1. Introduction
The current development in energy use is oriented towards reducing carbon consumption due to its environmental pollution. Hydrogen as a clean and sustainable energy carrier has gained more attention during the past decades. When hydrogen reacts with oxygen in fuel cells and internal combustion engines, a large amount of energy is released explosively in heat engines and quietly in fuel, releasing water as the product.
The present source of hydrogen comes mainly from synthesis gas, which is a mixture of H2, CO and CO2, and it is produced by breaking the strong C-H bonds (439 kJ/mol) of hydrocarbons in reforming reactions. Afterwards, hydrogen is purified and separated by different energy intensive steps. A membrane reactor (MR) technology can represent an energetically efficient option to the conventional processes, with the practical advantages of a smaller footprint and capital cost reduction. In this alternative reformer, the hydrogen is produced and continuously removed from the reaction side for permeation through a hydrogen perm-selective membrane, shifting the reaction towards further product formation. As a consequence, the conversion increases and pure hydrogen is produced at the same time.
In the specialized literature, porous carbon, silica and zeolite membranes have been used for hydrogen separation [
1,
2,
3]. Nevertheless, their separation characteristics are still not acceptable although their cost is relatively low. Dense membranes made of palladium (Pd) are able to separate hydrogen from a gas mixture by a solution-diffusion mechanism with a theoretically infinite hydrogen perm-selectivity [
4]. As it is well known, the cost of Pd-membranes is relatively high, but it can be reduced by depositing a thin Pd layer on a metallic or ceramic support [
5].
Despite of the great permeation characteristics of Pd, its application is restricted by some factors. Hydrogen embrittlement of Pd-membranes occurs when the operating conditions are below 300 °C and 2.0 MPa. In these conditions, hydrogen permeation through the membrane allows for the change of Pd lattice from α to β phase, and
vice versa. Several cycles from α to β phase can cause the formation of cracks in the membrane lattice [
6].
Furthermore, dense Pd-membranes are affected by surface contamination. Different studies have demonstrated the effects of different contaminants such as CO [
7,
8,
9,
10], H
2O [
11,
12,
13], Cl [
14], NH
3 [
15], certain hydrocarbons and sulfur compounds like H
2S [
16] on the membranes. In particular, by exposing H
2S to the Pd surface, sulfur is reversibly adsorbed on the palladium surface blocking the adsorption sites for hydrogen with a reduction in the hydrogen permeation. Sulfur can also react with the Pd surface producing Pd
4S which acts as a barrier to hydrogen permeation decreasing the permeance [
17]. The lattice constants of Pd and Pd
4S are highly different, so this can cause cracks in the membrane [
18].
In order to overcome these drawbacks, some studies proposed to change the crystal structure into nanostructure [
19]. However, most of the researches proposed an enhancement of the membranes stability by alloying them with other elements [
20,
21,
22]. Most efforts in this area are dedicated to Pd-Cu alloys because of their high resistance to sulfur poisoning [
23,
24,
25]. Lately, the Pd-Au membranes have been studied due to their higher hydrogen permeability with respect to Pd-Cu. In the pioneeristic study realized by McKinely
et al. [
26,
27], it was shown that the addition of Au content in the range 0wt% –20wt% to palladium membranes enhances the tolerance to sulfur compounds, minimizing the embrittlement phenomenon and improving the hydrogen permeability more than pure Pd. In the experiments done by Way
et al. [
28], the Pd-15%wt Au has exhibited a higher hydrogen permeating flux in the presence of H
2S than a Pd-6%wt Cu in the absence of H
2S. In particular, by using Pd-15%wt Au the hydrogen flux decreased 38% after the exposure to 5 ppm H
2S at 400 °C, while, with Pd-6%wt Cu, the hydrogen flux was 71% lower than that measured with Pd-Au in H
2S presence. Chen and Ma [
29] examined a Pd-8%wt Au on a porous metal support. In the temperature range between 350 and 500 °C, no sulfide was detected in the membrane even at 54.8 ppm H
2S exposure.
