New Trends on Antineoplastic Therapy Research: Bullfrog (Rana catesbeiana Shaw) Oil Nanostructured Systems
Abstract
:1. Introduction
2. Results and Discussion
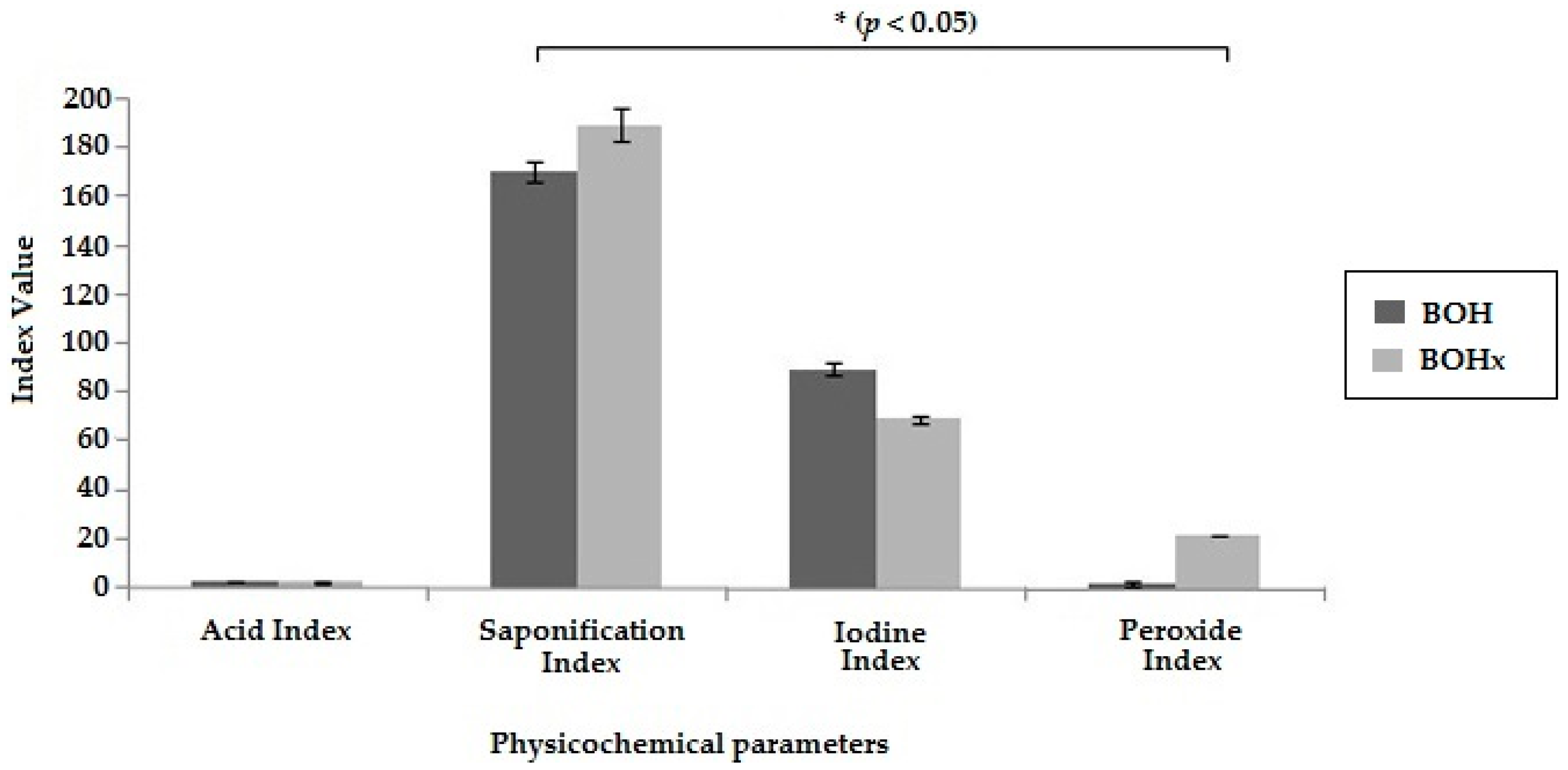
2.1. Extraction and Physicochemical Characterization of the Bullfrog Oil
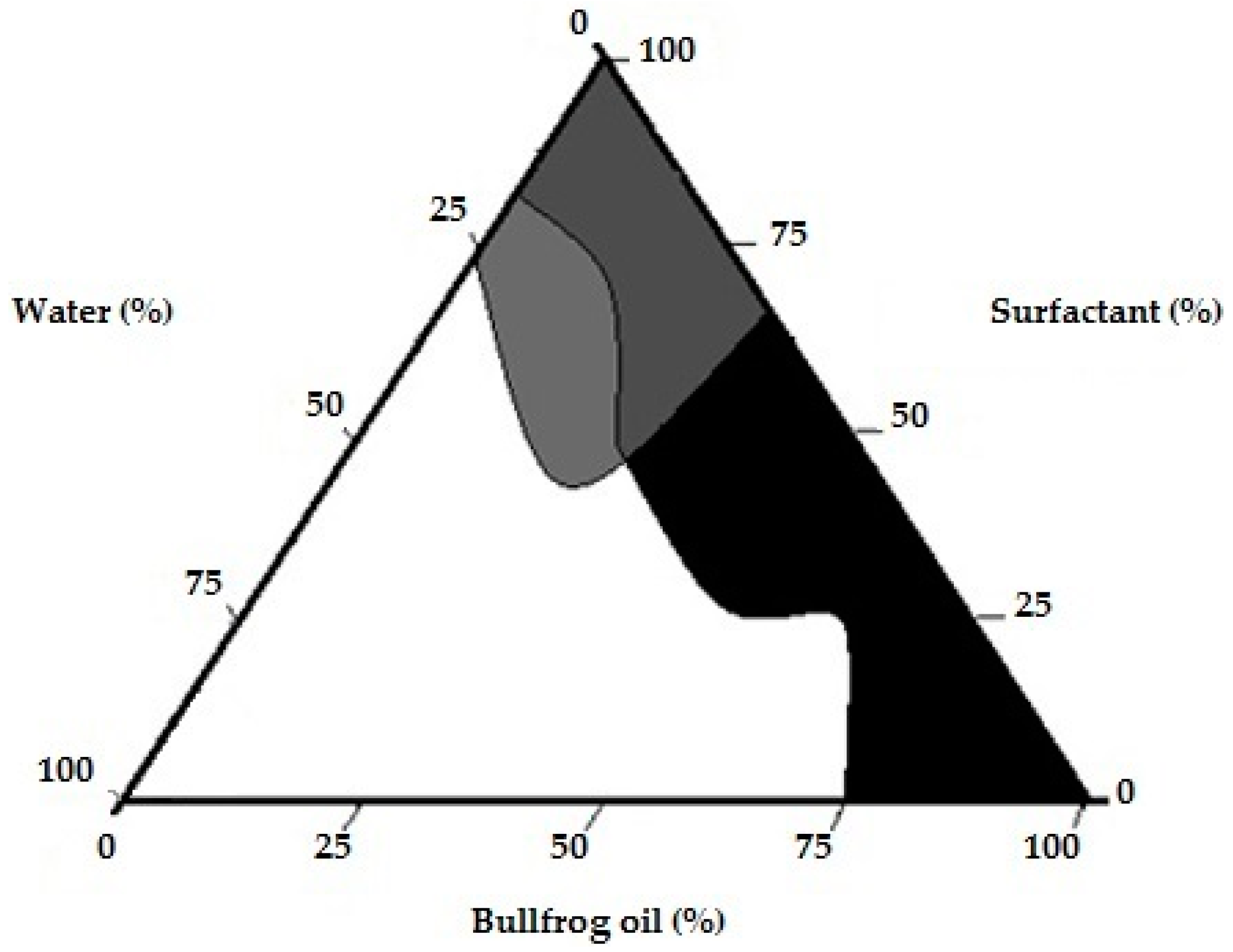
2.2. Pseudo-Ternary Phase Diagram (PTPD) for Bullfrog Oil Compositions
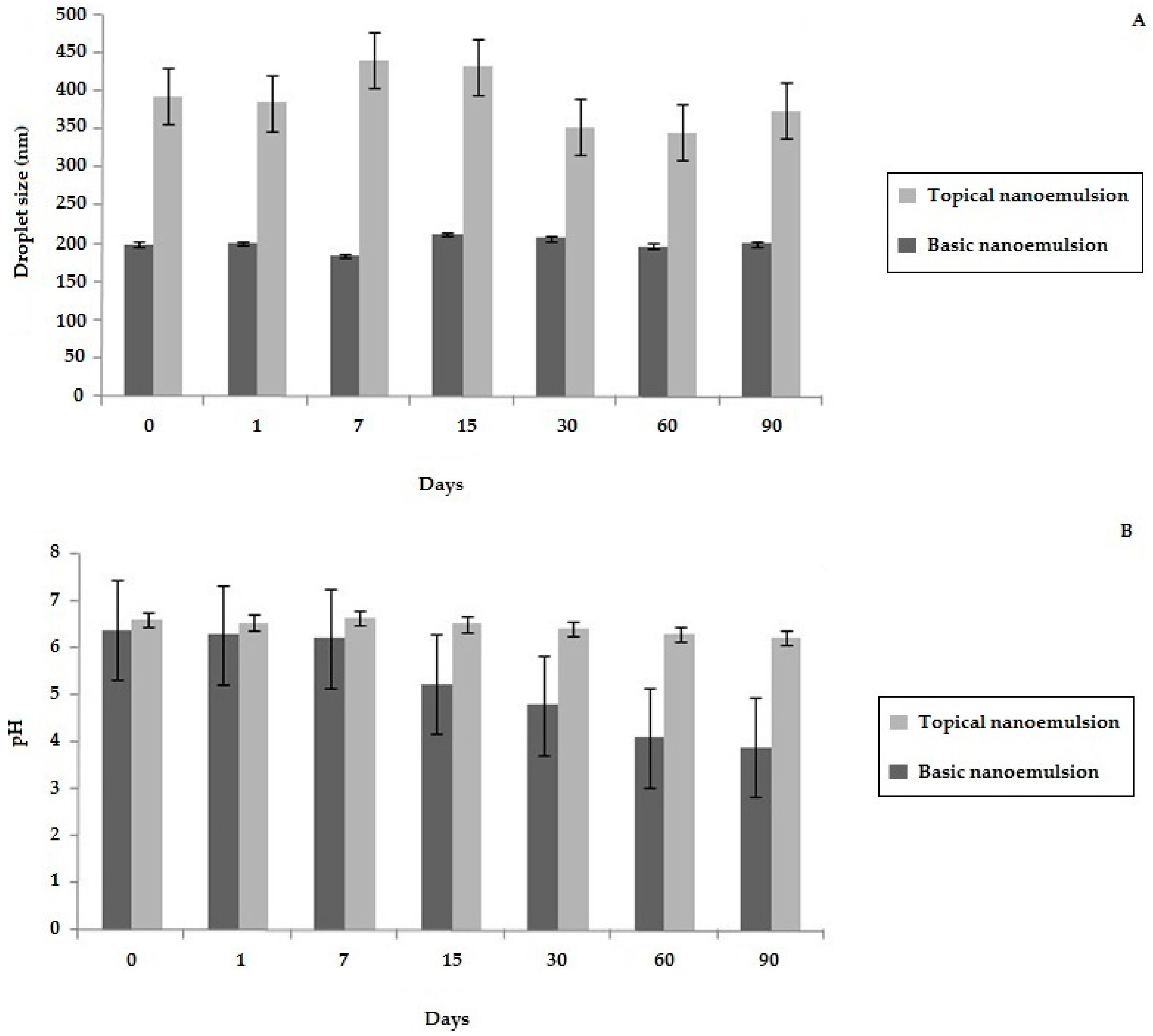
2.3. Development of a Topical Bullfrog Oil Nanoemulsion
2.4. Biocompatibility and Cytotoxicity Study
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Bullfrog Oil Extraction
4.2.2. Physicochemical Characterization of Bullfrog Oils
4.2.3. Gas-Chromatography—Mass Spectroscopy Analysis
4.2.4. HLBr Study
4.2.5. Construction of Pseudo-Ternary Phase Diagram
4.2.6. Development of Topical Nanoemulsion Based on Bullfrog Oil
4.3. Characterization of the Emulsions
4.3.1. Macroscopic Aspect
4.3.2. pH and Conductivity Evaluation
4.3.3. Droplet Size Distribution and Zeta Potential Analysis
4.4. Stability Study of the Emulsion
4.5. Biocompatibility and Cytotoxicity Study
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Felice, F.; Zambito, Y.; Belardinelli, E.; D’Onofrio, C.; Fabiano, A.; Balbarini, A.; di Stefano, R. Delivery of natural polyphenols by polymeric nanoparticles improves the resistance of endothelial progenitor cells to oxidative stress. Eur. J. Pharm. Sci. 2013, 50, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Bjorneboe, A.; Soyland, E.; Bjorneboe, G.E.A.; Rajka, G.; Drevon, C.A. Effect of dietary supplementation with eicosapentaenoic acid in the treatment of atopic-dermatitis. Br. J. Dermatol. 1987, 117, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.A.; Gurgel, L.A.; Campos, A.R.; Silveira, E.R.; Rao, V.S. Attenuation of ischemia/reperfusion-induced intestinal injury by oleo-resin from copaifera langsdorffii in rats. Life. Sci. 2004, 75, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.A.; Rao, V.S.; Gramosa, N.V.; Silveira, E.R. Gastroprotective effect of copaifera langsdorffii oleo-resin on experimental gastric ulcer models in rats. J. Ethnopharmacol. 1998, 62, 73–78. [Google Scholar] [CrossRef]
- Terano, T.; Salmon, J.A.; Higgs, G.A.; Moncada, S. Eicosapentaenoic acid as a modulator of inflammation—Effect on prostaglandin and leukotriene synthesis. Biochem. Pharmacol. 1986, 35, 779–785. [Google Scholar] [CrossRef]
