The Application of Ultrasound in 3D Bio-Printing
Abstract
:1. Introduction
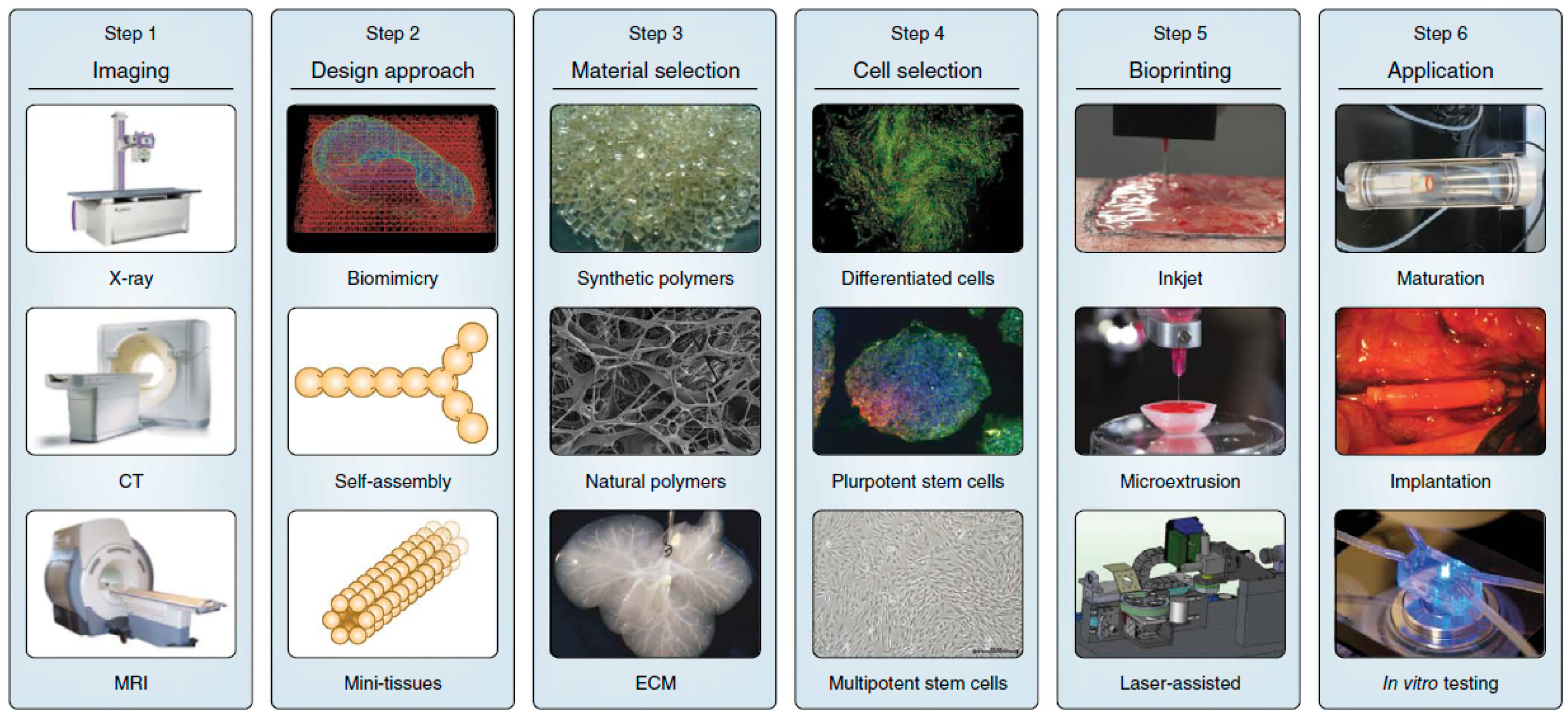
2. 3D Bioprinting
3. Bioink
3.1. Bioink and Its Preparation
3.2. Preparation Using Ultrasound Approach
3.3. Bioink Characterization
3.4. Medium of Bioink
4. Crosslinking
5. Tissue Fusion
6. Bioreactors
6.1. Post-Processing in 3D Bioprinting
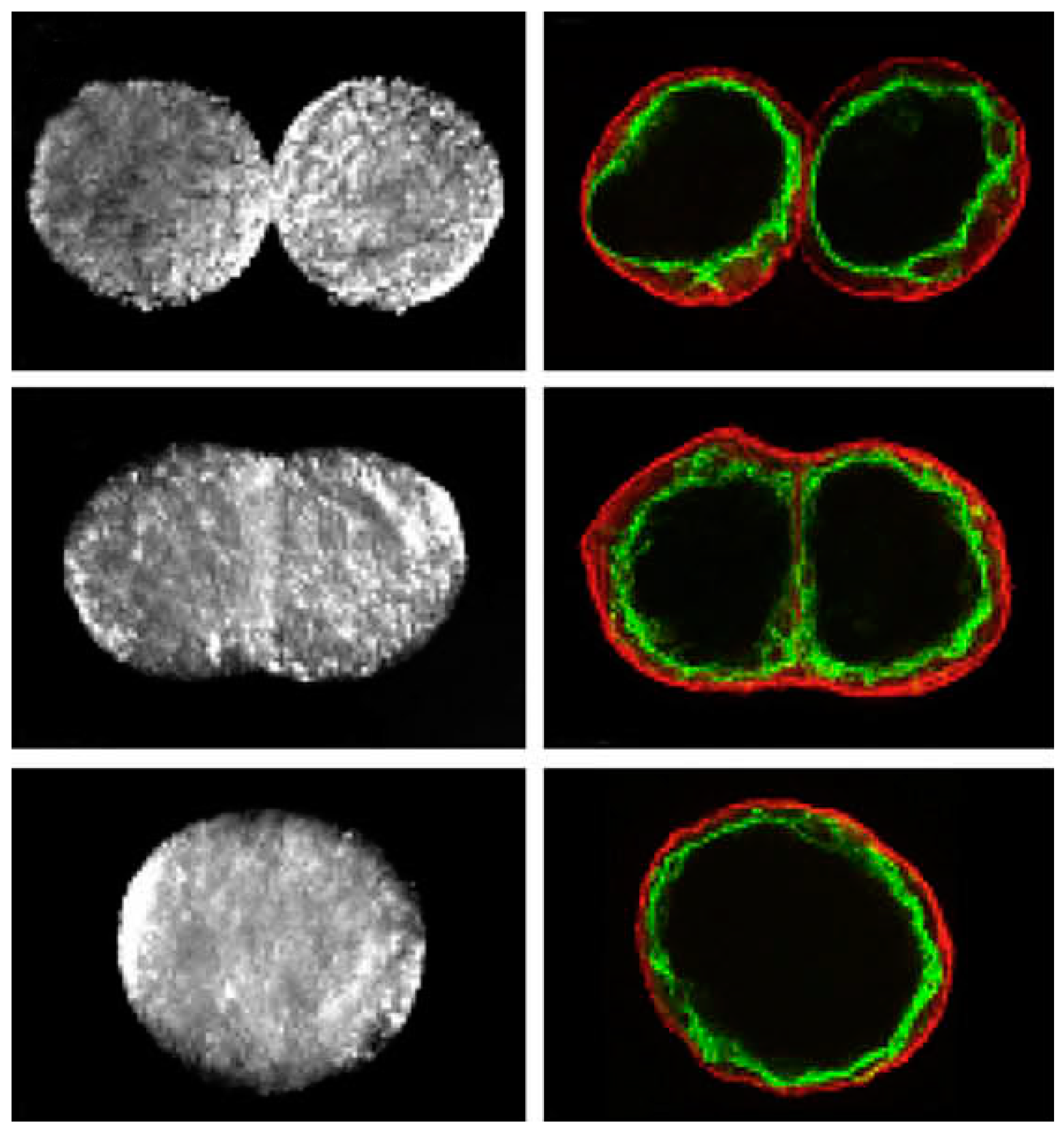
6.2. Vascularization of USWF-Induced Endothelial Cell Spheroids
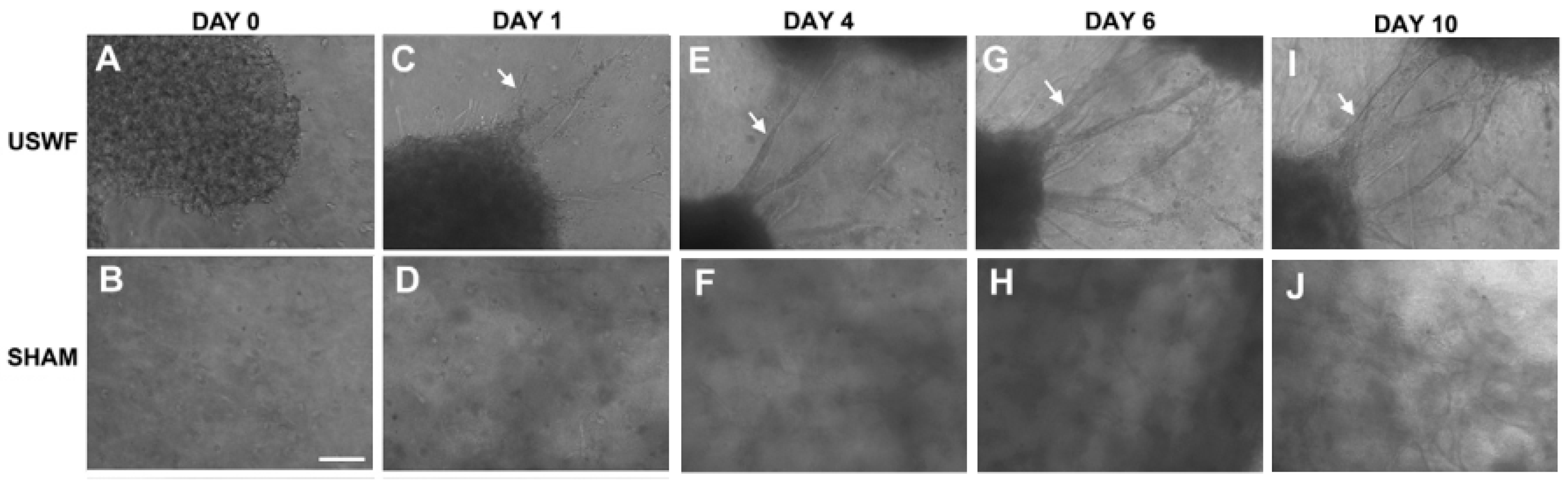
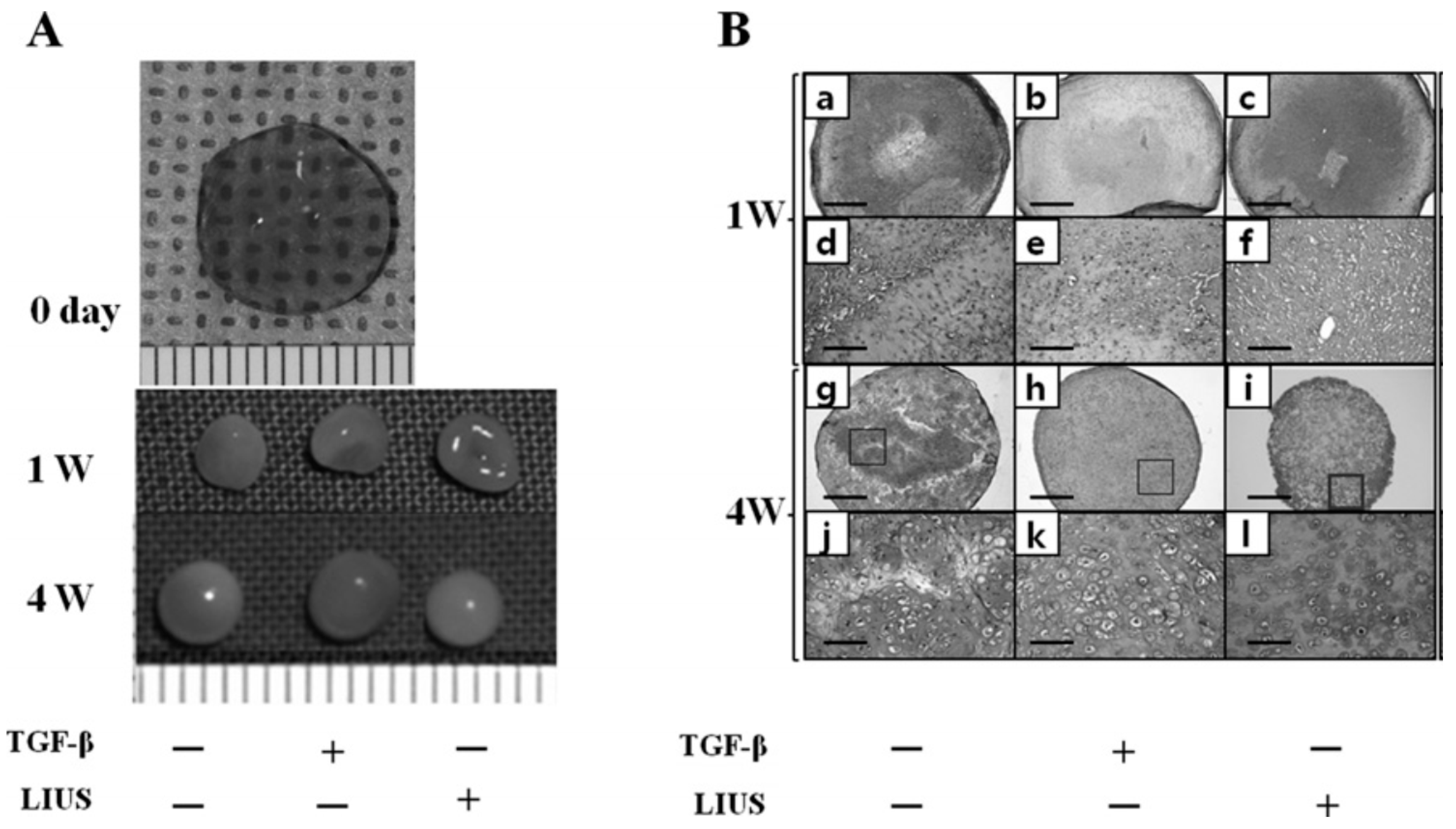
6.3. Effect of LIUS on Tissue Maturation
7. Discussion and Summary
Conflicts of Interest
References
- Murray, G.; Holden, R. Transplantation of kidneys, experimentally and in human cases. Am. J. Surg. 1954, 87, 508–515. [Google Scholar]
- Desmet, T.; Schacht, E.; Dubruel, P. Rapid prototyping as an elegant production tool for polymeric tissue engineering scaffolds: A review. In Tissue Engineering: Roles, Materials and Applications; Barnes, S.J., Harris, L., Eds.; Nova Science: New York, NY, USA, 2008; pp. 141–189. [Google Scholar]
- Bonassar, L.J.; Vacanti, C.A. Tissue engineering: the first decade and beyond. J. Cell. Biochem. 1998, 72 (Suppl. S30–S31), 297–303. [Google Scholar]
- Griffith, L.G.; Naughton, G. Tissue engineering—Current challenges and expanding opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Yu, Y. Bioprinting toward organ fabrication: Challenges and future trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar] [PubMed]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabricaiton and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Xu, T.; Damon, B.J.; Cui, X. Application of inkjet printing to tissue engineering. Biotechnol. J. 2006, 1, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.A.; Ivanova, O.S. 3D printing of multifunctional nanocomposites. Nano Today 2013, 8, 119–120. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R.; Borenstein, J.; Vacanti, J.P. Microscle technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. USA 2006, 103, 2480–2487. [Google Scholar] [CrossRef] [PubMed]
- Levenberg, S.; Rouwkema, J.; Macdonald, M.; Garfein, E.S.; Kohane, D.S.; Darland, D.C.; Marini, R.; van Blitterswijk, C.A.; Mulligan, R.C.; D’Amore, P.A.; et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005, 23, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.L.; Hudon, V.; Berthod, F.; Germain, L.; Auger, F.A. Inosculation of Tissue-Engineered Capillaries with the Host’s Vasculature in a Reconstructed Skin Transplanted on Mice. Am. J. Transplant. 2005, 5, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; West, J.L. Vascularization of engineered tissues: Approaches to promote angio-genesis in biomaterials. Curr. Top. Med. Chem. 2008, 8, 300–310. [Google Scholar] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 43, 3–12. [Google Scholar] [CrossRef]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting technology: A current state-of-the-art review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; de Carvalho Zavaglia, C.A.; Bernardes, L.F.; Lambert, C.S.; Kharmandayan, P.; Calderoni, D.; Maciel Filho, R. Customised titanium implant fabricated in additive manufacturing for craniomaxillofacial surgery: This paper discusses the design and fabrication of a metallic implant for the reconstruction of a large cranial defect. Virtual Phys. Prototyp. 2014, 9, 115–125. [Google Scholar] [CrossRef]
- Vaezi, M.; Yang, S. Extrusion-based additive manufacturing of PEEK for biomedical applications. Virtual Phys. Prototyp. 2015, 10, 123–135. [Google Scholar] [CrossRef]
