Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting
Abstract
:1. Introduction
2. Nature-Derived Bioinks
2.1. Alginate-Based Bioinks
Printability and Challenges
2.2. Gelatin-Based Bioinks
Printability and Cell Support
2.3. Collagen-Based Bioinks
Printability and Challenges
2.4. Fibrin-Based Bioinks
Printability and Challenges
2.5. Hyaluronic Acid-Based Bioinks
Printability and Challenges
2.6. Decellularized Extracellular Matrix
Printability and Challenges
2.7. Silk Based Bioinks
Printability and Challenges
2.8. Chitosan-Based Bioinks
Printability and Challenges
3. Synthetic Bioinks
3.1. PEG-Based Bioinks
Printability and Challenges
3.2. Pluronic Acid-Based Bioinks
Printability and Challenges
3.3. Synthetic Nanostructured Bioinks
Printability and Challenges
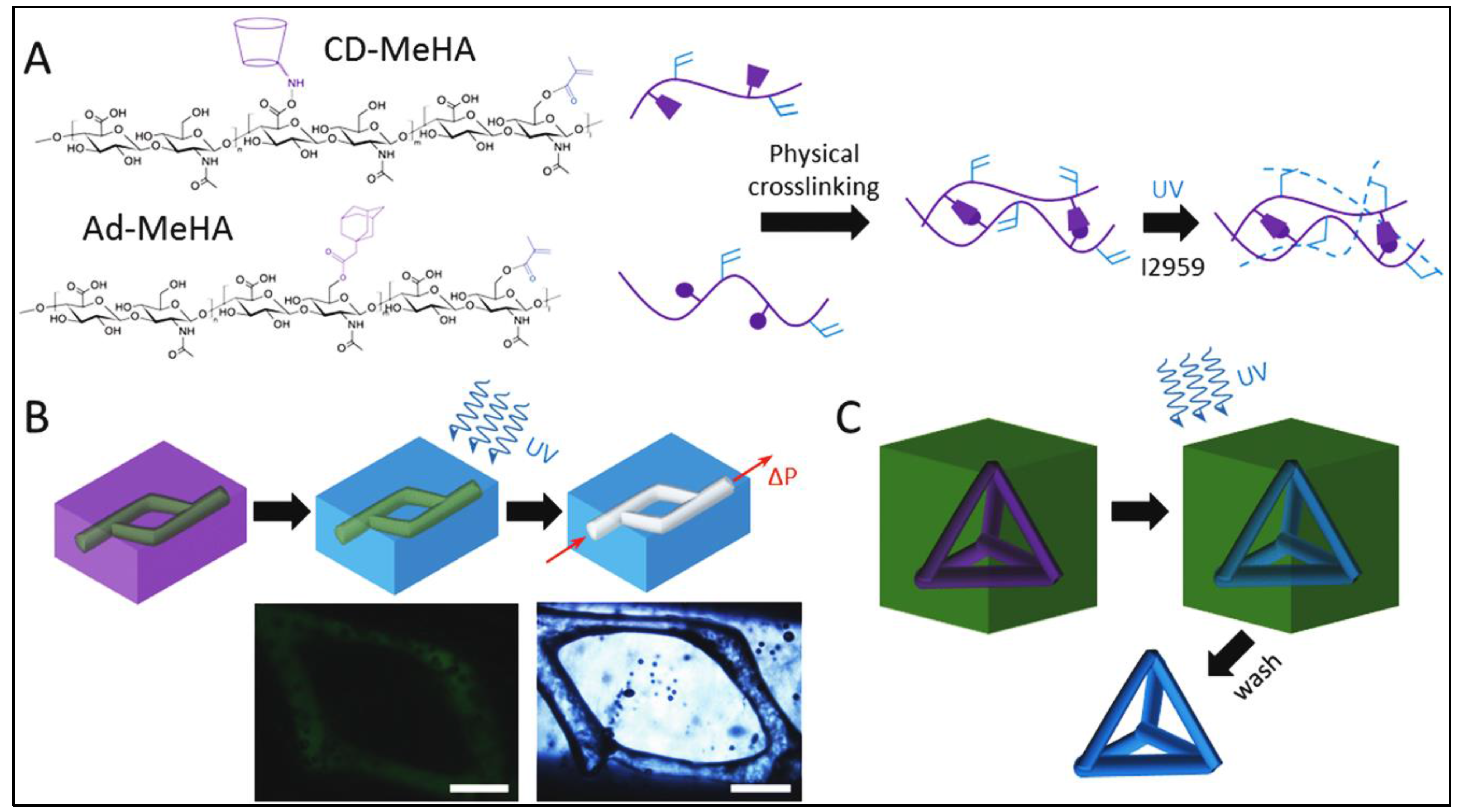
4. Self-Assembled Bioinks
Printability and Challenges
5. Summary and Conclusions
Acknowledgments
Conflicts of Interest
References
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- Chang, R.; Nam, J.; Sun, W. Direct cell writing of 3D microorgan for in vitro pharmacokinetic model. Tissue Eng. Part C Methods 2008, 14, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cui, H. Biodegradability and biocompatibility study of poly(chitosan-g-lactic acid) scaffolds. Molecules 2012, 17, 3243–3258. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Kim, M.H.; Lee, J.H.; Seliktar, D.; Cho, N.-J.; Tan, L.P. Modulation of Huh7. 5 spheroid formation and functionality using modified peg-based hydrogels of different stiffness. PLoS ONE 2015, 10, e0118123. [Google Scholar]
- Popa, E.; Santo, V.; Rodrigues, M.; Gomes, M. Magnetically-responsive hydrogels for modulation of chondrogenic commitment of human adipose-derived stem cells. Polymers 2016, 8, 28. [Google Scholar] [CrossRef]
- Alan, F.-J.; Catherine, F.; Dirk-Jan, C.; John, G.; Jason, K.; Aidan, C.; Wenmiao, S. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7, 044102. [Google Scholar]
- Chang, R.; Nam, J.; Sun, W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication–based direct cell writing. Tissue Eng. Part A 2008, 14, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Schuurman, W.; Wijnberg, H.M.; Prins, H.-J.; van Weeren, P.R.; Malda, J.; Alblas, J.; Dhert, W.J.A. Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng. Part C Methods 2011, 18, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kirihara, S.; Nakano, M. Freeform fabrication of magnetophotonic crystals with diamond lattices of oxide and metallic glasses for terahertz wave control by micro patterning stereolithography and low temperature sintering. Micromachines 2013, 4, 149–156. [Google Scholar] [CrossRef]
- Melchels, F.P.W. Preparation of Advanced Porous Structures by Stereolithography for Application in Tissue Engineering; University of Twente: Enschede, The Netherlands, 2010. [Google Scholar]