- Yaqoob, P. Monounsaturated fats and immune function. Proc. Nutr. Soc. 1998, 57, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Dossat, V.; Combes, D.; Marty, A. Lipase-catalysed transesterification of high oleic sunflower oil. Enzym. Microb. Technol. 2002, 30, 90–94. [Google Scholar] [CrossRef]
- Alencar, E.N.; Xavier, F.H.; Morais, A.R.V.; Dantas, T.R.F.; Dantas-Santos, N.; Verissimo, L.M.; Rehder, V.L.G.; Chaves, G.M.; Oliveira, A.G.; Egito, E.S.T. Chemical characterization and antimicrobial activity evaluation of natural oil nanostructured emulsions. J. Nanosci. Nanotechnol. 2015, 15, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.S.; Dantas, T.N.C.; Cunha, A.F.; Moura, E.F.; Maciel, M.A.M. Obtenção de um tensoativo aniônico a partir de óleo de rana catesbeiana shaw. Rev. de Ci. Vida. Seropética. 2010, 30, 85–97. [Google Scholar]
- Mendez, E.; Sanhueza, J.; Nieto, S.; Speisky, H.; Valenzuela, A. Fatty acid composition, extraction, fractionation, and stabilization of bullfrog (rana catesbeiana) oil. J. Am. Oil. Chem. Soc. 1998, 75, 67–71. [Google Scholar] [CrossRef]
- Andrade, L.N.; de Lima, T.M.; Curi, R.; Castrucci, A.M. Toxicity of fatty acids on murine and human melanoma cell lines. Toxicol. Vitro. 2005, 19, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, M.; Reznicek, G.; Kahlig, H.; Kotisch, H.; Resch, G.P.; Valenta, C. Topical delivery of acetyl hexapeptide-8 from different emulsions: Influence of emulsion composition and internal structure. Eur. J. Pharm. Sci. 2015, 68, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.A. Pharmaceutical Dosage Forms-Disperse Systems; St. John’s University: Jamaica, NY, USA, 1988. [Google Scholar]
- Griffin, W.C. Classification of surface-active agents by "hlb". J. Soc. Cosmet. Chem. 1949, 11, 1383–1390. [Google Scholar]
- Ferrari, M.; Maruno, M.; Nakano, A.K.; Rocha-Filho, P.A. In vivo evaluation of the photoprotective efficacy of o1/w/o2 multiple emulsions with andiroba oil (carapa guianensis). J. Disper. Sci. Technol. 2008, 29, 1203–1208. [Google Scholar] [CrossRef]
- Macedo, J.P.F.; Fernandes, L.L.; Formiga, F.R.; Reis, M.F.; Nagashima, T.; Soares, L.A.L.; Egito, E.S.T. Micro-emultocrit technique: A valuable tool for determination of critical hlb value of emulsions. AAPS. Pharmscitech. 2006, 7, 146–152. [Google Scholar]