- Dean, D.; Mott, E.; Luo, X.; Busso, M.; Wang, M.O.; Vorwald, C.; Siblani, A.; Fisher, J.P. Multiple initiators and dyes for continuous Digital Light Processing (cDLP) additive manufacture of resorbable bone tissue engineering scaffolds: A new method and new material to fabricate resorbable scaffold for bone tissue engineering via continuous Digital Light Processing. Virtual Phys Prototyp. 2014, 9, 3–9. [Google Scholar]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography; Google Patents: Arcadia, CA, USA, 1986. [Google Scholar]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.G.; Weiss, L.E. Tissue engineering with the aid of inkjet printers. Expert Opin. Biol. Ther. 2007, 7, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.L.; Malone, E.; Lipson, H.; Bonassar, L.J. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006, 12, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Reis, N.; Derby, B. Review: Bioprinting: A beginning. Tissue Eng. 2006, 12, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Lee, J.-S.; Kim, J.Y.; Cho, D.-W. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J. Micromech. Microeng. 2012, 22, 085014. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T.; D’Lima, D.D.; Lotz, M.K. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat. Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Iwami, K.; Noda, T.; Ishida, K.; Morishima, K.; Nakamura, M.; Umeda, N. Bio rapid prototyping by extruding/aspirating/refilling thermoreversible hydrogel. Biofabrication 2010, 2, 014108. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Rémy, M.; Bordenave, L.; Amédée, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet printing—Process and its applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Tekin, E.; Smith, P.J.; Schubert, U.S. Inkjet printing as a deposition and patterning tool for polymers and inorganic particles. Soft Matter 2008, 4, 703–713. [Google Scholar] [CrossRef]
- Fang, Y.; Frampton, J.P.; Raghavan, S.; Sabahi-Kaviani, R.; Luker, G.; Deng, C.X.; Takayama, S. Rapid generation of multiplexed cell cocultures using acoustic droplet ejection followed by aqueous two-phase exclusion patterning. Tissue Eng. Part C Methods 2012, 18, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.E.; Gough, J.E.; Derby, B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials 2008, 29, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Phillippi, J.A.; Miller, E.; Weiss, L.; Huard, J.; Waggoner, A.; Campbell, P. Microenvironments Engineered by Inkjet Bioprinting Spatially Direct Adult Stem Cells Toward Muscle- and Bone-Like Subpopulations. Stem Cells 2008, 26, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Choi, J.S.; Kim, B.S.; Choi, Y.C.; Cho, Y.W. Piezoelectric inkjet printing of polymers: Stem cell patterning on polymer substrates. Polymer 2010, 51, 2147–2154. [Google Scholar] [CrossRef]
- Parsa, S.; Gupta, M.; Loizeau, F.; Cheung, K.C. Effects of surfactant and gentle agitation on inkjet dispensing of living cells. Biofabrication 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Jones, N. Science in three dimensions: The print revolution. Nature 2012, 487, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Yeong, W.Y. A preliminary model of time-pressure dispensing system for bioprinting based on printing and material parameters: This paper reports a method to predict and control the width of hydrogel filament for bioprinting applications. Virtual Phys. Prototyp. 2015, 10, 3–8. [Google Scholar] [CrossRef]
- Chang, R.; Nam, J.; Sun, W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng. Part A 2008, 14, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Colina, M.; Serra, P.; Fernández-Pradas, J.M.; Sevilla, L.; Morenza, J.L. DNA deposition through laser induced forward transfer. Biosens. Bioelectron. 2005, 20, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng. Part C Methods 2010, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hopp, B.; Smausz, T.; Kresz, N.; Barna, N.; Bor, Z.; Kolozsvári, L.; Chrisey, D.B.; Szabó, A.; Nógrádi, A. Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng. 2005, 11, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Ringeisen, B.R.; Kim, H.; Barron, J.A.; Krizman, D.B.; Chrisey, D.B.; Jackman, S.; Auyeung, R.; Spargo, B.J. Laser printing of pluripotent embryonal carcinoma cells. Tissue Eng. 2004, 10, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef]
