New Inducible Nitric Oxide Synthase and Cyclooxygenase-2 Inhibitors, Nalidixic Acid Linked to Isatin Schiff Bases via Certain l-Amino Acid Bridges
Abstract
:1. Introduction
2. Results
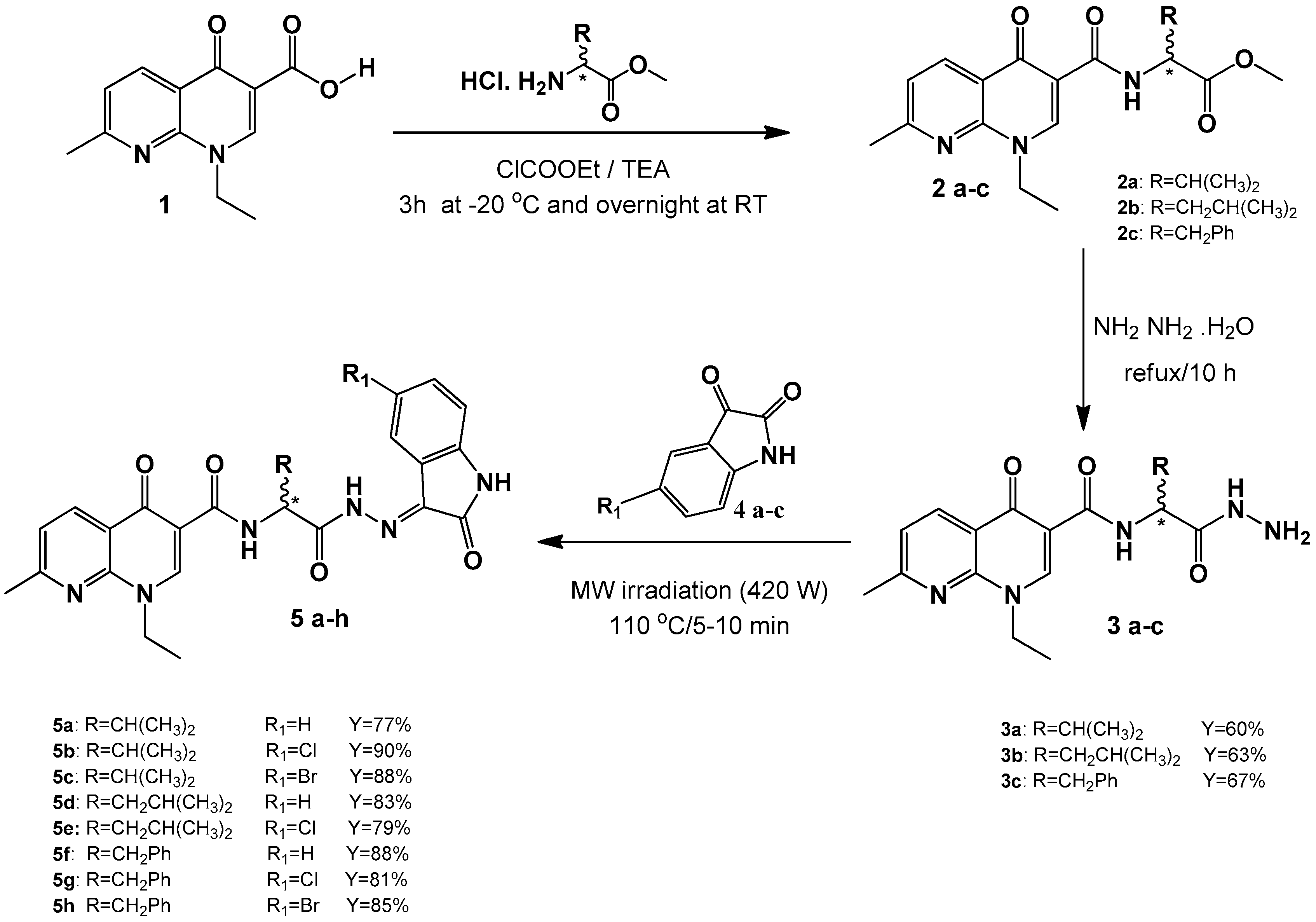
2.1. Chemistry
2.2. The Anti-Inflammatory Activity
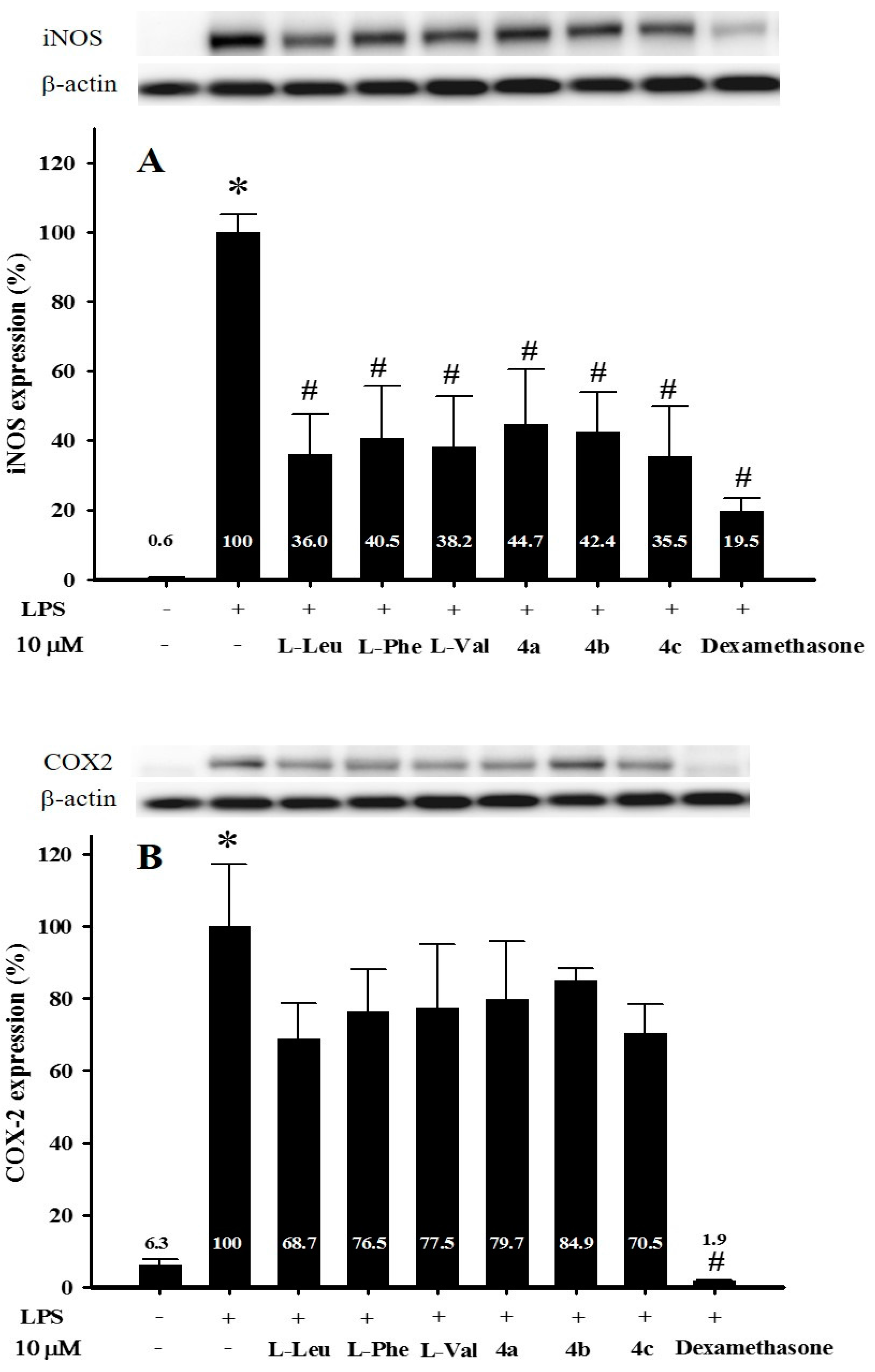
2.2.1. The Effect of Amino Acids and Isatins on iNOS and COX-2 Expression
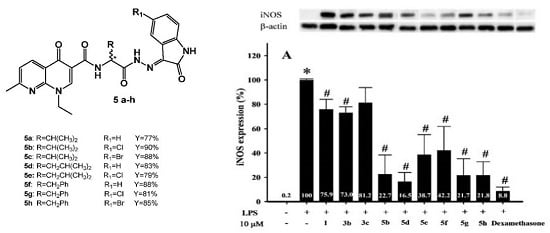
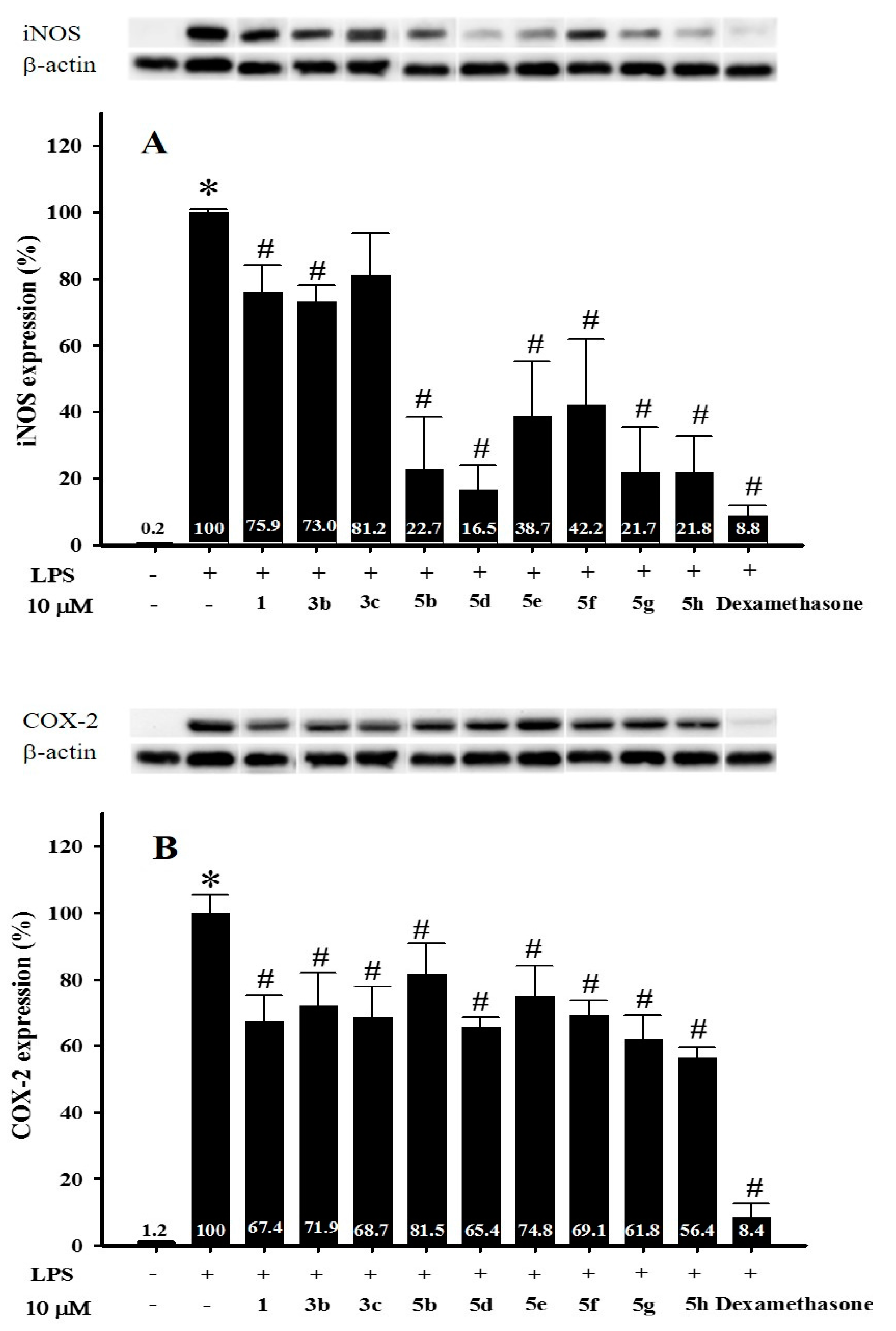
2.2.2. The Effect of Nalidixic Acid and Its Conjugates on iNOS and COX-2 Expression
3. Discussion
4. Materials and Methods
4.1. General
4.2. Synthesis of Nalidixic Acid Carboxamides of Amino Acids Esters (2a–c)
4.3. Synthesis of Hydrazide Derivatives (3a–c)
4.4. Synthesis of Schiff Bases (5a–h)
4.5. Cell Viability Assay
4.6. In Vitro Anti-inflammatory Assay
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BAA | Branched amino acids |
| COX-2 | Cyclooxygenase-2 |
| iNOS | Inducible nitric oxide Synthase |
| LPS | E. coli lipopolysaccharide |
| NSAIDs | nonsteroidal antiinflammatory drugs |
| NMR | Nuclear magnetic resonance |
| MW | Microwave |
References
- Emmerson, A.M.; Jones, A.M. The quinolones: Decades of development and use. J. Antimicrob. Chemother. 2003, 51, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Sato, K.; Fujii, T.; Hirai, K.; Inoue, M.; Iyobe, S.; Mitsuhashi, S. Some properties of subunits of DNA gyrase from Pseudomonas aeruginosa PAO1 and its nalidixic acid-resistant mutant. J. Bacteriol. 1987, 169, 2322–2325. [Google Scholar] [PubMed]
- Aboul-Fadl, T.; Bin-Jubair, F.A.; Aboul-Wafa, O. Schiff bases of indoline-2,3-dione (isatin) derivatives and nalidixic acid carbohydrazide, synthesis, antitubercular activity and pharmacophoric model building. Eur. J. Med. Chem. 2010, 45, 4578–4586. