Ultrasound-Assisted Enantioselective Esterification of Ibuprofen Catalyzed by a Flower-Like Nanobioreactor
Abstract
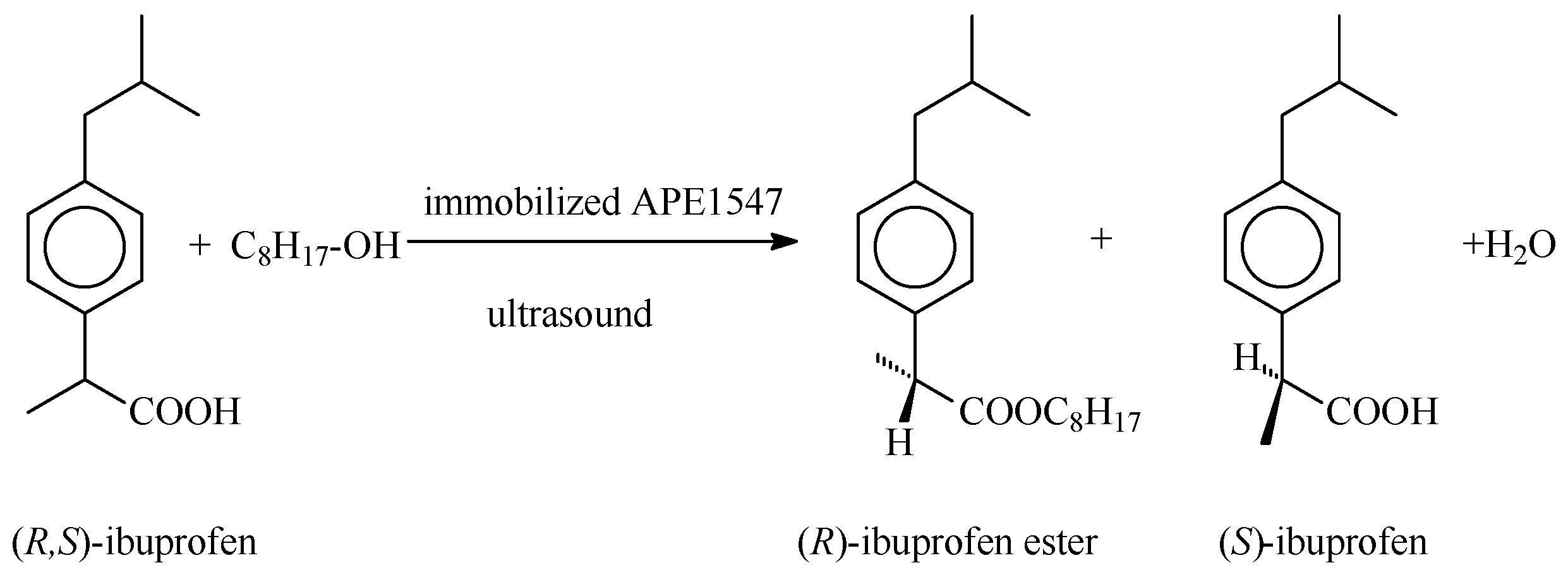
:1. Introduction
2. Results and Discussion
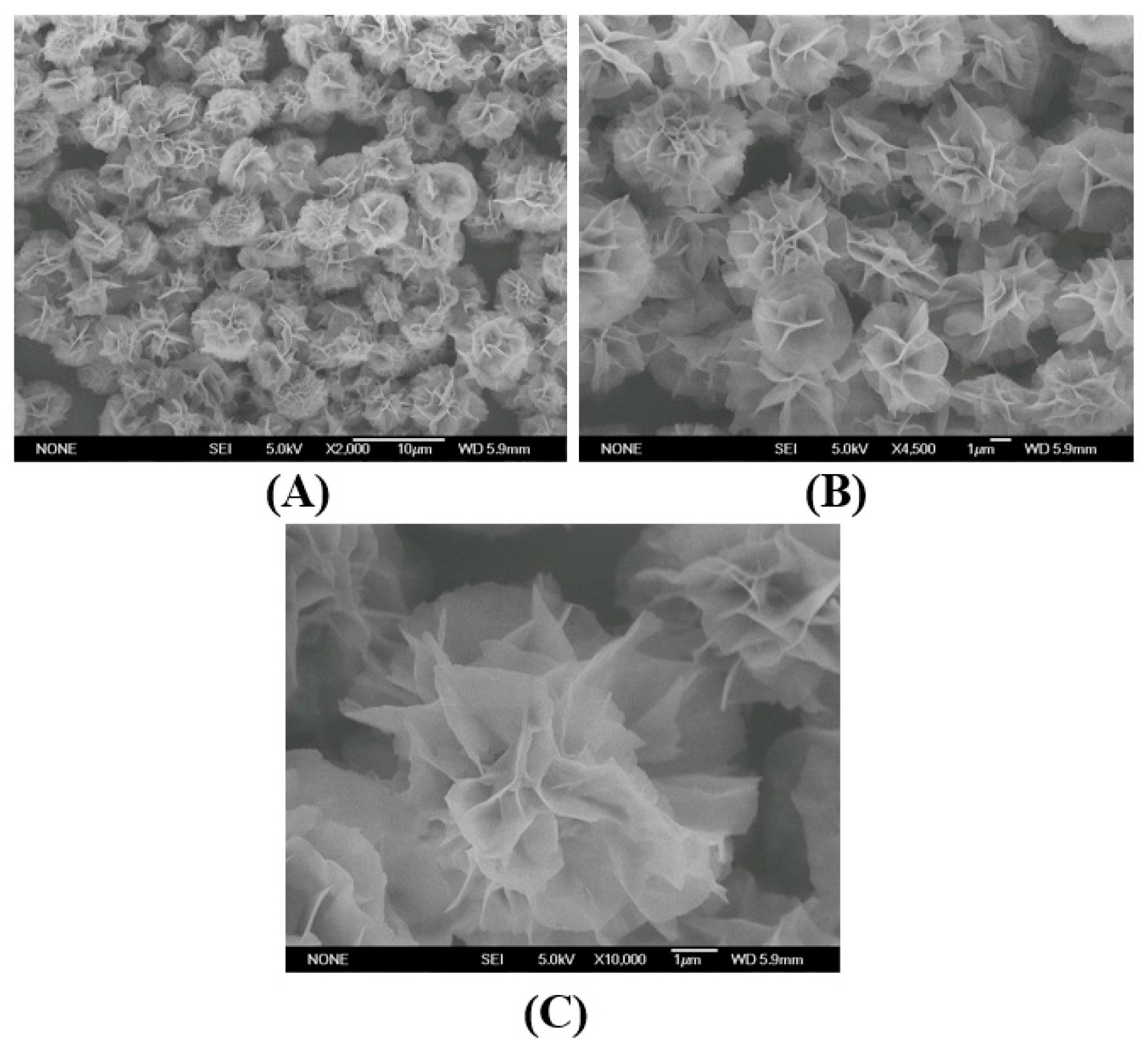
2.1. The Morphologies of the Immobilized Samples
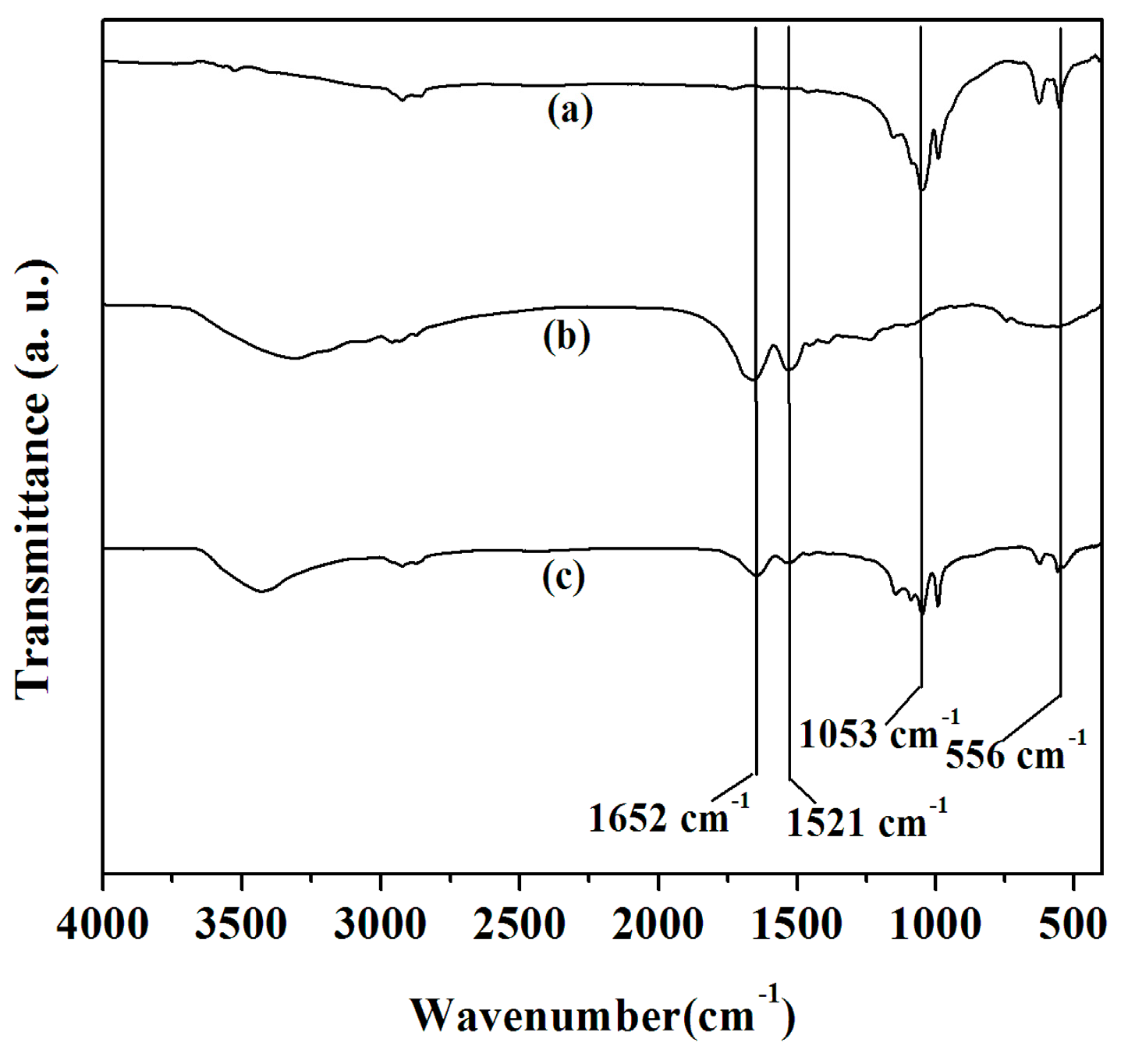
2.2. FTIR
2.3. Effect of Ultrasound Power
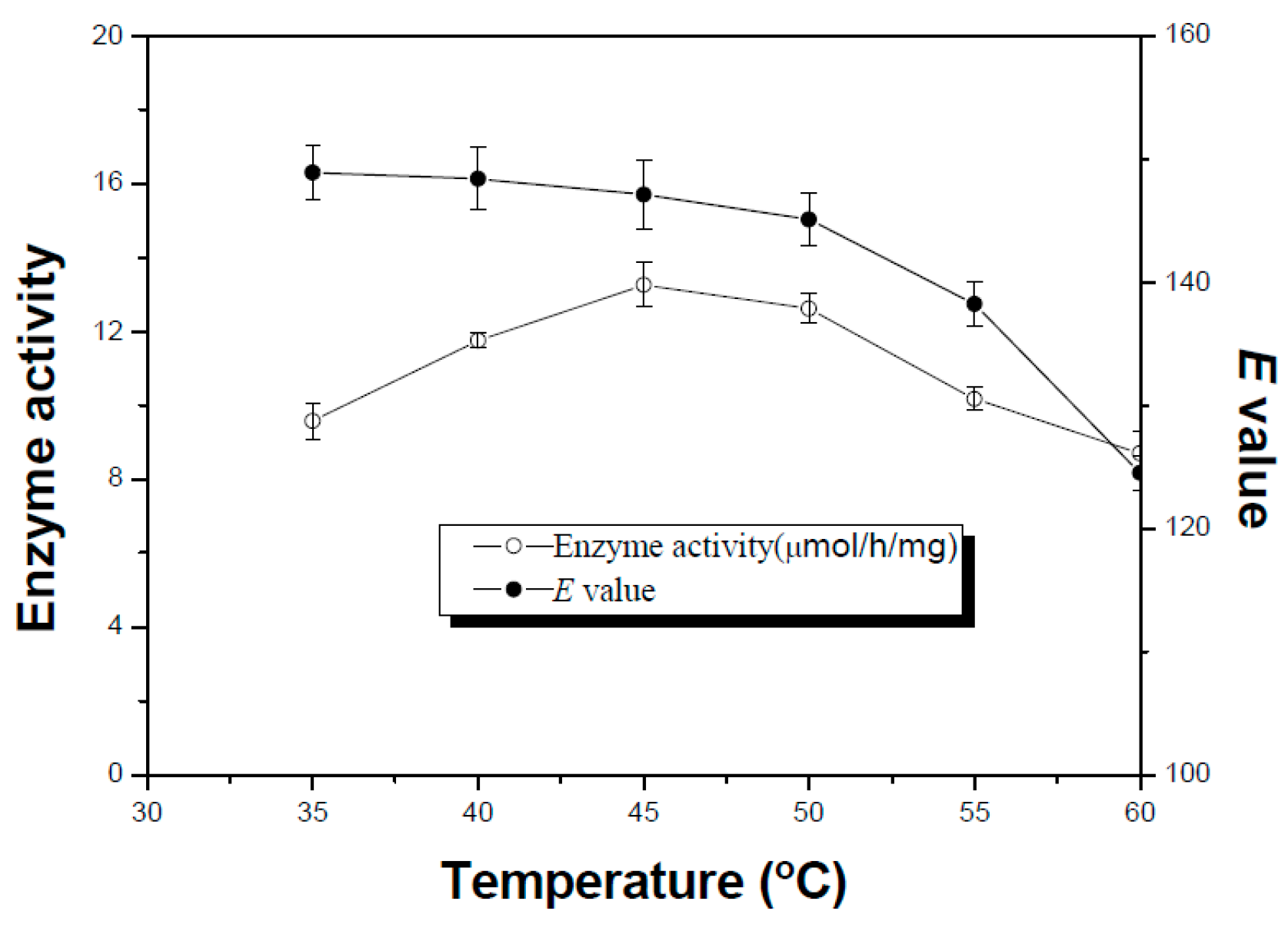
2.4. Effect of Temperature
2.5. Effect of Water Activity
2.6. Reusability and Stability
3. Experimental
3.1. Materials
3.2. Ultrasound Equipment
3.3. Preparation of the Nanobioreactor
3.4. Control and Measurement of Water Activity
3.5. Enzyme-Catalyzed Esterification of Ibuprofen
3.6. Characterization of the Prepared Nanobioreactor
3.7. Recycling the Enzyme
3.8. High Performance Liquid Chromatography (HPLC) Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adams, S.; Bresloff, P.; Mason, C. Pharmacological differences between the optical isomers of ibuprofen: Evidence for metabolic inversion of the (−)-isomer. J. Pharm. Pharmacol. 1976, 28, 256–257. [Google Scholar] [CrossRef] [PubMed]
- Pollard, D.J.; Woodley, J.M. Biocatalysis for pharmaceutical intermediates: The future is now. Trends Biotechnol. 