Some studies have compared hydrogen permeation in Pd-Au alloys with pure Pd. Sonwane
et al. [
30] predicted that the hydrogen permeability in Pd-Au membrane increased by increasing the Au content, having a maximum value at 12wt% Au and showing a hydrogen permeability 1.8 times greater than pure Pd at 180 °C. In other investigations, Gryaznov [
31] found that the hydrogen permeability of Pd-10%wt Au at 500 °C is 2.2 times higher than that of pure Pd. Ma
et al. [
32] investigated the performance of several Pd-Au membranes with different Au content at (4.2wt%–16.7wt%) from 250 to 450 °C, confirming that the membranes with 4.2wt% and 5.4wt% Au show higher hydrogen permeability than pure Pd.
However, the stability of Pd-Au membranes under long-term operation has been tested only in a few studies, including the experimental efforts of Guazzone
et al. and Mardilovich
et al., who analyzed the long-term permeation test results of Pd and Pd-Au composite membranes under desulfurized coal-derived syngas at pilot scale [
33,
34,
35].
The objective of the present work is to investigate the long-term stability characteristics of hydrogen permeation of a composite membrane constituted of a Pd-Au (Au 7wt%) dense layer supported on a porous stainless steel (PSS) support, meanwhile evaluating the H2/N2, H2/CO2, H2/CH4 and H2/He ideal selectivities.
Furthermore, at stable value of hydrogen permeance, the composite Pd-Au membrane was allocated in a MR module for carrying out methane steam reforming (MSR) reaction for producing hydrogen and to analyze the effect of coke formation during the course of experiments.
3. Materials and Methods
3.1. Membrane Preparation
Different techniques can be adopted for depositing palladium or its alloys onto porous substrates such as magnetron sputtering [
38], spray pyrolysis [
39], chemical vapor deposition (CVD) [
40], physical vapor deposition [
39,
41] and electroless plating deposition (ELP) [
42,
43]. In this study, a dense Pd-Au layer (~7 μm thick) was deposited onto a PSS tubular support via the ELP method.
The support was supplied by Pall AccuSep (New York, NY, USA) having 1.0 cm O.D. (AISI 316L porous tube) with an active length around 4 cm. The porous support was welded to two stainless steel tubes, and one of them is closed for facilitating the membrane housing in the MR module. Then, the total length of the membrane tube was 20 cm. The support was oxidized for 12 h at 500 °C. It was then graded with preactivated Al2O3 particles and cemented with Pd in order to decrease the surface pore size and narrow the surface pore size distribution and create the intermediate layer for avoiding intermetallic diffusion.
The surface activation was then carried out using the standard SnCl
2-PdCl
2 activation procedure and a thin layer of pure Pd was deposited by electroless plating technique. Successively, the Au deposition was performed by the method described in detail in Chen and Ma [
29]. After each step of membrane preparation, the He permeance at room temperature was also measured (
Figure 8a,b).
Hence, the Pd-Au/PSS membrane was allocated in the module and fixed by means of graphite gaskets and 3.0 g of Ni/Al2O3 commercial catalyst was packed in the annulus of the MR. To prevent the movement of catalyst particles, glass spheres were placed at each side of the catalytic bed.
The MR was flowed with pure N2 at ambient temperature to check the presence of leakages. A thermocouple was used to measure the temperature of the MR module, which was heated up by using two electrical tapes up to achieving the required temperatures (300–420 °C). The retentate pressure was adjusted by utilizing a back-pressure regulator placed at the outlet side of this stream, while the permeate pressure was always kept at 100 kPa.