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Kumashiro, R.; Yamamoto, M.; Willett, N.P.; Lindsay, D.D. Regulation of inflammation-primed activation of macrophages by two serum factors, vitamin D3-binding protein and albumin. Infect. Immun. 1993, 61, 5388–5391. [Google Scholar] [PubMed]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. J. Tech. Methods Pathol. 2000, 80, 617–653. [Google Scholar] [CrossRef]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.K.; Tamaoki, J. Antibiotics as Anti-Inflammatory and Immunomodulatory Agents; Birkhäuser Verlag: Basel, Switzerland, 2005. [Google Scholar]
- Huang, H.C.; Shieh, C.C.; Yu, W.L.; Cheng, K.C.; Chen, C.C.; Chang, S.T.; Chuang, Y.C. Comparing the protective effects of ciprofloxacin, moxifloxacin and levofloxacin in mice with lipopolysaccharide-induced acute lung injuries. Respirology 2008, 13, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Suthar, S.K.; Jaiswal, V.; Lohan, S.; Bansal, S.; Chaudhary, A.; Tiwari, A.; Alex, A.T.; Joesph, A. Novel quinolone substituted thiazolidin-4-ones as anti-inflammatory, anticancer agents: Design, synthesis and biological screening. Eur. J. Med. Chem. 2013, 63, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Pervez, H.; Rauf, A.; Khan, K.M.; Supuran, C.T. Isatin-derived Antibacterial and Antifungal Compounds and their Transition Metal Complexes. J. Enzym. Inhib. Med. Chem. 2004, 19, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, S.N.; Smitha, S.; Jyoti, M.; Sridhar, S.K. Biological activities of isatin and its derivatives. Acta Pharm. 2005, 55, 27–46. [Google Scholar] [PubMed]
- Matheus, M.E.; Violante, F.D.A.; Garden, S.J.; Pinto, A.C.; Fernandes, P.D. Isatins inhibit cyclooxygenase-2 and inducible nitric oxide synthase in a mouse macrophage cell line. Eur. J. Pharmacol. 2007, 556, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.N.; Pendse, V.K.; Khanna, N.K. Anti-inflammatory and analgesic properties of four amino-acids. Indian J. Physiol. Pharmacol. 1984, 28, 299–305. [Google Scholar] [PubMed]