2007, 25, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Dordick, J.; Hauer, B.; Kiener, A.; Wubbolts, M.; Witholt, B. Industrial biocatalysis today and tomorrow. Nature 2001, 409, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.N. Biocatalytic synthesis of intermediates for the synthesis of chiral drug substances. Curr. Opin. Biotechnol. 2001, 12, 587–604. [Google Scholar] [CrossRef]
- Ghanem, A.; Aboul-Enein, H.Y. Application of lipases in kinetic resolution of racemates. Chirality 2005, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-T.; Xun, E.-N.; Wang, J.-X.; Wang, R.; Wei, X.-F.; Wang, L.; Wang, Z. Enantioselective esterification of ibuprofen by a novel thermophilic biocatalyst: APE1547. Biotechnol. Bioprocess Eng. 2011, 16, 638–644. [Google Scholar] [CrossRef]
- Zhao, D.; Yue, H.; Chen, G.; Jiang, L.; Zhang, H.; Wang, Z.; Liu, G. Enzymatic resolution of ibuprofen in an organic solvent under ultrasound irradiation. Biotechnol. Appl. Biochem. 2014, 61, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lorente, G.; Palomo, J.M.; Fuentes, M.; Mateo, C.; Guisán, J.M.; Fernández-Lafuente, R. Self-assembly of Pseudomonas fluorescens lipase into bimolecular aggregates dramatically affects functional properties. Biotechnol. Bioeng. 