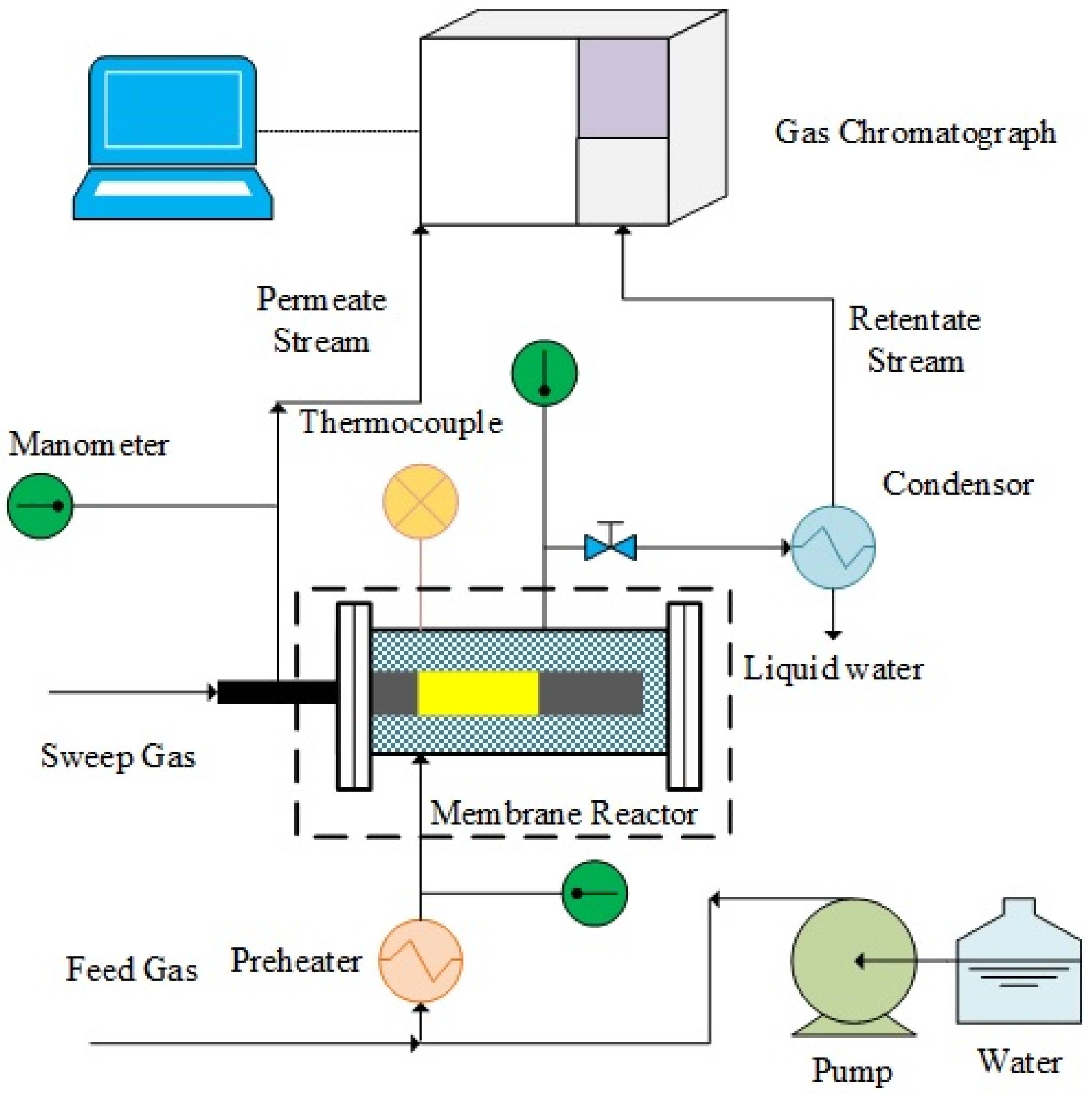
A scheme of the experimental setup used in this work is illustrated in
Figure 9. In the case of permeation tests, pure gases (H
2, N
2, He, CH
4 or CO
2) were flowed into the MR module and the permeating flow rate was measured by means of bubble flow meters.
During the reaction tests of MSR, methane was mixed with deionized water (steam/methane = 3.5/1), which was pumped by a P680 HPLC pump (Dreieich, Germany), in a preheated chamber and, then, injected to the reaction zone. N2 was also used as an internal standard gas and as a sweep gas with a flow rate of ~25 mL/min, flowed in counter-current mode with respect to the feed. To remove the unreacted water from the retentate and, eventually, from the permeate streams, they were passed through a condenser. Then, the retentate and permeate dry streams were analyzed by means of a temperature programmed HP 6890 gas chromatograph (GC) (Foster City, CA, USA). Each experimental point reported in this work was constituted of, at least, ten reaction cycles at the same operating conditions in order to ensure the reproducibility of the results.
3.2. Permeation Tests
Pure gases such as H2, N2, He, CH4 or CO2 were flowed into the membrane reactor module before reaction tests for studying the permeation characteristics of the supported Pd-Au membrane.
The pure gases were provided by cylinders.
To analyze the hydrogen permeation characteristics of the Pd-Au/PSS membrane, Equation (3) was used as:
where
JH2 is the hydrogen flux permeating through the membrane,
PH2 the hydrogen permeance,
pH2,reaction and
pH2,permeate the hydrogen partial pressures in the reaction and permeate sides, respectively.
n is variable in the range 0.5–1 depending on the rate limiting step of hydrogen diffusion. More details about the deviation of Sieverts–Fick law (
n = 0.5) were explained elsewhere [
44].
