Macromol. 2012, 50, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Andriamanantena, R.W.; Artaud, J.; Gaydou, E.M.; Iatrides, M.C. Fatty acid and sterol compositions of Malagasy Tamarind Kernel Oils. JAOCS 1983, 60, 1318–1321. [Google Scholar] [CrossRef]
- Kaur, H.; Yadav, S.; Ahuja, M.; Dilbaghi, N. Synthesis, characterization and evalution of thiolated tamarind seed polysaccharide as a mucoadhesive polymer. Carbohydr. Polym. 2012, 90, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Saettone, M.F.; Burgalassis, S.; Giannaccini, B.; Boldrini, E. Ophthalmic Solutions Viscosified with Tamarind Seed Polysaccharides. US6056950 A, 2 May 2000. [Google Scholar]
- Jana, S.; Saha, A.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Aceclofenac-loaded chitosan-tamarind seed polysaccharide interpenetrating polymeric network microparticles. Colloids Surf. B Biointerfaces 2013, 105, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Kumar, V.; Sharma, P. Carboxymethylation of Tamarind kernel powder. Carbohydr. Polym. 2007, 69, 251–255. [Google Scholar] [CrossRef]
- Singh, R.; Malviya, R.; Sharma, P.K. Extraction and Characterization of Tamarind Seed Polysaccharide as a Pharmaceutical Excipient. Pharmacogn. J. 2011, 3, 17–19. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, R.; Nayak, P.L. Tamarind Seed Polysachharide: A Versatile Biopolymer for Mucoadhesive Applications. JPBMS 2010, 8, 1–12. [Google Scholar]
- Berretta, G.U.; Balzano, F.; Vanni, L.; Sansò, M. Mucoadhesive properties of tamarind-seed polysaccharide/hyaluronic acid mixture: A nuclear magnetic resonance spectroscopy investigation. Carbohydr. Polym. 2013, 91, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P. The oxalic acid content of Indian foods. Qual. Plant. Mater. 1973, 22, 335–347. [Google Scholar] [CrossRef]
- Kaewkumsan, P.; Honggr, J.; Sawadee, B. The use of tamarind kernel powder substitute commercial pectin. Khon Kaen Agric. J. 2014, 42 (Suppl. S1), 641–645. [Google Scholar]
- Chandramouli, Y.; Firoz, S.; Vikram, A.; Mahitha, B.; Yasmeen, B.R.; Hemanthpavankumar, K. Tamarind seed polysaccharides (TSP)-An adaptable excipient for novel drug delivery system. IJPPDR 2012, 2, 57–63. [Google Scholar]