- Matsumoto, K.; Koba, T.; Hamada, K.; Sakurai, M.; Higuchi, T.; Miyata, H. Branched-chain amino acid supplementation attenuates muscle soreness, muscle damage and inflammation during an intensive training program. J. Sport Med. Phys. Fit. 2009, 49, 424–431. [Google Scholar]
- Burstein, S.; McQuain, C.; Salmonsen, R.; Seicol, B. N-Amino acid linoleoyl conjugates: Anti-inflammatory activities. Bioorg. Med. Chem. Lett. 2012, 22, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Stefani, H.A.; Botteselle, G.V.; Zukerman-Schpector, J.; Caracelli, I.; da Silva Correa, D.; Farsky, S.H.; Machado, I.D.; Santin, J.R.; Hebeda, C.B. Synthesis, anti-inflammatory activity and molecular docking studies of 2,5-diarylfuran amino acid derivatives. Eur. J. Med. Chem. 2012, 47, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, M.; Tang, Y.R.; Wang, C.; Zhang, Z.; Peng, S. N-[2-(5,5-Dimethyl-1,3-dioxane-2-yl)ethyl]amino acids: Their synthesis, anti-inflammatory evaluation and QSAR analysis. Eur. J. Med. Chem. 2008, 43, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Yin, L.L.; Cai, M.J.; Zhang, W.Y.; Huang, Y.; Wang, X.; Zhu, X.Z.; Shen, J.K. Design, synthesis, and anti-inflammatory evaluation of a series of novel amino acid-binding 1,5-diarylpyrazole derivatives. Acta Pharmacol. Sin. 2005, 26, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, N.M.; Naglah, A.M.; Al-Omar, M.A.; Amr, A.E.-G.E. Synthesis and Reactions of New Chiral Linear Dipeptide Candidates Using Nalidixic Acid as Starting Material. Z. Naturforschung B. 2014, 69b, 728–736. [Google Scholar] [CrossRef]