- Lin, R.Z.; Chang, H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Jakab, K.; Norotte, C.; Damon, B.; Marga, F.; Neagu, A.; Besch-Williford, C.L.; Kachurin, A.; Church, K.H.; Park, H.; Mironov, V.; et al. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng. Part A 2008, 14, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Boncheva, M. Beyond molecules: Self-assembly of mesoscopic and macroscopic components. Proc. Natl. Acad. Sci. USA 2002, 99, 4769–4774. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Yuhas, J.M.; Li, A.P.; Martinez, A.O.; Ladman, A.J. A simplified method for production and growth of multicellular tumor spheroids. Cancer Res. 1977, 37, 3639–3643. [Google Scholar] [PubMed]
- Hamilton, G. Multicellular spheroids as an in vitro tumor model. Cancer Lett. 1998, 131, 29–34. [Google Scholar] [CrossRef]
- Khaitan, D.; Chandna, S.; Arya, M.; Dwarakanath, B. Establishment and characterization of multicellular spheroids from a human glioma cell line; Implications for tumor therapy. J. Transl. Med. 2006, 4. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Pérez, J.; Ballesteros, P.; Cerdán, S. Microscopic images of intraspheroidal pH by 1H magnetic resonance chemical shift imaging of pH sensitive indicators. Magn. Reson. Mater. Phys. Biol. Med. 2005, 18, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Curcio, E.; Salerno, S.; Barbieri, G.; de Bartolo, L.; Drioli, E.; Bader, A. Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system. Biomaterials 2007, 28, 5487–5497. [Google Scholar] [CrossRef] [PubMed]
- Rieke, M.; Gottwald, E.; Weibezahn, K.-F.; Layer, P.G. Tissue reconstruction in 3D-spheroids from rodent retina in a motion-free, bioreactor-based microstructure. Lab Chip 2008, 8, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.P.; Chai, P.; Dean, D.M.; Morgan, J.R. Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. 2007, 13, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Achilli, T.-M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Timmins, N.E.; Nielsen, L.K. Generation of multicellular tumor spheroids by the hanging-drop method. Tissue Eng. 2007, 141–151. [Google Scholar]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.-S.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Toh, Y.-C.; Zhang, C.; Zhang, J.; Khong, Y.M.; Chang, S.; Samper, V.D.; van Noort, D.; Hutmacher, D.W.; Yu, H. A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip 2007, 7, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.K.; Shum, H.C.; Rowat, A.C.; Lee, D.; Agresti, J.J.; Utada, A.S.; Chu, L.-Y.; Kim, J.-W.; Fernandez-Nieves, A.; Martinez, C.J.; et al. Designer emulsions using microfluidics. Mater. Today 2008, 11, 18–27. [Google Scholar] [CrossRef]
- Sebastian, A.; Buckle, A.M.; Markx, G.H. Tissue engineering with electric fields: Immobilization of mammalian cells in multilayer aggregates using dielectrophoresis. Biotechnol. Bioeng. 2007, 98, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Okochi, M.; Honda, H. Application of magnetic force-based cell patterning for controlling cell-cell interactions in angiogenesis. Biotechnol. Bioeng. 2009, 102, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Okochi, M.; Takano, S.; Isaji, Y.; Senga, T.; Hamaguchi, M.; Honda, H. Three-dimensional cell culture array using magnetic force-based cell patterning for analysis of invasive capacity of BALB/3T3/v-src. Lab Chip 2009, 9, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Bazou, D.; Foster, G.A.; Ralphs, J.R.; Coakley, W.T. Molecular adhesion development in a neural cell monolayer forming in an ultrasound trap. Mol. Membr. Biol. 2005, 22, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Bazou, D.; Kuznetsova, L.A.; Coakley, W.T. Physical enviroment of 2-D animal cell aggregates formed in a short pathlength ultrasound standing wave trap. Ultrasound Med. Biol. 2005, 31, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Coakley, W.T.; Bardsley, D.W.; Grundy, M.A.; Zamani, F.; Clarke, D.J. Cell manipulation in ultrasonic standing wave fields. J. Chem. Technol. Biotechnol. 1989, 44, 43–62. [Google Scholar] [CrossRef]