- Bimakr, M.; Rahman, R.L.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I.S.M. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (mentha spicata l.) leaves. Food. Bioprod. Proces. 2011, 89, 67–72. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; The Society: Champaign, IL, USA, 1989. [Google Scholar]
- Knothe, G. Structure indices in fa chemistry. How relevant is the iodine value? J. Am. Oil. Chem. Soc. 2002, 79, 847–854. [Google Scholar]
- Adebowale, K.O.; Adedire, C.O. Chemical composition and insecticidal properties of the underutilized jatropha curcas seed oil. Afr. J. Biotechnol. 2006, 5, 901–906. [Google Scholar]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations a review of current status. Chem. Eng. Process. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Abramovic, H.; Abram, V. Physico-chemical properties, composition and oxidative stability of camelina sativa oil. Food. Technol. Biotechnol. 2005, 43, 63–70. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food. Sci. Food. Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Silva, L.P.; Miyasaka, C.K.; Martins, E.F.; Leite, J.R.S.A.; Lacava, Z.G.M.; Curi, R.; Azevedo, R.B. Effect of bullfrog (rana catesbeiana) oil administered by gavage on the fatty acid composition and oxidative stress of mouse liver. Braz. J. Med. Biol. Res. 2004, 37, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, R.; Morham, S.G.; Tiano, H.F.; Loftin, C.D.; Ghanayem, B.I.; Chulada, P.C.; Mahler, J.F.; Lee, C.A.; Goulding, E.H.; Kluckman, K.D.; et al. Prostaglandin-synthase-1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric-ulceration. Cell 1995, 83, 483–492. [Google Scholar] [CrossRef]
- Lopes, L.R.; Laurindo, F.R.M.; Mancini, J.; Curi, R.; Sannomiya, P. Nadph-oxidase activity and lipid peroxidation in neutrophils from rats fed fat-rich diets. Cell. Biochem. Funct. 1999, 17, 57–64. [Google Scholar] [CrossRef]
- O’Shea, M.; Bassaganya-Riera, J.; Mohede, I.C.M. Immunomodulatory properties of conjugated linoleic acid. Am. J. Clin. Nutr. 2004, 79, 1199s–1206s. [Google Scholar] [PubMed]
- Ziboh, V.A.; Miller, C.C.; Cho, Y.H. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: Generation of antiinflammatory and antiproliferative metabolites. Am. J. Clin. Nutr. 2000, 71, 361s–366s. [Google Scholar] [PubMed]