2003, 82, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.; Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett 2009, 31, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Du, C.; Zhao, B.; Li, C.; Wang, P.; Wang, Z.; Tang, J.; Wang, L. Improvement of the enantioselectivity and activity of lipase from Pseudomonas sp. via adsorption on a hydrophobic support: Kinetic resolution of 2-octanol. Biocatal. Biotransform. 2009, 27, 340–347. [Google Scholar] [CrossRef]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzym. Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Bansode, S.R.; Rathod, V.K. Ultrasound assisted lipase catalysed synthesis of isoamyl butyrate. Process. Biochem. 2014, 49, 1297–1303. [Google Scholar] [CrossRef]
- Miyahara, M.; Vinu, A.; Ariga, K. Immobilization of Lysozyme onto pore-engineered mesoporous AlSBA-15. J. Nanosci. Nanotechnol. 2006, 6, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Jung, D. Biocatalysis with enzymes immobilized on mesoporous hosts: The status quo and future trends. J. Mater. Chem. 2010, 20, 844–857. [Google Scholar] [CrossRef]
- Wu, Z.; Li, X.; Li, F.; Yue, H.; He, C.; Xie, F.; Wang, Z. Enantioselective transesterification of (R,S)-2-pentanol catalyzed by a new flower-like nanobioreactor. RSC Adv. 2014, 4, 33998–34002. [Google Scholar] [CrossRef]
- Cho, I.S.; Kim, D.W.; Lee, S.; Kwak, C.H.; Bae, S.T.; Noh, J.H.; Yoon, S.H.; Jung, H.S.; Kim, D.W.; Hong, K.S. Synthesis of Cu2PO4OH hierarchical superstructures with photocatalytic activity in visible light. Adv. Funct. Mater. 2008, 18, 2154–2162. [Google Scholar] [CrossRef]