- Naglah, A.M.; Kalmouch, A.; Awad, H.M.; AlOmar, M.A.; Amr, A.E. Antimicrobial evaluation and microwaveassisted synthesis of some Schiff's bases from isatin with amino acid conjugates of nalidixic acid. Amino Acids 2015, 47, 1632–1632. [Google Scholar]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Okada, S.; Wakiguchi, H.; Kudo, K.; Kittaka, S.; Hara, M.; Furukawa, S. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin. Exp. Immunol. 2012, 167, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.F.; Hsieh, Y.-T.; Wen, Z.-H.; Wu, Y.-C.; Sheu, J.-H. Polyoxygenated sterols from the Formosan soft coral Sinularia gibberosa. J. Nat. Prod. 2006, 69, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Joullié, M.M.; Lassen, K.M. Evolution of amide bond formation. Arkivoc 2010, VIII, 189–250. [Google Scholar]
- Dewan, S.K. Microwave effect in organic reactions. Indian J. Chem. 2006, 45B, 2337–2340. [Google Scholar] [CrossRef]
- Hewett, J.A.; Roth, R.A. Hepatic and extrahepatic pathobiology of bacterial lipopolysaccharides. Pharmacol. Rev. 1993, 45, 382–411. [Google Scholar] [PubMed]
- Kubes, P.; McCafferty, D.M. Nitric oxide and intestinal inflammation. Am. J. Med. 2000, 109, 150–158. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Hamalainen, M.; Kankaanranta, H.; Moilanen, E. Dexamethasone inhibits inducible nitric-oxide synthase expression and nitric oxide production by destabilizing mRNA in lipopolysaccharide-treated macrophages. Mol. Pharmacol. 2002, 62, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Koistinaho, J.; Koponen, S.; Chan, P.H. Expression of cyclooxygenase-2 mRNA after global ischemia is regulated by AMPA receptors and glucocorticoids. Stroke J. Cereb. Circ. 1999, 30, 1900–1905. [Google Scholar] [CrossRef]