- Belhaj, N.; Dupuis, F.; Arab-Tehrany, E.; Denis, F.M.; Paris, C.; Lartaud, I.; Linder, M. Formulation, characterization and pharmacokinetic studies of coenzyme q(10) pufa’s nanoemulsions. Eur. J. Pharm. Sci. 2012, 47, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Dhanawat, M.; Dash, B.; Nagarwal, R.C.; Shrivastava, S.K. Codrug: An efficient approach for drug optimization. Eur. J. Pharm. Sci. 2010, 41, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Chandramohan, G.; Mariajancyrani, J.; Shanmugasundaram, P. Gc-ms analysis of phytochemical constituents in ethanolic bark extract of ficus religiosa linn. Int. J. Pharm. Pharm. Sci. 2014, 6, 457–460. [Google Scholar]
- Sarada, K.; Jothibai Margret, R.; Mohan, V.R. GC–MS determination of bioactive components of naringi crenulata (roxb) nicolson. Intern. J. ChemTech. Research. 2011, 3, 1548–1555. [Google Scholar]
- Kelley, D.S. Modulation of human immune and inflammatory responses by dietary fatty acids. Nutrition. 2001, 17, 669–673. [Google Scholar] [CrossRef]
- ICI Americas, I. The Hlb System: A Time-Saving Guide to Emulsifier Selection; ICI Americas, Incorporated: London, UK, 1984. [Google Scholar]
- Barel, A.O.; Paye, M.; Maibach, H.I. Handbook of Cosmetic Science and Technology, 4th ed.; Taylor & Francis: Boca Raton, FL, USA, 2014. [Google Scholar]
- Geusens, B.; Strobbe, T.; Bracke, S.; Dynoodt, P.; Sanders, N.; van Gele, M.; Lambert, J. Lipid-mediated gene delivery to the skin. Eur. J. Pharm. Sci. 2011, 43, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, I.; Norlen, L.; Bagatolli, L.A. Direct visualization of lipid domains in human skin stratum corneum’s lipid membranes: Effect of ph and temperature. Biophys. J. 2007, 93, 3142–3155. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, L.R.; Fernandes, F.R.; Costa, D.F.; Frézard, F.; Afonso, L.C.C.; Ferreira, L.A.M. Nanoemulsions loaded with amphotericin b: A new approach for the treatment of leishmaniasis. Eur. J. Pharm. Sci. 2015, 70, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.J.; Zhang, C.G.; Yao, Q.; Wang, Y.Q.; Tian, B.; Tang, X.; Wang, Y.J. Improving cabazitaxel chemical stability in parenteral lipid emulsions using cholesterol. Eur. J. Pharm. Sci. 2014, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Masmoudi, H.; le Dreu, Y.; Piccerelle, P.; Kister, J. The evaluation of cosmetic and pharmaceutical emulsions aging process using classical techniques and a new method: Ftir. Int. J. Pharm. 