- Yang, W.-J.; Griffiths, P.R.; Byler, D.M.; Susi, H. Protein conformation by infrared spectroscopy: Resolution enhancement by Fourier self-deconvolution. Appl. Spectrosc. 1985, 39, 282–287. [Google Scholar] [CrossRef]
- Li, F.; Zhao, D.; Chen, G.; Zhang, H.; Yue, H.; Wang, L.; Wang, Z. Enantioselective transesterification of N-hydroxymethyl vince lactam catalyzed by lipase under ultrasound irradiation. Biocatal. Biotransform. 2013, 31, 299–304. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Wang, C.; Yang, F.; Zhang, H.; Yue, H.; Wang, L. Lipase catalyzed synthesis of 3,3[prime or minute]-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione). RSC Adv. 2014, 4, 35686–35689. [Google Scholar] [CrossRef]
- Phillips, R.S. Temperature modulation of the stereochemistry of enzymatic catalysis: Prospects for exploitation. Trends Biotechnol. 1996, 14, 13–16. [Google Scholar] [CrossRef]
- Ducret, A.; Trani, M.; Lortie, R. Lipase-catalyzed enantioselective esterification of ibuprofen in organic solvents under controlled water activity. Enzym. Microb. Technol. 1998, 22, 212–216. [Google Scholar] [CrossRef]