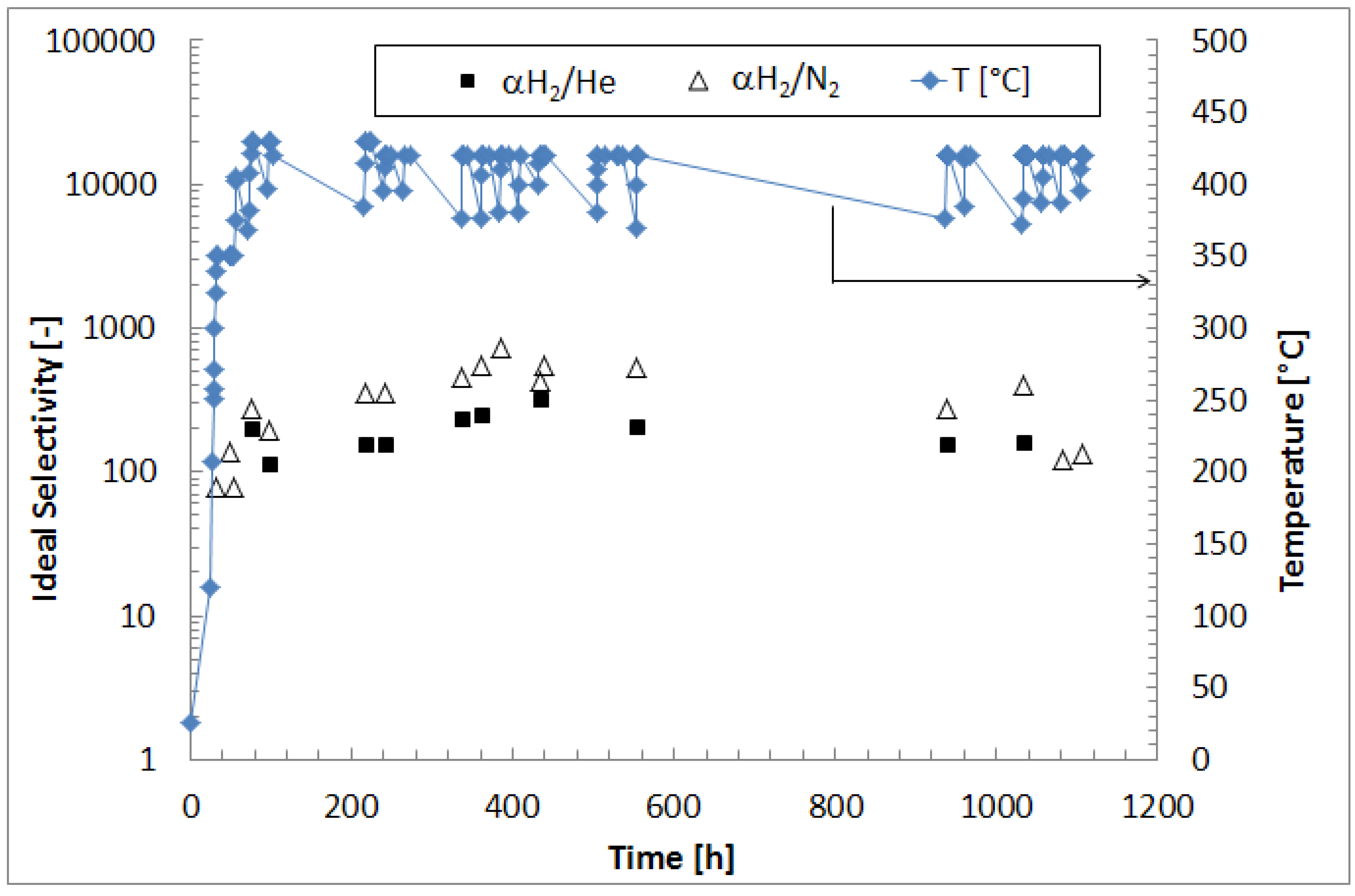
The hydrogen over other pure gas ideal selectivity (α
H2/i) was expressed as the ratio of the H
2 permeating flux over the permeating flux of another pure gas of interest at the same transmembrane pressure, as reported in Equation (4):