- Suárez-González, D.; Barnhart, K.; Migneco, F.; Flanagan, C.; Hollister, S.J.; Murphy, W.L. Controllable mineral coatings on PCL scaffolds as carriers for growth factor release. Biomaterials 2012, 33, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Geng, P.; Li, G.; Zhao, D.; Zhang, H.; Zhao, J. Influence of layer thickness and raster angle on the mechanical properties of 3D-printed PEEK and a comparative mechanical study between PEEK and ABS. Materials 2015, 8, 5834–5846. [Google Scholar] [CrossRef]
- Vaezi, M.; Yang, S. Extrusion-based additive manufacturing of PEEK for biomedical applications. Virtual Phys. Prototyp. 2015, 10, 123–135. [Google Scholar] [CrossRef]
- Nakamura, M.; Nishiyama, Y.; Henmi, C.; Yamaguchi, K.; Mochizuki, S.; Koki, T.; Nakagawa, H. Inkjet Bioprinting as an Effective Tool for Tissue Fabrication. In Proceedings of the NIP & Digital Fabrication Conference, Denver, CO, USA, 17–22 September 2006.
- Sánchez-Salcedo, S.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Preventing bacterial adhesion on scaffolds for bone tissue engineering. Int. J. Bioprinting 2016, 2, 20–34. [Google Scholar]
- Kirchmajer, D.M.; Gorkin III, R. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar] [CrossRef]
- Tseng, Y.; Kole, T.P.; Wirtz, D. Micromechanical mapping of live cells by multiple-particle-tracking microrheology. Biophys. J. 2002, 83, 3162–3176. [Google Scholar] [CrossRef]
- Kilpatrick, J.I.; Revenko, I.; Rodriguez, B.J. Nanomechanics of cells and biomaterials studied by atomic force microscopy. Adv. Healthc. Mater. 2015, 4, 2456–2474. [Google Scholar] [CrossRef] [PubMed]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J.r. Strategies and molecular design criteria for 3D printable hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yao, R.; Mao, S.; Chen, X.; Na, J.; Sun, W. Three-dimensional bioprinting of embryonic stem cells directs highly uniform embryoid body formation. Biofabrication 2015, 7, 044101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef] [PubMed]
- Tobias, Z.; Bapi, S.; Aldo, R.B.; Rainer, D. Evaluation of an alginate–gelatine crosslinked hydrogel for bioplotting. Biofabrication 2015, 7, 025001. [Google Scholar]
- Wang, X.-F.; Lu, P.-J.; Song, Y.; Sun, Y.-C.; Wang, Y.-G.; Wang, Y. Nano hydroxyapatite particles promote osteogenesis in a three-dimensional bio-printing construct consisting of alginate/gelatin/HASCS. RSC Adv. 2016, 6, 6832–6842. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Yan, Y.; Yao, R.; Ge, Y. An cell-assembly derived physiological 3D model of the metabolic syndrome, based on adipose-derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials 2010, 31, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.; Dhert, W.J.; Hutmacher, D.W.; Malda, J. Development and characterisation of a new bioink for additive tissue manufacturing. J. Mater. Chem. B 2014, 2, 2282–2289. [Google Scholar] [CrossRef]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.; Hutmacher, D.W.; Melchels, F.P.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; van Weeren, P.R.; Dhert, W.J.