- Dyson, M.; Pond, J.; Woodward, B.; Broadbent, J. The production of blood cell stasis and endothelial damage in the blood vessels of chick embryos treated with ultrasound in a stationary wave field. Ultrasound Med. Biol. 1974, 1, 133–148. [Google Scholar] [CrossRef]
- Whitworth, G.; Coakley, W. Particle column formation in a stationary ultrasonic field. J. Acoust. Soc. Am. 1992, 91, 79–85. [Google Scholar] [CrossRef]
- Gor’Kov, L. On the Forces Acting on a Small Particle in an Acoustical Field in an Ideal Fluid. Sov. Phys. Dokl. 1962, 6, 773. [Google Scholar]
- Kuznetsova, L.A.; Bazou, D.; Edwards, G.O.; Coakley, W.T. Multiple three-dimensional mammalian cell aggregates formed away from solid substrata in ultrasound standing waves. Biotechnol. Prog. 2009, 25, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Vacanti, J.P.; Langer, R. Progress in tissue engineering. Sci. Am. 2009, 300, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bazou, D. Biochemical properties of encapsulated high-density 3-D HepG2 aggregates formed in an ultrasound trap for application in hepatotoxicity studies. Cell Biol. Toxicol. 2010, 26, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Bazou, D.; Coakley, W.T.; Hayes, A.; Jackson, S.K. Long-term viability and proliferation of alginate-encapsulated 3-D HepG2 aggregates formed in an ultrasound trap. Toxicol. Vitro 2008, 22, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Garvin, K.A.; Hocking, D.C.; Dalecki, D. Controlling the spatial organization of cells and extracellular matrix proteins in engineered tissues using ultrasound standing wave fields. Ultrasound Med. Biol. 2010, 36, 1919–1932. [Google Scholar] [CrossRef] [PubMed]
- Garvin, K.A.; Dalecki, D.; Hocking, D.C. Vascularization of three-dimensional collagen hydrogels using ultrasound standing wave fields. Ultrasound Med. Biol. 2011, 37, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kuznetsova, L.A.; Edwards, G.O.; Xu, J.; Ma, M.; Purcell, W.M.; Jackson, S.K.; Coakley, W.T. Functional three-dimensional HepG2 aggregate cultures generated from an ultrasound trap: Comparison with HepG2 spheroids. J. Cell. Biochem. 2007, 102, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Braeckmans, K.; Peeters, L.; Sanders, N.N.; De Smedt, S.C.; Demeester, J. Three-dimensional fluorescence recovery after photobleaching with the confocal scanning laser microscope. Biophys. J. 2003, 85, 2240–2252. [Google Scholar] [CrossRef]
- Bartholomä, P.; Reininger-Mack, A.; Zhang, Z.; Thielecke, H.; Robitzki, A. A more aggressive breast cancer spheroid model coupled to an electronic capillary sensor system for a high-content screening of cytotoxic agents in cancer therapy: 3-Dimensional in vitro tumor spheroids as a screening model. J. Biomol. Screen. 2005, 10, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Tasoglu, S.; Demirci, U. Bioprinting for stem cell research. Trends Biotechnol. 2013, 31, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Nuttelman, C.R.; Rice, M.A.; Rydholm, A.E.; Salinas, C.N.; Shah, D.N.; Anseth, K.S. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Prog. Polym. Sci. 2008, 33, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pomares, J.M.; Foty, R.A. Tissue fusion and cell sorting in embryonic development and disease: Biomedical implications. Bioessays 2006, 28, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.; Poole, T. Liquid behavior of embryonic tissues. In Cell Behaviour; Bellairs, R., Curtis, A.S.G., Dunn, G., Eds.; Cambridge University Press: Cambridge, UK, 1982; pp. 583–607. [Google Scholar]