2005, 289, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Xavier, F.H.; Silva, K.G.H.; Farias, I.E.G.; Morais, A.R.V.; Alencar, E.N.; Araujo, I.B.; Oliveira, A.G.; Egito, E.S.T. Prospective study for the development of emulsion systems containing natural oil products. J. Drug. Deliv. Sci. Technol. 2012, 22, 367–372. [Google Scholar] [CrossRef]
- Latreille, B.; Paquin, P. Evaluation of emulsion stability by centrifugation with conductivity measurements. J. Food. Sci. 1990, 55, 1666–1672. [Google Scholar] [CrossRef]
- Mondal, N.; Samanta, A.; Pal, T.K.; Ghosal, S.K. Effect of different formulation variables on some particle characteristics of poly (dl-lactide-co-glycolide) nanoparticles. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2008, 128, 595–601. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Formariz, T.P.; Chiavacci, L.A.; Sarmento, V.H.V.; Santilli, C.V.; Egito, E.S.T.; Oliveira, A.G. The mixtures of components of a formulation at the moment of constructing a phase pseudoternary diagram, even if kept hlbc, not always will produce a dispersed and homogeneous system. Colloids Surf. B Biointerfaces. 2007, 60, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, G.P.; Scanlon, M.G. Characterization of alcohol-containing dairy emulsions: Pseudo-ternary phase diagrams of sodium caseinate solution-oil-ethanol systems. Food. Res. Int. 2013, 53, 49–55. [Google Scholar] [CrossRef]
- Moghimipour, E.; Salimi, A.; Leis, F. Preparation and evaluation of tretinoin microemulsion based on pseudo-ternary phase diagram. Adv. Pharm. Bull. 2012, 2, 141–147. [Google Scholar] [PubMed]
- Nanjwade, V.K.; Katare, O.P.; Manvi, F.V.; Nanjwade, B.K. Lipid-nanoemulsions as drug delivery carriers for poorly-water soluble drug. Int. J. Drug. Dev. Res. 2013, 5, 333–338. [Google Scholar]