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, L.; Vermonden, T.; Malda, J.; Censi, R.; Dhert, W.J.; Alhaique, F.; Hennink, W.E.; Matricardi, P. In situ forming IPN hydrogels of calcium alginate and dextran-HEMA for biomedical applications. Acta Biomater. 2011, 7, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; Prestwich, G.D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 2010, 31, 6173–6181. [Google Scholar] [CrossRef] [PubMed]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv. Mater. 2015, 27, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv. Mater. 2015. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateos-Timoneda, M.A. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 2014, 6, 035020. [Google Scholar] [CrossRef] [PubMed]
- Nupura, S.B.; Vijayan, M.; Solange, M.; Ali, T.; Masoumeh, G.; Mario, M.; Qi, L.; Yu Shrike, Z.; Su Ryon, S.; Giovanni, C.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar]
- Itoh, M.; Nakayama, K.; Noguchi, R.; Kamohara, K.; Furukawa, K.; Uchihashi, K.; Toda, S.; Oyama, J.-I.; Node, K.; Morita, S. Scaffold-free tubular tissues created by a bio-3D printer undergo remodeling and endothelialization when implanted in rat aortae. PloS one 2015, 10, e0136681. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Jang, K.-M.; Hahn, S.K.; Park, J.Y.; Jung, H.; Oh, K.; Park, K.M.; Yeom, J.; Park, S.H.; Kim, S.W. Three-dimensional bioprinting of multilayered constructs containing human mesenchymal stromal cells for osteochondral tissue regeneration in the rabbit knee joint. Biofabrication 2016, 8, 014102. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Faulkner-Jones, A.; Dun, A.R.; Jin, J.; Chen, P.; Xing, Y.; Yang, Z.; Li, Z.; Shu, W.; Liu, D. Rapid formation of a supramolecular polypeptide–DNA hydrogel for in situ three-dimensional multilayer bioprinting. Angew. Chem. Int. Ed. 2015, 54, 3957–3961. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.; Gandhi, M.; Khalil, S.; Yan, K.C.; Marcolongo, M.; Barbee, K.; Sun, W. Characterization of cell viability during bioprinting processes. Biotechnol. J. 2009, 4, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yao, R.; Chen, X.; Na, J.; Sun, W. 3D printing of HEK 293FT cell-laden hydrogel into macroporous constructs with high cell viability and normal biological functions. Biofabrication 2015, 7, 015010. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.; Son, A.R.; Hamid, Q.; Wang, C.; Lui, Y.; Sun, W. Mesenchymal stem cell printing and process regulated cell properties. Biofabrication 2015, 7, 044106. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Khristov, V.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Malda, J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 2011, 3, 021001. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Shirahama, H.; Cho, N.-J.; Tan, L.P. Efficient and controllable synthesis of highly substituted gelatin methacrylamide for mechanically stiff hydrogels. RSC Adv. 2015, 5, 106094–106097. [Google Scholar] [CrossRef]