- Oh, Y.C.; Jeong, Y.H.; Cho, W.K.; Ha, J.H.; Gu, M.J.; Ma, J.Y. Anti-inflammatory and analgesic effects of pyeongwisan on LPS-stimulated murine macrophages and mouse models of acetic acid-induced writhing response and xylene-induced ear edema. Int. J. Mol. Sci. 2015, 16, 1232–1251. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Palumbo, A.; Prota, G. Adrenalin oxidation revisited. New products beyond the adrenochrome stage. Tetrahedron 1988, 44, 6441–6446. [Google Scholar] [CrossRef]
- Hossain, M.M.; Islam, N.; Khan, R.; Islam, M.R. Cytotoxicity study of dimethylisatin and its heterocyclic derivatives. Bangladesh J. Pharmacol. 2007, 2, 66–70. [Google Scholar] [CrossRef]
- Sin, N.; Venables, B.L.; Combrink, K.D.; Gulgeze, H.B.; Yu, K.L.; Civiello, R.L.; Thuring, J.; Wang, X.A.; Yang, Z.; Zadjura, L.; et al. Respiratory syncytial virus fusion inhibitors. Part 7: Structure-activity relationships associated with a series of isatin oximes that demonstrate antiviral activity in vivo. Bioorg. Med. Chem. Lett. 2009, 19, 4857–4862. [Google Scholar] [CrossRef] [PubMed]
- Selvam, P.; Chandramohan, M.; De Clercq, E.; Witvrouw, M.; Pannecouque, C. Synthesis and anti-HIV activity of 4-[(1,2-dihydro-2-oxo-3H-indol-3-ylidene) amino]-N(4,6-dimethyl-2-pyrimidinyl)-benzene sulfonamide and its derivatives. Eur. J. Pharm. Sci. 2001, 14, 313–316. [Google Scholar] [CrossRef]
- Mathur, G.; Nain, S. Recent Advancement in Synthesis of Isatin as Anticonvulsant Agents. Rev. Med. Chem. 2014, 4, 417–427. [Google Scholar]
- Sharma, S.K.; Karthikeyan, C.; Hari Narayana Moorthy, N.S.; Jain, D.K.; Trivedi, P. Synthesis and evaluation of some amino acid conjugates of pirprofen. Biomed. Prev. Nutr. 2013, 3, 241–246. [Google Scholar] [CrossRef]
- Jarapula, R.; Gangarapu, K.; Manda, S.; Rekulapally, S. Synthesis, In Vivo Anti-Inflammatory Activity, and Molecular Docking Studies of New Isatin Derivatives. Int. J. Med. Chem. 2016, 2016, 2181027. [Google Scholar] [PubMed]