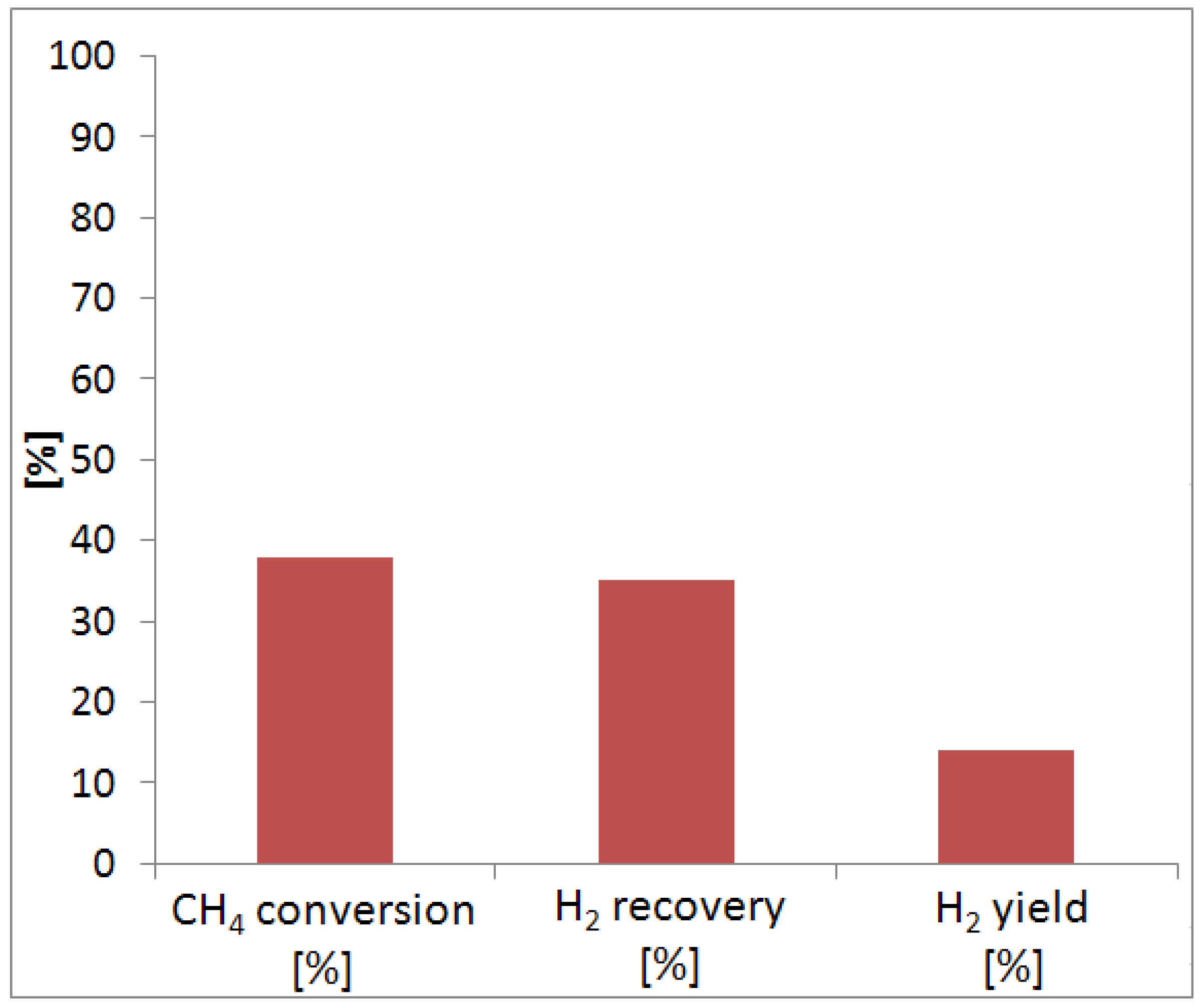
Methane conversion was described by using Equation (5) during MSR reaction:
where CO
out and CO
2out represent the outlet molar flow rates of CO and CO
2, while CH
4,in represents the molar flow rate of methane in the reaction side.