- Shanjani, Y.; Pan, C.; Elomaa, L.; Yang, Y. A novel bioprinting method and system for forming hybrid tissue engineering constructs. Biofabrication 2015, 7, 045008. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Shim, J.-H.; Lee, J.-S.; Cho, D.-W. 3D printing of cell-laden constructs for heterogeneous tissue regeneration. Manuf. Lett. 2013, 1, 49–53. [Google Scholar] [CrossRef]
- Shim, J.-H.; Lee, J.-S.; Kim, J.Y.; Cho, D.-W. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J. Micromech. Microeng. 2012, 22, 085014. [Google Scholar] [CrossRef]
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An additive manufacturing-based PCL–alginate–chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Jung-Seob, L.; Jung Min, H.; Jin Woo, J.; Jin-Hyung, S.; Jeong-Hoon, O.; Dong-Woo, C. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication 2014, 6, 024103. [Google Scholar]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 035006. [Google Scholar] [CrossRef] [PubMed]
- Boere, K.W.; Visser, J.; Seyednejad, H.; Rahimian, S.; Gawlitta, D.; van Steenbergen, M.J.; Dhert, W.J.; Hennink, W.E.; Vermonden, T.; Malda, J. Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomater. 2014, 10, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Schacht, K.; Jüngst, T.; Schweinlin, M.; Ewald, A.; Groll, J.; Scheibel, T. Biofabrication of cell-loaded 3D spider silk constructs. Angew. Chem. Int. Ed. 2015, 54, 2816–2820. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Richards, D.J.; Pollard, S.; Tan, Y.; Rodriguez, J.; Visconti, R.P.; Trusk, T.C.; Yost, M.J.; Yao, H.; Markwald, R.R. Engineering alginate as bioink for bioprinting. Acta Biomater. 2014, 10, 4323–4331. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Ha, D.-H.; Jang, J.; Han, H.H.; Rhie, J.-W.; Cho, D.-W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 2015, 62, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int. J. Biopr. 2016, 2, 1. [Google Scholar] [CrossRef]
- Ribas, J.; Sadeghi, H.; Manbachi, A.; Leijten, J.; Brinegar, K.; Zhang, Y.S.; Ferreira, L.; Khademhosseini, A. Cardiovascular organ-on-a-chip platforms for drug discovery and development. Appl. In Vitro Toxicol. 2016. [Google Scholar] [CrossRef]
- Gaetani, R.; Feyen, D.A.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.; Sluijter, J.P. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015, 61, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; McCoard, L.; Xu, X.; Oottamasathien, S.; Prestwich, G.D. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng. Part A 2010, 16, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.J.; Melchels, F.P.; Malda, J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 2013, 5, 035007. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.W.; Chor, Y.T.; Feng, W.; Subbu, S.V.; Lay, P.T. Engineered polymeric biomaterials for tissue engineering. Curr. Tissue Eng. 2012, 1, 41–53. [Google Scholar]