- Steinberg, M.S. Reconstruction of tissues by dissociated cells. Science 1963, 141, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.S. Differential adhesion in morphogenesis: A modern view. Curr. Opin. Genet. Dev. 2007, 17, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.M.; Morgan, J.R. Cytoskeletal-mediated tension modulates the directed self-assembly of microtissues. Tissue Eng. Part A 2008, 14, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Jakab, K.; Damon, B.; Marga, F.; Doaga, O.; Mironov, V.; Kosztin, I.; Markwald, R.; Forgacs, G. Relating cell and tissue mechanics: Implications and applications. Dev. Dyn. 2008, 237, 2438–2449. [Google Scholar] [CrossRef] [PubMed]
- Neagu, A.; Jakab, K.; Jamison, R.; Forgacs, G. Role of physical mechanisms in biological self-organization. Phys. Rev. Lett. 2005, 95, 178104. [Google Scholar] [CrossRef] [PubMed]
- Khatiwala, C.; Law, R.; Shepherd, B.; Dorfman, S.; Csete, M. 3D cell bioprinting for regenerative medicine research and therapies. Gene Ther. Regul. 2012, 7. [Google Scholar] [CrossRef]
- Tan, Q.; Steiner, R.; Yang, L.; Welti, M.; Neuenschwander, P.; Hillinger, S.; Weder, W. Accelerated angiogenesis by continuous medium flow with vascular endothelial growth factor inside tissue-engineered trachea. Eur. J. Cardio-Thorac. Surg. 2007, 31, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Nik, N.; Amoabediny, G.; Pouran, B.; Tabesh, H.; Shokrgozar, M.A.; Haghighipour, N.; Khatibi, N.; Anisi, F.; Mottaghy, K.; Zandieh-Doulabi, B. Engineering parameters in bioreactor’s design: A critical aspect in tissue engineering. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, Y.; Pan, Y.; Xiong, Z.; Liu, H.; Cheng, J.; Liu, F.; Lin, F.; Wu, R.; Zhang, R. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, Y.; Zhang, R. Recent trends and challenges in complex organ manufacturing. Tissue Eng. Part B Rev. 2009, 16, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, X.; Pan, Y.; Liu, H.; Cheng, J.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials 2005, 26, 5864–5871. [Google Scholar] [CrossRef] [PubMed]
- Limpanuphap, S.; Derby, B. Manufacture of biomaterials by a novel printing process. J. Mater. Sci. Mater. Med. 2002, 13, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.-H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Liu, Y.; Shu, X.Z.; Prestwich, G.D. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials 2005, 26, 6054–6067. [Google Scholar] [CrossRef] [PubMed]
- Peattie, R.A.; Rieke, E.R.; Hewett, E.M.; Fisher, R.J.; Shu, X.Z.; Prestwich, G.D. Dual growth factor-induced angiogenesis in vivo using hyaluronan hydrogel implants. Biomaterials 2006, 27, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Korff, T.; Augustin, H.G. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J. Cell Biol. 1998, 143, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Girton, T.; Oegema, T.; Tranquillo, R. Exploiting glycation to stiffen and strengthen tissue equivalents for tissue engineering. J. Biomed. Mater. Res. 1999, 46, 87–92. [Google Scholar] [CrossRef]
- Pelttari, K.; Winter, A.; Steck, E.; Goetzke, K.; Hennig, T.; Ochs, B.G.; Aigner, T.; Richter, W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006, 54, 3254–3266. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Breit, S.; Parsch, D.; Benz, K.; Steck, E.; Hauner, H.; Weber, R.M.; Ewerbeck, V.; Richter, W. Cartilage-like gene expression in differentiated human stem cell spheroids: A comparison of bone marrow-derived and adipose tissue–derived stromal cells. Arthritis Rheum. 2003, 48, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Merrilees, M.J.; Braun, K.; Beaumont, B.; Lemire, J.; Clowes, A.W.; Hinek, A.; Wight, T.N. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ. Res. 2006, 98, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.A.; Damon, B.; Mironov, V.; Kasyanov, V.; Ramamurthi, A.; Moreno-Rodriguez, R.; Trusk, T.; Potts, J.D.; Goodwin, R.L.; Davis, J.; et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J. Cell. Biochem. 