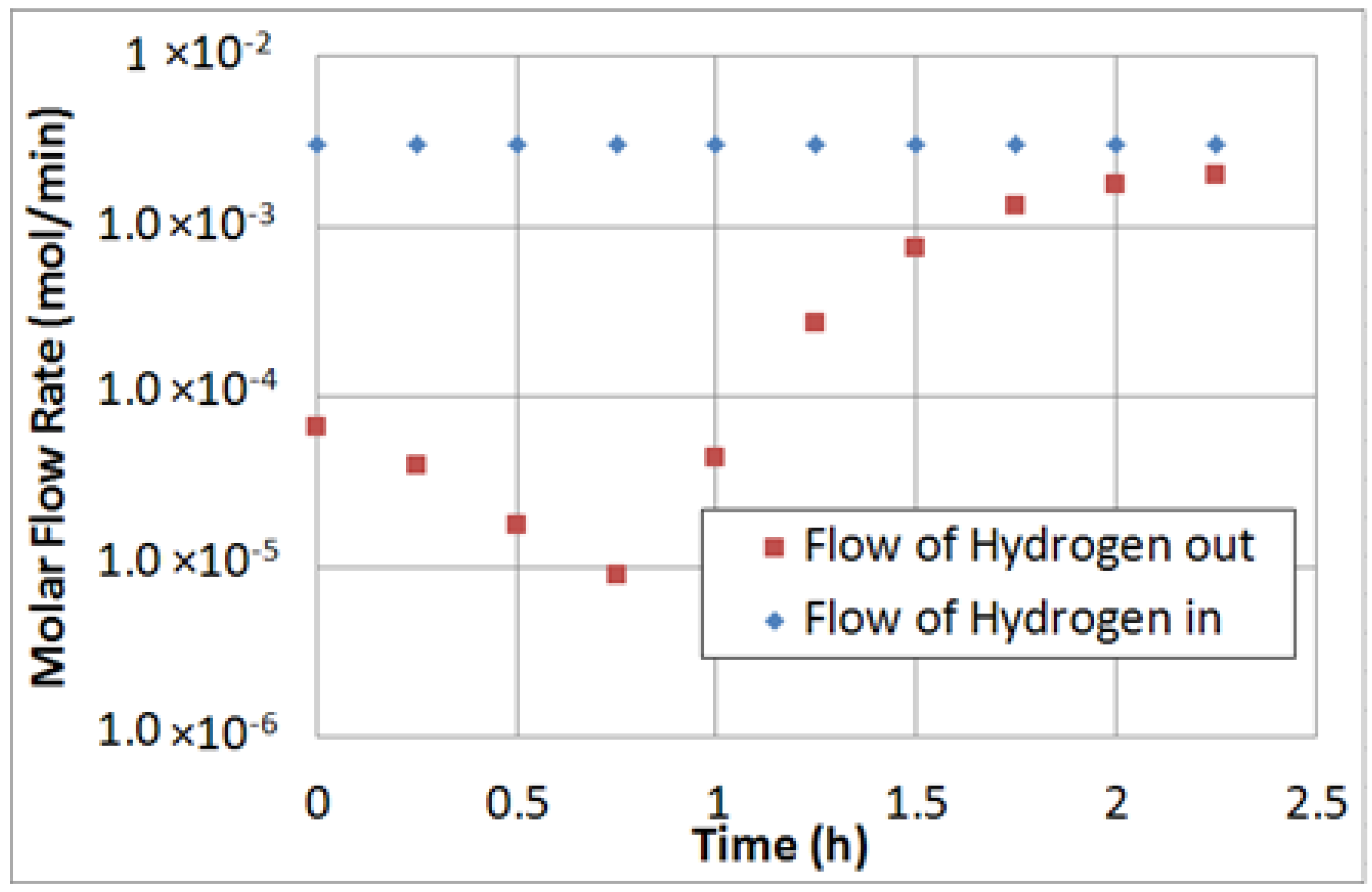
Hydrogen recovery was defined by Equation (6):
where H
2-perm and H
2-ret are the molar flow rates of hydrogen in the permeate and retentate streams, respectively.