- Wolf, M.T.; Daly, K.A.; Brennan-Pierce, E.P.; Johnson, S.A.; Carruthers, C.; D’Amore, A.; Nagarkar, S.P.; Velankar, S.S.; Badylak, S.F. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 2012, 33, 7028–7038. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; He, J.; Nichol, J.W.; Wang, L.; Hutson, C.B.; Wang, B.; Du, Y.; Fan, H.; Khademhosseini, A. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011, 7, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.B.; Andrade, J.D. Blood compatibility of polyethylene oxide surfaces. Prog. Polym. Sci. 1995, 20, 1043–1079. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Du, K.T.; Fang, Q.; Gu, Y.; Mihardja, S.S.; Sievers, R.E.; Wu, J.C.; Lee, R.J. The use of human mesenchymal stem cells encapsulated in rgd modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials 2010, 31, 7012–7020. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Cardoso, J.C.; Manoharan, V.; Cristino, A.L.; Bhise, N.S.; Araujo, W.A.; Zorlutuna, P.; Vrana, N.E.; Ghaemmaghami, A.M.; Dokmeci, M.R. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 2014, 6, 024105. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, X.; Yan, Y.; Zheng, W.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Rapid prototyping three-dimensional cell/gelatin/fibrinogen constructs for medical regeneration. J. Bioact. Compat. Polym. 2007, 22, 363–377. [Google Scholar] [CrossRef]
- Das, S.; Pati, F.; Choi, Y.-J.; Rijal, G.; Shim, J.-H.; Kim, S.W.; Ray, A.R.; Cho, D.-W.; Ghosh, S. Bioprintable, cell-laden silk fibroin–gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015, 11, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Wüst, S.; Godla, M.E.; Müller, R.; Hofmann, S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014, 10, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Kapetanovic, E.; Hockaday, L.; Butcher, J. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014, 10, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Irvine, S.A.; Agrawal, A.; Lee, B.H.; Chua, H.Y.; Low, K.Y.; Lau, B.C.; Machluf, M.; Venkatraman, S. Printing cell-laden gelatin constructs by free-form fabrication and enzymatic protein crosslinking. Biomed. Microdevices 2015, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duarte Campos, D.F.; Blaeser, A.; Korsten, A.; Neuss, S.; Jäkel, J.; Vogt, M.; Fischer, H. The stiffness and structure of three-dimensional printed hydrogels direct the differentiation of mesenchymal stromal cells toward adipogenic and osteogenic lineages. Tissue Eng. Part A 2014, 21, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, X.; Wang, J.; Wang, Y.; Shi, B.; Huang, C.; Yang, X.; Liu, T. Novel chitosan/collagen scaffold containing transforming growth factor-β1 DNA for periodontal tissue engineering. Biochem. Biophys. Res. Commun. 2006, 344, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Binder, K.W.; Albanna, M.Z.; Dice, D.; Zhao, W.; Yoo, J.J.; Atala, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2012, 5, 015001. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, J.-C.; Shim, J.-H.; Lee, J.-S.; Park, H.; Kim, S.W.; Doh, J.; Cho, D.