- Patel, H.K.; Barot, B.S.; Parejiya, P.B.; Shelat, P.K.; Shukla, A. Topical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo: Ex vivo permeation and skin irritation studies. Colloids Surf. B Biointerfaces. 2013, 102, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, H.N.; Dalrymple, D.M.; Serajuddin, A.T.M. A comparative evaluation of mono-, di- and triglyceride of medium chain fatty acids by lipid/surfactant/water phase diagram, solubility determination and dispersion testing for application in pharmaceutical dosage form development. Pharm. Res. 2012, 29, 285–305. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter. 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Do Vale Morais, A.R.; do Nascimento Alencar, É.; Júnior, F.H.X.; de Oliveira, C.M.; Marcelino, H.R.; Barratt, G.; Fessi, H.; do Egito, E.S.T.; Elaissari, A. Freeze-drying of emulsified systems: A review. Int. J. Pharm. 2016, 503, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.V.; Popovich, N.G.; Ansel, H.C. Ansel’s Pharmaceutical Dosage Forms and Drug deliVery Systems; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Asghar, S.; Salmani, J.M.M.; Hassan, W.; Xie, Y.; Meng, F.F.; Su, Z.G.; Sun, M.J.; Xiao, Y.Y.; Ping, Q.N. A facile approach for crosslinker free nano self assembly of protein for anti-tumor drug delivery: Factors’ optimization, characterization and in vitro evaluation. Eur. J. Pharm. Sci. 2014, 63, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Lopez-Viota, M.; Clares, B.; Ruiz, M.A. Stability of fenbendazole suspensions for veterinary use correlation between zeta potential and sedimentation. Eur. J. Pharm. Sci. 2008, 34, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Roland, I.; Piel, G.; Delattre, L.; Evrard, B. Systematic characterization of oil-in-water emulsions for formulation design. Int. J. Pharm. 2003, 263, 85–94. [Google Scholar] [CrossRef]
- Aulton, M.E.; Taylor, K.M.G. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines; Elsevier Health Science: New York, NY, USA, 2013. [Google Scholar]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. American Pharmacist Association: Handbook of Pharmaceutical Excipients; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Machado, L.A.; Alencar, E.N.; Morais, A.R.V.; Xavier-Júnior, F.H.; Souza, A.M.S.; Cruz, A.K.M.; Genre, J.; Rocha, H.A.O.; Egito, E.S.T. Cytotoxicity evaluation of a topic emulsion based in the bullfrog (rana catesbeiana shaw) oil. In Proceedings of AAPS Biotechnology Conference, Orlando, FL, USA, 25 October 2015; p. 1.