4. Conclusions
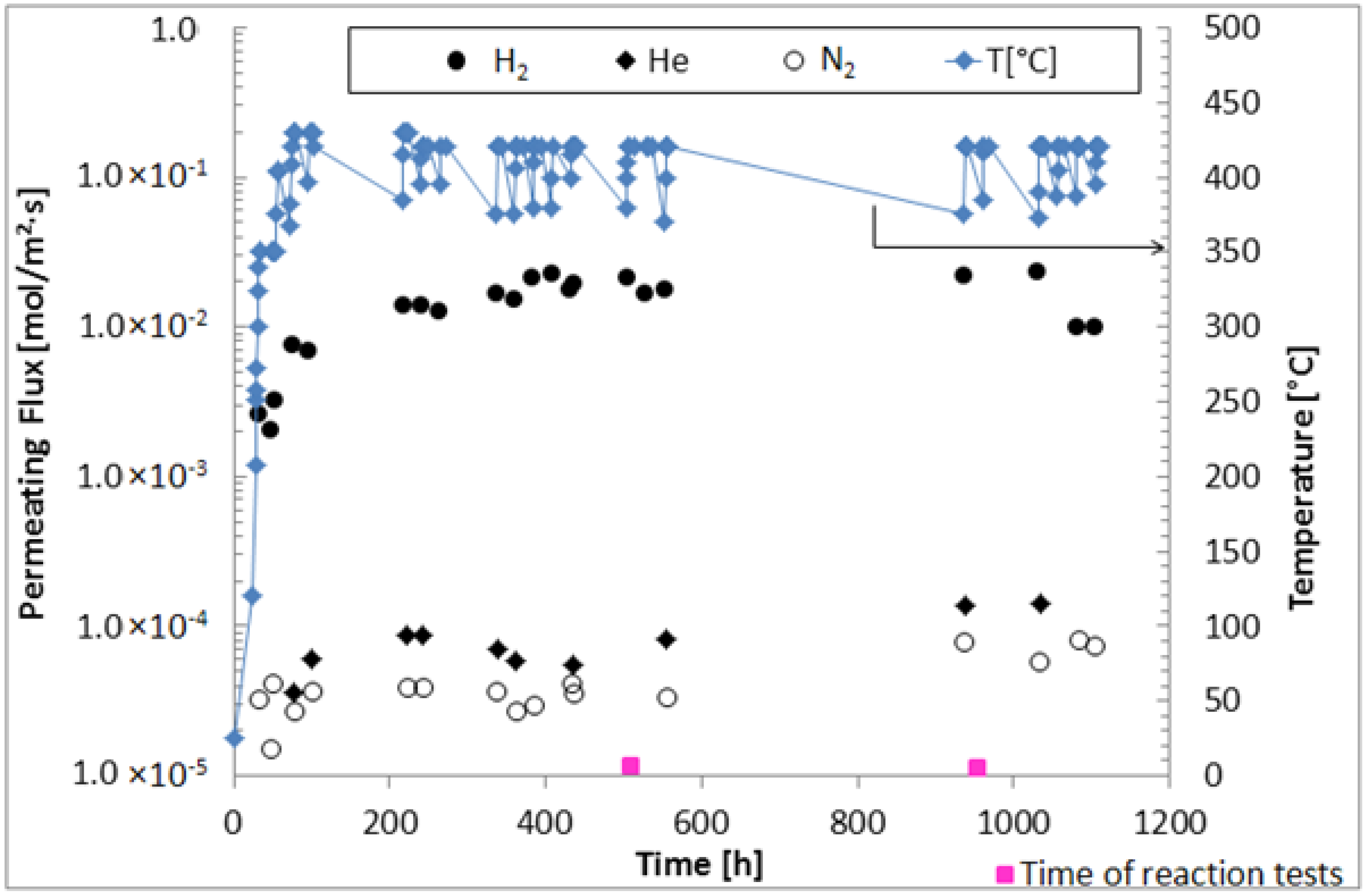
We investigated the performance of a supported Pd-Au membrane in terms of hydrogen permeance and ideal selectivity in pure gas permeation tests under long time continuous operation. The hydrogen permeance increased till achieving steady state conditions after around 400 h. Hence, at 50 kPa of transmembrane pressure and 420 °C, the composite membrane reached H2/N2 ideal selectivity of around 500 with an hydrogen permeance of 2.3 × 10−2 mol/m2·s, remaining stable up to 1100 h under operation.
During MSR reaction tests, 40% of methane conversion and 35% of hydrogen recovery were reached in the MR. However, coke was formed during the reaction tests, and it was probably responsible of the irreversible loss of hydrogen permeation characteristics of the membrane. The MR was cooled at ambient temperature, and we observed that the color of the membrane surface moved from gold to gray. In the future, we will better investigate this effect, making more stable the alloy during the reaction tests, meanwhile, improving the hydrogen permeation selectivity.