- Khalifa, N.M.; Naglah, A.M.; Al-Omar, M.A.; Abo-Ghalia, M.H.; Amr, A.E.-G.E. Synthesis and Reactions of New Chiral Linear Carboxamides with an Incorporated Peptide Linkage Using Nalidixic Acid and Amino Acids as Starting Materials. Z. Naturforschung B. 2014, 69, 351–361. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wang, H.M.; Wen, Y.S.; Liu, W.; Li, P.H.; Chiu, C.C.; Chen, P.C.; Huang, C.Y.; Sheu, J.H.; Wen, Z.H. 4-(Phenylsulfanyl)butan-2-One Suppresses Melanin Synthesis and Melanosome Maturation in Vitro and in Vivo. Int. J. Mol. Sci. 2015, 16, 20240–20257. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.M.; Lai, C.C.; Huang, L.J.; Kuo, T.C.; Chao, C.M.; Lin, W.W. The anti-inflammatory carbazole, LCY-2-CHO, inhibits lipopolysaccharide-induced inflammatory mediator expression through inhibition of the p38 mitogen-activated protein kinase signaling pathway in macrophages. Br. J. Pharmacol. 2004, 141, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naglah, A.M.; Ahmed, A.F.; Wen, Z.-H.; Al-Omar, M.A.; Amr, A.E.-G.E.; Kalmouch, A. New Inducible Nitric Oxide Synthase and Cyclooxygenase-2 Inhibitors, Nalidixic Acid Linked to Isatin Schiff Bases via Certain l-Amino Acid Bridges. Molecules 2016, 21, 498. https://doi.org/10.3390/molecules21040498
Naglah AM, Ahmed AF, Wen Z-H, Al-Omar MA, Amr AE-GE, Kalmouch A. New Inducible Nitric Oxide Synthase and Cyclooxygenase-2 Inhibitors, Nalidixic Acid Linked to Isatin Schiff Bases via Certain l-Amino Acid Bridges. Molecules. 2016; 21(4):498. https://doi.org/10.3390/molecules21040498
Chicago/Turabian StyleNaglah, Ahmed M., Atallah F. Ahmed, Zhi-Hong Wen, Mohamed A. Al-Omar, Abd El-Galil E. Amr, and Atef Kalmouch. 2016. "New Inducible Nitric Oxide Synthase and Cyclooxygenase-2 Inhibitors, Nalidixic Acid Linked to Isatin Schiff Bases via Certain l-Amino Acid Bridges" Molecules 21, no. 4: 498. https://doi.org/10.3390/molecules21040498