- US Pharmacopeial Convention. USP35 NF30, 2012: US Pharmacopoeia National Formulary; United States Pharmacopeial: Rockville, MD, USA, 2011. [Google Scholar]
- Yu, W.; Egito, E.S.T.; Barratt, G.; Fessi, H.; Devissaguet, J.P.; Puisieux, F. A novel-approach to the preparation of injectable emulsions by a spontaneous emulsification process. Int. J. Pharm. 1993, 89, 139–146. [Google Scholar] [CrossRef]
- Gosenca, M.; Bester-Rogac, M.; Gasperlin, M. Lecithin based lamellar liquid crystals as a physiologically acceptable dermal delivery system for ascorbyl palmitate. Eur. J. Pharm. Sci. 2013, 50, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.P.; Nagarsenker, M.S. Self-nanoemulsifying granules of ezetimibe: Design, optimization and evaluation. Eur. J. Pharm. Sci. 2008, 35, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Woodman, T.J.; Mansour, S.; Mortada, N.D.; Geneidi, A.S.; Guy, R.H. Microemulsion formulations for the transdermal delivery of testosterone. Eur. J. Pharma. Sci. 2010, 40, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sample Availability: Not available.

| Substance | Retention Time (min) | Concentration (%) |
|---|---|---|
| Myristic acid | 10.3 | 1.4 |
| Arachidonic acid | 12.0 | 8.4 |
| Palmitic acid | 12.2 | 10.3 |
| EPA, Timnodonic acid | 13.7 | 17.6 |
| Oleic acid | 13.7 | 29.9 |
| Stearic acid | 14 | 2.5 |
| DHA, Cervonic acid | 16.5 | 0.8 |
| Cholesterol | 20.6 | 9.5 |
| Ethyl iso-allocholate | 27.7 | 3.5 |
| Total | 83.9 | |
| Not identified | 16.1 |
| HLB | Droplet Size (nm) ± SD | Polydispersity | Micro-Emultocrit (%) ± SD | pH ± SD | Conductivity (S/cm) |
|---|---|---|---|---|---|
| 4.5 | PS | PS | PS | PS | PS |
| 5.5 | PS | PS | PS | PS | PS |
| 6.5 | PS | PS | PS | PS | PS |
| 7.5 | PS | PS | PS | PS | PS |
| 8.5 | 266.7 ± 14.2 | 0.235 | 4.1 ± 1.3 | 4.2 ± 0.6 | 94.4 |
| 9.5 | 257.7 ± 13.7 | 0.261 | 3.6 ± 1.2 | 3.9 ± 0.5 | 121.2 |
| 10.5 | 295.9 ± 24.8 | 0.268 | 4.3 ± 1.5 | 4.9 ± 1.0 | 107.7 |
| 11.5 | PS | PS | PS | PS | PS |
| 12.5 | 259.8 ± 8.7 | 0.218 | 3.0 ± 0.6 | 4.2 ± 0.6 | 100.3 |
| 13.5 | 275.6 ± 11.9 | 0.190 | 2.8 ± 0.7 | 3.8 ± 0.6 | 175.7 |
| 14.5 | 324.3 ± 26.0 | 0.230 | 3.6 ± 0.8 | 4.0 ± 0.4 | 101.4 |
| 15.5 | PS | PS | PS | PS | PS |
| HLB | Droplet Size (nm) ± SD | Polydispersity | Micro-Emultocrit (%) ± SD | pH ± SD | Conductivity (S/cm) |
|---|---|---|---|---|---|
| 12.1 | 212.0 ± 13.6 | 0.213 | 2.4 ± 0.5 | 5.2 ± 1.4 | 91.2 |