- Gao, R.; Feng, Y.; Ishikawa, K.; Ishida, H.; Ando, S.; Kosugi, Y.; Cao, S. Cloning, purification and properties of a hyperthermophilic esterase from archaeon Aeropyrum pernix K1. J. Mol. Catal. B Enzym. 2003, 24, 1–8. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds ((S)-ibuprofen, 99% ee) are available from the authors.


| Reaction Cycle | Relative Activity (%) | E value |
|---|---|---|
| 1 | 100 | 147.1 |
| 2 | 99.6 | 150.9 |
| 3 | 99.5 | 148.4 |
| 4 | 98.7 | 146.3 |
| 5 | 99.2 | 151.4 |
| 6 | 99.6 | 147.2 |
| 7 | 98.8 | 148.5 |
| 8 | 98.7 | 151.3 |
| 9 | 99.1 | 146.5 |
| 10 | 99.5 | 147.7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, B.; Fan, H.; Wu, Z.; Zheng, L.; Wang, L.; Wang, Z.; Chen, G. Ultrasound-Assisted Enantioselective Esterification of Ibuprofen Catalyzed by a Flower-Like Nanobioreactor. Molecules 2016, 21, 565. https://doi.org/10.3390/molecules21050565
An B, Fan H, Wu Z, Zheng L, Wang L, Wang Z, Chen G. Ultrasound-Assisted Enantioselective Esterification of Ibuprofen Catalyzed by a Flower-Like Nanobioreactor. Molecules. 2016; 21(5):565. https://doi.org/10.3390/molecules21050565
Chicago/Turabian StyleAn, Baiyi, Hailin Fan, Zhuofu Wu, Lu Zheng, Lei Wang, Zhi Wang, and Guang Chen. 2016. "Ultrasound-Assisted Enantioselective Esterification of Ibuprofen Catalyzed by a Flower-Like Nanobioreactor" Molecules 21, no. 5: 565. https://doi.org/10.3390/molecules21050565