2007, 101, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Wenger, A.; Stahl, A.; Weber, H.; Finkenzeller, G.; Augustin, H.; Stark, G.; Kneser, U. Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng. 2004, 10, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Boer, J.D.; Blitterswijk, C.A.V. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006, 12, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Alajati, A.; Laib, A.M.; Weber, H.; Boos, A.M.; Bartol, A.; Ikenberg, K.; Korff, T.; Zentgraf, H.; Obodozie, C.; Graeser, R.; et al. Spheroid-based engineering of a human vasculature in mice. Nat. Methods 2008, 5, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kamei, M.; Saunders, W.B.; Bayless, K.J.; Dye, L.; Davis, G.E.; Weinstein, B.M. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 2006, 442, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Hoying, J.B.; Boswell, C.A.; Williams, S.K. Angiogenic potential of microvessel fragments established in three-dimensional collagen gels. Vitro Cell. Dev. Biol. Anim. 1996, 32, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [PubMed]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Angele, P.; Yoo, J.; Smith, C.; Mansour, J.; Jepsen, K.; Nerlich, M.; Johnstone, B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J. Orthop. Res. 2003, 21, 451–457. [Google Scholar] [CrossRef]
- Huang, C.; Charles, Y.; Hagar, K.L.; Frost, L.E.; Sun, Y.; Cheung, H.S. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 2004, 22, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Park, S.R.; Choi, B.H.; Min, B.-H. Low-intensity ultrasound (LIUS) as an innovative tool for chondrogenesis of mesenchymal stem cells (MSCs). Organogenesis 2007, 3, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Woo, J.I.; Min, B.H.; Park, S.R. Low-intensity ultrasound stimulates the viability and matrix gene expression of human articular chondrocytes in alginate bead culture. J. Biomed. Mater. Res. Part A 2006, 79, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Min, B.-H.; Choi, B.H.; Park, S.R. Low intensity ultrasound as a supporter of cartilage regeneration and its engineering. Biotechnol. Bioprocess Eng. 2007, 12, 22–31. [Google Scholar] [CrossRef]
- Cook, S.D.; Salkeld, S.L.; Popich-Patron, L.S.; Ryaby, J.P.; Jones, D.G.; Barrack, R.L. Improved cartilage repair after treatment with low-intensity pulsed ultrasound. Clin. Orthop. Relat. Res. 2001, 391, S231–S243. [Google Scholar] [CrossRef]
- Choi, J.W.; Choi, B.H.; Park, S.H.; Pai, K.S.; Li, T.Z.; Min, B.H.; Park, S.R. Mechanical Stimulation by Ultrasound Enhances Chondrogenic Differentiation of Mesenchymal Stem Cells in a Fibrin-Hyaluronic Acid Hydrogel. Artif. Organs 2013, 37, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, B.H.; Min, B.H.; Son, Y.S.; Park, S.R. Low-intensity Ultrasound Stimulation Enhances Chondrogenic Differentiation in Alginate Culture of Mesenchymal Stem Cells. Artif. Organs 2006, 30, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, B.H.; Min, B.-H.; Park, S.R. Low-intensity ultrasound inhibits apoptosis and enhances viability of human mesenchymal stem cells in three-dimensional alginate culture during chondrogenic differentiation. Tissue Eng. 2007, 13, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Schmelz, A.; Seufferlein, T.; Li, Y.; Zhao, J.; Bachem, M.G. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. J. Biol. Chem. 2004, 279, 54463–54469. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Choi, M.H.; Kwak, M.; Min, B.; Woo, Z.; Park, S. Mechanotransduction pathways of low-intensity ultrasound in C-28/I2 human chondrocyte cell line. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2007, 221, 527–535. [Google Scholar] [CrossRef]