| 12.2 | 202.7 ± 21.6 | 0.22 | 2.4 ± 0.5 | 5.1 ± 1.5 | 100.8 |
| 12.5 | 202.9 ± 29.8 | 0.215 | 2.2 ± 0.7 | 5.4 ± 1.2 | 87.3 |
| 12.7 | 199.6 ± 16.6 | 0.232 | 2.7 ± 0.4 | 5.1 ± 1.5 | 108.7 |
| 12.9 | 209.3 ± 18.4 | 0.234 | 2.1 ± 0.3 | 5.3 ± 1.4 | 97.4 |
| 13.0 | 196.4 ± 17.3 | 0.215 | 2.0 ± 0.0 | 5.2 ± 1.4 | 90.5 |
| 13.3 | 201.9 ± 15.0 | 0.215 | 2.5 ± 0.5 | 5.0 ± 1.5 | 100.1 |
| 13.5 | 208.1 ± 19.9 | 0.245 | 2.4 ± 0.5 | 5.1 ± 1.4 | 120.5 |
| 13.7 | 194.4 ± 11.7 | 0.225 | 2.4 ± 0.5 | 5.0 ± 1.5 | 72.5 |
| 13.9 | 193.7 ± 18.5 | 0.248 | 2.5 ± 0.5 | 5.0 ± 1.5 | 112.0 |
| 14.0 | 188.4 ± 22.2 | 0.261 | 2.7 ± 0.5 | 5.0 ± 1.5 | 120.4 |
| Excipients | % (w/w) | Function | |
|---|---|---|---|
| Aqueous phase | Tween® 20 | 5.03 | Surfactant |
| Propylene glycol | 4.00 | Humectant | |
| Germall | 0.30 | Antimicrobial preservative | |
| Xanthan gum | 1.00 | Stabilizing agent | |
| Distillated Water | 62.55 | Dispenser agent | |
| Oily phase | Span® 80 | 2.97 | Surfactant |
| Butylhydroxytoluene | 0.10 | Antioxidant | |
| Cetostearyl alcohol ethoxylate | 8.00 | Viscosity-increasing agent | |
| Isopropyl palmitate | 4.00 | Emollient and skin penetrant | |
| Bullfrog oil | 12.00 | Oil | |
| After preparation | Fragrance | 0.05 | Essence |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral-Machado, L.; Xavier-Júnior, F.H.; Rutckeviski, R.; Morais, A.R.V.; Alencar, É.N.; Dantas, T.R.F.; Cruz, A.K.M.; Genre, J.; Da Silva-Junior, A.A.; Pedrosa, M.F.F.; et al. New Trends on Antineoplastic Therapy Research: Bullfrog (Rana catesbeiana Shaw) Oil Nanostructured Systems. Molecules 2016, 21, 585. https://doi.org/10.3390/molecules21050585
Amaral-Machado L, Xavier-Júnior FH, Rutckeviski R, Morais ARV, Alencar ÉN, Dantas TRF, Cruz AKM, Genre J, Da Silva-Junior AA, Pedrosa MFF, et al. New Trends on Antineoplastic Therapy Research: Bullfrog (Rana catesbeiana Shaw) Oil Nanostructured Systems. Molecules. 2016; 21(5):585. https://doi.org/10.3390/molecules21050585
Chicago/Turabian StyleAmaral-Machado, Lucas, Francisco H. Xavier-Júnior, Renata Rutckeviski, Andreza R. V. Morais, Éverton N. Alencar, Teresa R. F. Dantas, Ana K. M. Cruz, Julieta Genre, Arnóbio A. Da Silva-Junior, Matheus F. F. Pedrosa, and et al. 2016. "New Trends on Antineoplastic Therapy Research: Bullfrog (Rana catesbeiana Shaw) Oil Nanostructured Systems" Molecules 21, no. 5: 585. https://doi.org/10.3390/molecules21050585