- Khanna, M. Polyose from seeds of Tamarindus indica of unique property and immense pharmaceutical use. Trends Carbohydr. Chem. 1997, 4, 79–81. [Google Scholar]
- Sattle, A.; Agrawal, S. Solubility enhancement potential of Tamarind seed polysaccharide as pharmaceutical excipient. Int. J. Pharm. Biol. Arch. 2012, 3, 456–459. [Google Scholar]
- Singh, D.; Wangchu, L.; Moond, S.K. Processed products of Tamarind. Nat. Prod. Radiance 2007, 6, 315–321. [Google Scholar]
- Sudjaroen, Y.; Haubner, R.; Wurtele, G.; Hull, W.E. Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem. Toxicol. 2005, 43, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
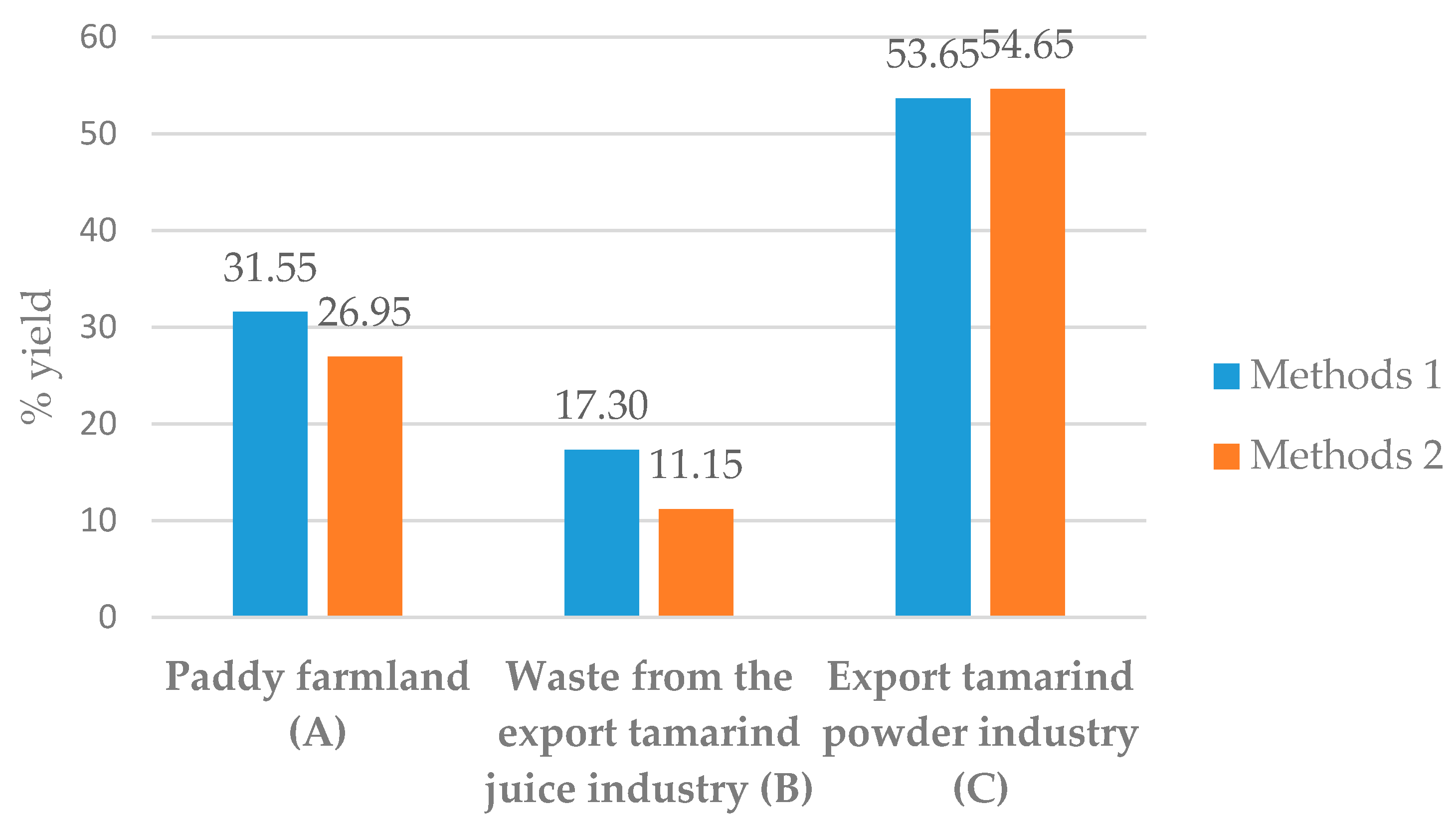


| Methods | Tamarind Seed Sources | |||
|---|---|---|---|---|
| Paddy Farmland (A) | Waste from the Export Tamarind Juice Industry (B) | Export Tamarind Powder Industry (C) | ||
| Methods 1 | Weight (grams) | 20.00 | 20.00 | 20.00 |
| Supernatant (mL) | 173.75 | 202.50 | 208.00 | |
| TSP Weight (grams) | 6.31 | 3.46 | 10.73 | |
| % yield | 31.55 | 17.30 | 53.65 | |
| Methods 2 | Weight (grams) | 20.00 | 20.00 | 20.00 |
| Supernatant (mL) | 222.75 | 239.50 | 173.25 | |
| TSP Weight (grams) | 5.39 | 2.23 | 10.93 | |
| % yield | 26.95 | 11.15 | 54.65 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chawananorasest, K.; Saengtongdee, P.; Kaemchantuek, P. Extraction and Characterization of Tamarind (Tamarind indica L.) Seed Polysaccharides (TSP) from Three Difference Sources. Molecules 2016, 21, 775. https://doi.org/10.3390/molecules21060775
Chawananorasest K, Saengtongdee P, Kaemchantuek P. Extraction and Characterization of Tamarind (Tamarind indica L.) Seed Polysaccharides (TSP) from Three Difference Sources. Molecules. 2016; 21(6):775. https://doi.org/10.3390/molecules21060775
Chicago/Turabian StyleChawananorasest, Khanittha, Patsuda Saengtongdee, and Praphakorn Kaemchantuek. 2016. "Extraction and Characterization of Tamarind (Tamarind indica L.) Seed Polysaccharides (TSP) from Three Difference Sources" Molecules 21, no. 6: 775. https://doi.org/10.3390/molecules21060775