-W. A comparative study on collagen type i and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 2014, 6, 035004. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.-S.; Dai, G.; Karande, P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods 2013, 20, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Stone, A.L.; Parkhill, R.L.; Stewart, R.L.; Simpkins, M.W.; Kachurin, A.M.; Warren, W.L.; Williams, S.K. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng. 2004, 10, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. A 2013, 101, 272–284. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, E.; Schacht, K.; Jungst, T.; Groll, J.; Scheibel, T. Biofabrication of 3D constructs: Fabrication technologies and spider silk proteins as bioinks. Pure Appl. Chem. 2015, 87, 737–749. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Ozbolat, I.T. Direct bioprinting of vessel-like tubular microfluidic channels. J. Nanotechnol. Eng. Med. 2013, 4, 020902. [Google Scholar] [CrossRef]
- Peppas, N.A.; Keys, K.B.; Torres-Lugo, M.; Lowman, A.M. Poly (ethylene glycol)-containing hydrogels in drug delivery. J. Control. Release 1999, 62, 81–87. [Google Scholar] [CrossRef]
- Cui, X.; Breitenkamp, K.; Finn, M.; Lotz, M.; D'Lima, D.D. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Tello, P.; Camacho, F.; Blazquez, G. Density and viscosity of concentrated aqueous solutions of polyethylene glycol. J. Chem. Eng. Data 1994, 39, 611–614. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; DeConinck, A.; Lewis, J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011, 23. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Swennen, I.; Girones, J.; Moroni, L.; Van Blitterswijk, C.A.; Schacht, E.; Alblas, J.; Dhert, W.J. Evaluation of photocrosslinked lutrol hydrogel for tissue printing applications. Biomacromolecules 2009, 10, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; De Wijn, J.R.; Verbout, A.J.; Alblas, J.; Dhert, W.J. Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Eng. Part A 2008, 14, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Lee, H.; Kim, Y.; Yoo, S.Y.; Chung, W.-J.; Kim, G. Phage as versatile nanoink for printing 3-D cell-laden scaffolds. Acta Biomater. 2016, 29, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, N.; Bruce, M.A.; Wu, J.C.; Butte, M.J. Atomic force mechanobiology of pluripotent stem cell-derived cardiomyocytes. PLoS ONE 2012, 7, e37559. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.G.; Starodubtseva, M.N.; Yegorenkov, N.I.; Chizhik, S.A.; Zhdanov, R.I. Atomic force microscopy probing of cell elasticity. Micron 2007, 38, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Padula, D.; Popov, C.; Mutschler, W.; Clausen-Schaumann, H.; Schieker, M. Researching into the cellular shape, volume and elasticity of mesenchymal stem cells, osteoblasts and osteosarcoma cells by atomic force microscopy. J. Cell. Mol. Med. 2008, 12, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Khoo, Z.X.; Teoh, J.E.M.; Liu, Y.; Chua, C.K.; Yang, S.; An, J.; Leong, K.F.; Yeong, W.Y. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual Phys. Prototyp. 2015, 10, 103–122. [Google Scholar] [CrossRef]