- Cui, J.H.; Park, K.; Park, S.R.; Min, B.-H. Effects of low-intensity ultrasound on chondrogenic differentiation of mesenchymal stem cells embedded in polyglycolic acid: An in vivo study. Tissue Eng. 2006, 12, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Kasyanov, V.; Markwald, R.R. Organ printing: From bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 2011, 22, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.J.; Zaccarelli, E.; Ciulla, F.; Schofield, A.B.; Sciortino, F.; Weitz, D.A. Gelation of particles with short-range attraction. Nature 2008, 453, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Kasyanov, V.; Markwald, R.R. Nanotechnology in vascular tissue engineering: From nanoscaffolding towards rapid vessel biofabrication. Trends Biotechnol. 2008, 26, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, H.K.; Philp, D.; Hoffman, M.P. Role of the extracellular matrix in morphogenesis. Curr. Opin. Biotechnol. 2003, 14, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.A.; Chen, C.S. Deconstructing dimensionality. Science 2013, 339, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Frieboes, H.B.; Edgerton, M.E.; Fruehauf, J.P.; Rose, F.R.; Worrall, L.K.; Gatenby, R.A.; Ferrari, M.; Cristini, V. Prediction of drug response in breast cancer using integrative experimental/computational modeling. Cancer Res. 2009, 69, 4484–4492. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Groneberg, D.A.; Grosse-Siestrup, C.; Fischer, A. In vitro models to study hepatotoxicity. Toxicol. Pathol. 2002, 30, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D platform to explore cell—ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B. Three-Dimensional Tissue Culture Models in Cancer Biology. Semin. Cancer Biol. 2005, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Vargo-Gogola, T.; Rosen, J.M. Modelling breast cancer: One size does not fit all. Nat. Rev. Cancer 2007, 7, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Ridky, T.W.; Chow, J.M.; Wong, D.J.; Khavari, P.A. Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat. Med. 2010, 16, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Schrobback, K.; Klein, T.J.; Crawford, R.; Upton, Z.; Malda, J.; Leavesley, D.I. Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res. 2012, 347, 649–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramis-Conde, I.; Drasdo, D.; Anderson, A.R.; Chaplain, M.A. Modeling the influence of the E-cadherin-β-catenin pathway in cancer cell invasion: A multiscale approach. Biophys. J. 2008, 95, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Demuth, T.; Mobley, D.; Berens, M.; Sander, L. A Mathematical Model of Glioblastoma Tumor Spheroid Invasion in a Three-Dimensional in vitro experiment. J. Biophys. 2007, 921, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Song, S.-J.; Lee, J.Y.; Jang, H.; Choi, J.; Sun, K.; Park, Y. Cellular behavior in micropatterned hydrogels by bioprinting system depended on the cell types and cellular interaction. J. Biosci. Bioeng. 2013, 116, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; Prestwich, G.D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 2010, 31, 6173–6181. [Google Scholar] [CrossRef] [PubMed]
- Soucy, P.A.; Romer, L.H. Endothelial cell adhesion, signaling, and morphogenesis in fibroblast-derived matrix. Matrix Biol. 2009, 28, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Noble, P.B.; Walton, P.A.; Laird, D.W.; Chauvin, P.J.; Tabah, R.J.; Black, M.; Zänker, K.S. Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer Res. 1995, 55, 4557–4560. [Google Scholar] [PubMed]
- An, J.; Chua, C.K.; Mironov, V. A perspective on 4D bioprinting. Int. J. Bioprint. 2016, 2, 3–5. [Google Scholar] [CrossRef]
- Khoo, Z.X.; Teoh, J.E.M.; Liu, Y.; Chua, C.K.; Yang, S.; An, J.; Leong, K.F.; Yeong, W.Y. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual Phys. Prototyp. 2015, 10, 103–122. [Google Scholar] [CrossRef]