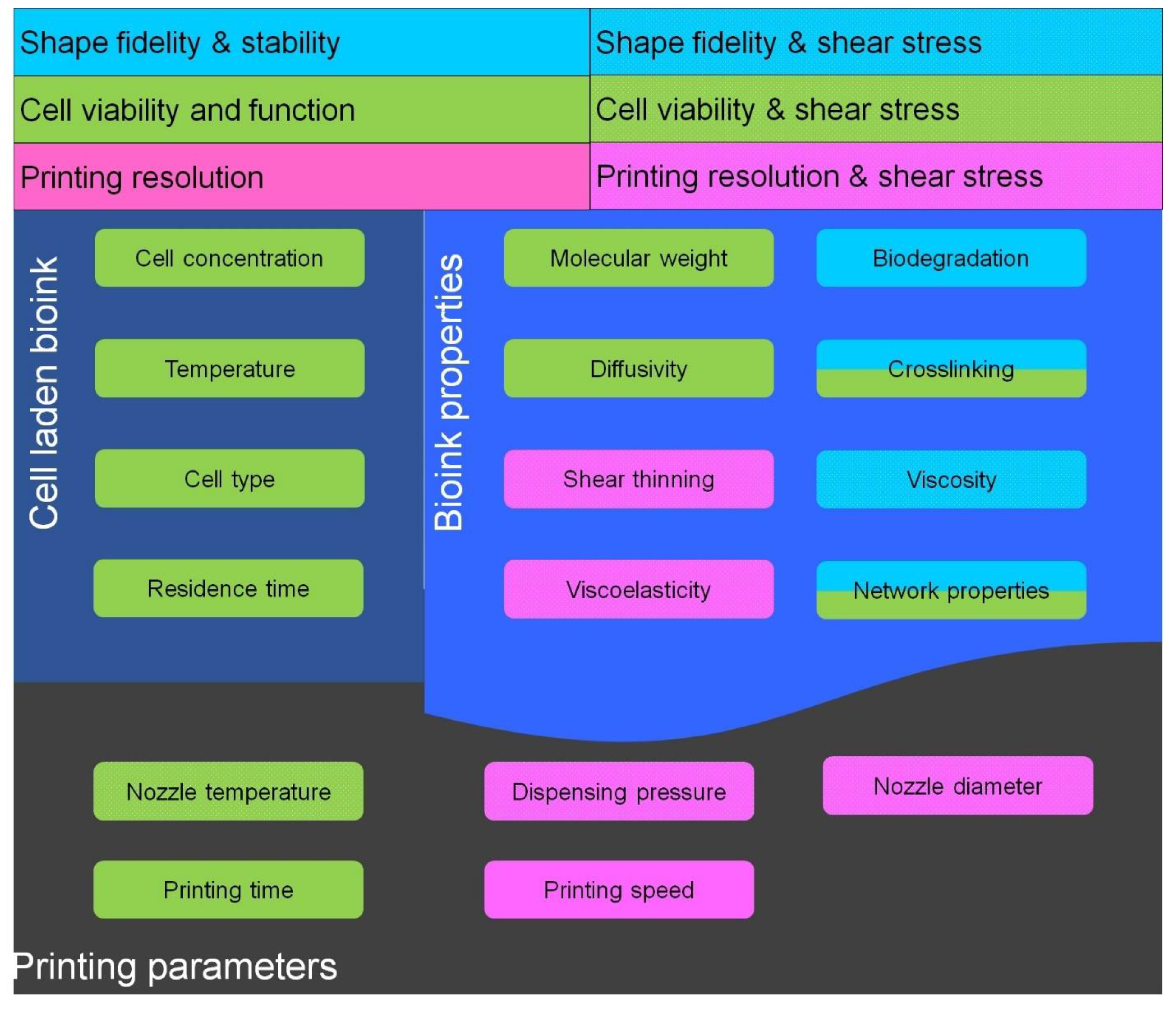
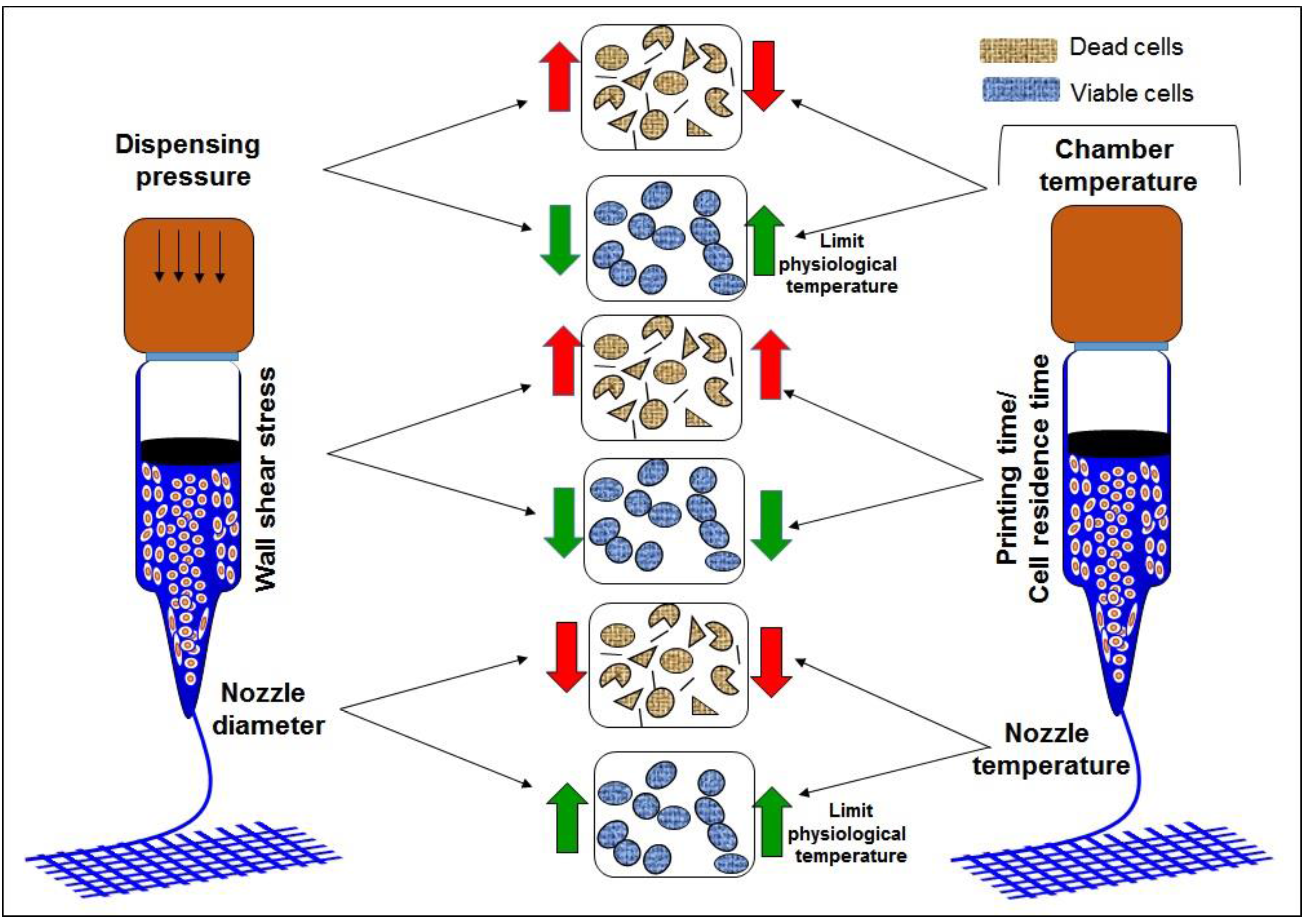
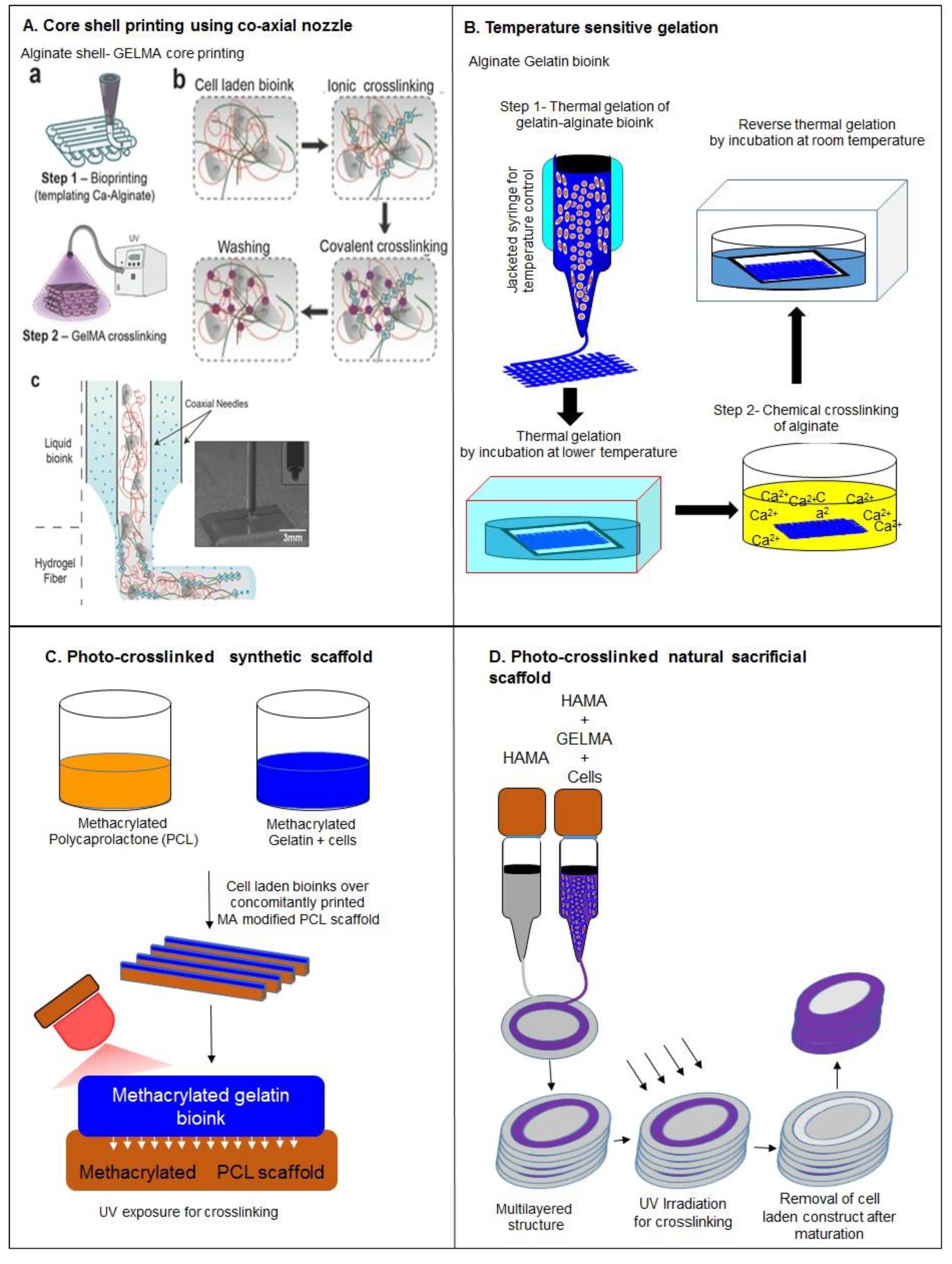



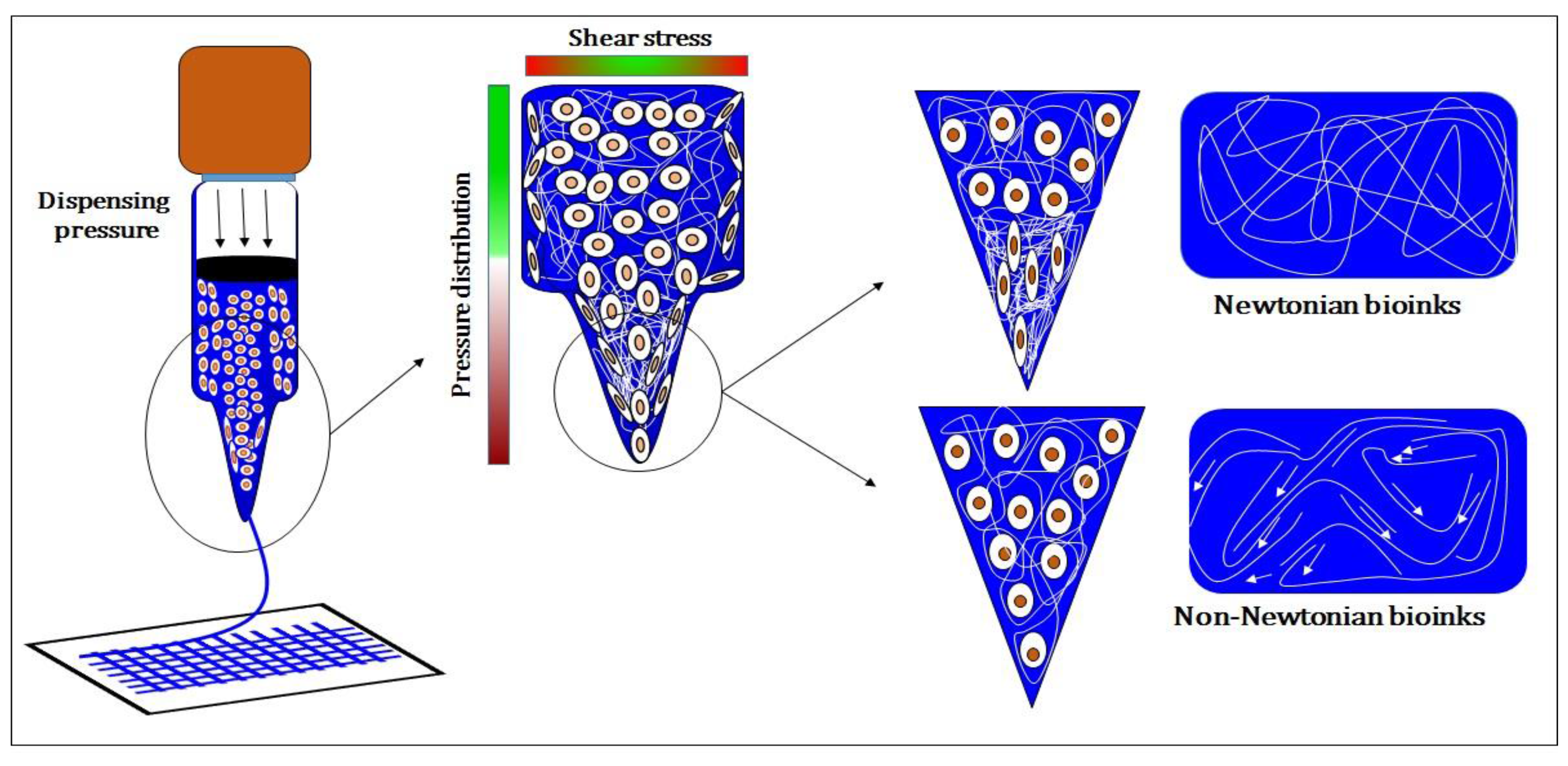
| SNo. | Motivation | Strategies | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| 1 | To improve bioink printability | Blending | Improve the viscosity of bioink | Decreases cell viability | [23,24,25,26,27,28,29] |
| Interpenetrating hydrogels | Improve viscosity of bioink | Decreases cell viability | [30,31] | ||
| Partial crosslinking by synthetic polymers | Improve viscosity, resolution | Involves synthetic cross-linkers which limits cell viability and have lower overall mechanical properties | [32,33] | ||
| Co-axial nozzle | Printing of low viscosity bioinks, improve resolution, facilitate heterogeneous construct fabrication, enable multimaterial deposition | Low cell viability during bioprinting | [34] | ||
| Cell loaded micro-carriers laden bioink | Low viscosity bioinks can be printed, improve mechanical strength of bioink, good cell viability and function, enable heterogeneous construct fabrication | Limited resolution | [35] | ||
| Cell spheroids laden bioink | Efficient control over dimensions of printed construct, reduce shear stress during printing and consistent diffusion of bioactive molecules and good cell viability | Large constructs cannot be fabricated, comparatively large structures which limits bioprinting resolution, unable to form multilayered structures | [36,37] | ||
| Nanoparticles loaded bioinks | Improve viscosity with shear thinning properties, better resolution, safeguarding of cells from printing stress and cell function | Low cell viability after bioprinting | [26,38] | ||
| 2 | To improve shape fidelity | Modification with Self assembled moieties | Improve safe-guarding of cells from printing stresses | Difficult to control properties of self-assembled bioink | [22,39,40] |
| Optimization to quantify shear stress during bioprinting | Improve cell viability during bioprinting and determine printing parameters for better resolution | Optimization was established for limited bioinks and are not applicable for other bioinks | [2,9,23,24,41,42,43,44,45] | ||
| Photo-chemical Crosslinking | Increased mechanical strength to hold printed 3D structure | Decrease cell viability | [29,46,47,48] | ||
| Ionic crosslinking | Increase mechanical strength | Inefficient for multilayered constructs, decreases cell viability | [25,43,45,49,50] | ||
| Concomitantly printed scaffolds | Improve mechanical strength and efficient diffusivity of construct | Involve synthetic bioinks | [48,51] | ||
| Sacrificial scaffolds | Facilitate printing of complex shapes, efficient diffusion characteristics | Toxicity in case of synthetic scaffolds | [49,52,53,54] | ||
| Bioink for nano-porous scaffolds | Improve diffusion of nutrients and shape fidelity | Toxic synthetic polymers | [55] | ||
| Crosslinking with supportive scaffold | Improve mechanical strength and stability, implantable constructs due to efficient degradation | Rapid degradation | [56] | ||
| 3 | To achieve good cell viability and function | RGD modification | Improve cell adhesion and viability | - | [57,58] |
| Blending with biopolymers with cell binding domains | Improve cell binding as well as viability | Decrease printability | [33,48] | ||
| Decellularized ECM | Provide native microenvironment to laden cells | Low viscosity and poor resolution | [59,60] | ||
| Printing temperature | Improve cell viability during bioprinting | Decrease the printability of bioink | [23] | ||
| Vascularized constructs | Better diffusion characteristics which improves the cell viability | - | [53,55] |
| Source | Bioinks | Crosslinking Mechanism(s) | Shape Fidelity | Printing Resolution | Cell Supportive Ability | Drawbacks | Reference |
|---|---|---|---|---|---|---|---|
| Nature derived bioink | Alginate | Ionic | Medium | 400–600 μm (by blending) | Low | Lack cell binding domains | [31] |
| Gelatin | Covalent, enzymatic, physical and thermal | Medium | 350–450 μm (partial crosslinking and blending) | High | Low viscosity and mechanical integrity | [44,50,54,56,61,62] | |
| Collagen I | Physical | Low | 1000 μm | High | Low viscosity and mechanical integrity | [21] | |
| Fibrin | Enzyme based | High | - | Medium | Fast biodegradation and irreversible gelation | [48,58] | |
| Hyaluronic acid | Physical or covalent | High | 730 ± 28 μm (Semi-IPN) | High | Low stability | [50,63] | |
| Decellularied extracellular matrix | Physical | Low | - | High | Low viscosity and mechanical strength | [59] | |
| Silk | Enzymatic or physical | High | 280–320 μm | Low | Lack cell binding domains, Low cell viability | [64,65] | |
| Chitosan | Ionic | low | 400 μm (blending) | Medium | Low mechanical integrity, low cell viability | [60,66] | |
| Synthetic bioink | PEG | Covalent | - | - | Medium | Lack cell binding domains | [44] |
| Pluronic acid | Covalent | High | 150 μm | Low | Lack cell binding domains, Low cell viability and mechanical strength | [67] | |
| Nanostructured bioinks | Physical | Medium | - | Medium | - | [68,69] | |
| Self-Assembled bioink | Cd-Ad HA | Non-covalent | Medium | 222.6 ± 29.7 μm (supporting gel) | Medium | Low mechanical strength | [28] |
| DNA-peptide | Non-covalent | Medium | 500 μm | Medium | Low mechanical integrity and viscosity | [70] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panwar, A.; Tan, L.P. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685. https://doi.org/10.3390/molecules21060685
Panwar A, Tan LP. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules. 2016; 21(6):685. https://doi.org/10.3390/molecules21060685
Chicago/Turabian StylePanwar, Amit, and Lay Poh Tan. 2016. "Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting" Molecules 21, no. 6: 685. https://doi.org/10.3390/molecules21060685