- Gillette, B.M.; Jensen, J.A.; Wang, M.; Tchao, J.; Sia, S.K. Dynamic Hydrogels: Switching of 3D Microenvironments Using Two-Component Naturally Derived Extracellular Matrices. Adv. Mater. 2010, 22, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.A.; Swartz, M.A. Mechanobiology in the third dimension. Ann. Biomed. Eng. 2005, 33, 1469–1490. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Jiang, J.; Yanase, T.; Nishi, Y.; Morgan, J.R. Connexon-mediated cell adhesion drives microtissue self-assembly. FASEB J. 2011, 25, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Tzanakakis, E.S.; Hansen, L.K.; Hu, W.-S. The role of actin filaments and microtubules in hepatocyte spheroid self-assembly. Cell Motil. Cytoskelet. 2001, 48, 175–189. [Google Scholar] [CrossRef]
- Zhang, J.; Betson, M.; Erasmus, J.; Zeikos, K.; Bailly, M.; Cramer, L.P.; Braga, V.M. Actin at cell-cell junctions is composed of two dynamic and functional populations. J. Cell Sci. 2005, 118, 5549–5562. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Gabbiani, G. Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotechnol. 2003, 14, 538–546. [Google Scholar] [CrossRef] [PubMed]
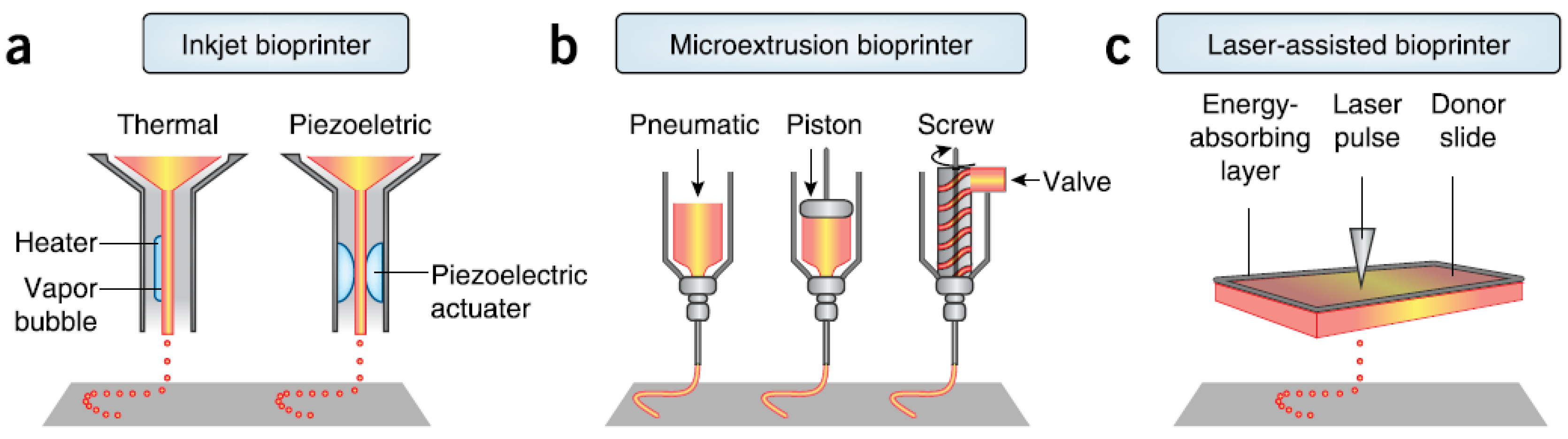




| Specification | Inkjet | Microextrusion | Laser Assisted | |
|---|---|---|---|---|
| Resolution | medium, 50 μm wide | medium-low, 5 μm–mm wide | high, μm wide | |
| Droplet size | 50–300 μm | 100–1000 μm | >20 μm | |
| Printing speed | fast (1–10,000 droplets/s) | slow (10–50 μm/s) | medium-fast (200–1600 mm/s) | |
| Materials | liquids, hydrogels | hydrogels, cell aggregates | cell in media | |
| Material viscosity | 3.5–12 mPa/s | 30–6 × 107 mPa/s | 1–300 mPa/s | |
| Cell density | low, <106 cells/mL | High, cell spheroids | medium, 108 cells/mL | |
| Multicellular feasibility | yes | yes | yes | |
| Preparation time | short | short-medium | long | |
| Mechanical integrity | low | high | low | |
| Fabrication time | long | long-medium | short | |
| Cell viability | high, >85% | medium-high, 40%–80% | medium, >95% | |
| Throughput | high | medium | low-medium | |
| Single-cell printing | low | medium | high | |
| Gelation speed | high | medium | high | |
| Printer price | low | medium | high | |
| Commercial availability | yes | yes | yes | |
| Advantages | affordable, versatile | multiple compositions, good mechanical properties | high accuracy, single cell manipulation, high-viscosity material | |
| Disadvantages | low viscosity, low strength | shear stress on nozzle tip, relatively low accuracy | cell unfriendly, low scalability, low viscosity in 3D build-up | |
| Imaging | Sonography has much lower resolution than MRI and CT to illustrate the composition and structure of tissue clearly. But it can monitor the condition of bioprinted parts in vivo in real time at much lower cost. |
| Bioink preparation | USWF can generate various types of cell spheroids efficiently and quickly in a high cell viability using easy operation. Design of the device and optimization of operating parameters need specific knowledge, acoustics. |
| Tissue fusion | Acoustic field may be beneficial in enhancing the tissue fusion by the acoustic radiation force or mechanical vibration, which needs more experimental evidence. |
| Tissue maturation | LIUS could enhance the differentiation of stem cells effectively and highly compatible with the current bioreactors and tissue maturation approaches. Appropriate control of the release of growth factor at different stages of tissue maturation using different cell types is challenging. |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y. The Application of Ultrasound in 3D Bio-Printing. Molecules 2016, 21, 590. https://doi.org/10.3390/molecules21050590
Zhou Y. The Application of Ultrasound in 3D Bio-Printing. Molecules. 2016; 21(5):590. https://doi.org/10.3390/molecules21050590
Chicago/Turabian StyleZhou, Yufeng. 2016. "The Application of Ultrasound in 3D Bio-Printing" Molecules 21, no. 5: 590. https://doi.org/10.3390/molecules21050590






