Effect of Sodium Dodecyl Sulfate Adsorption on the Behavior of Water inside Single Walled Carbon Nanotubes with Dissipative Particle Dynamics Simulation
Abstract
:1. Introduction
2. Results and Discussion
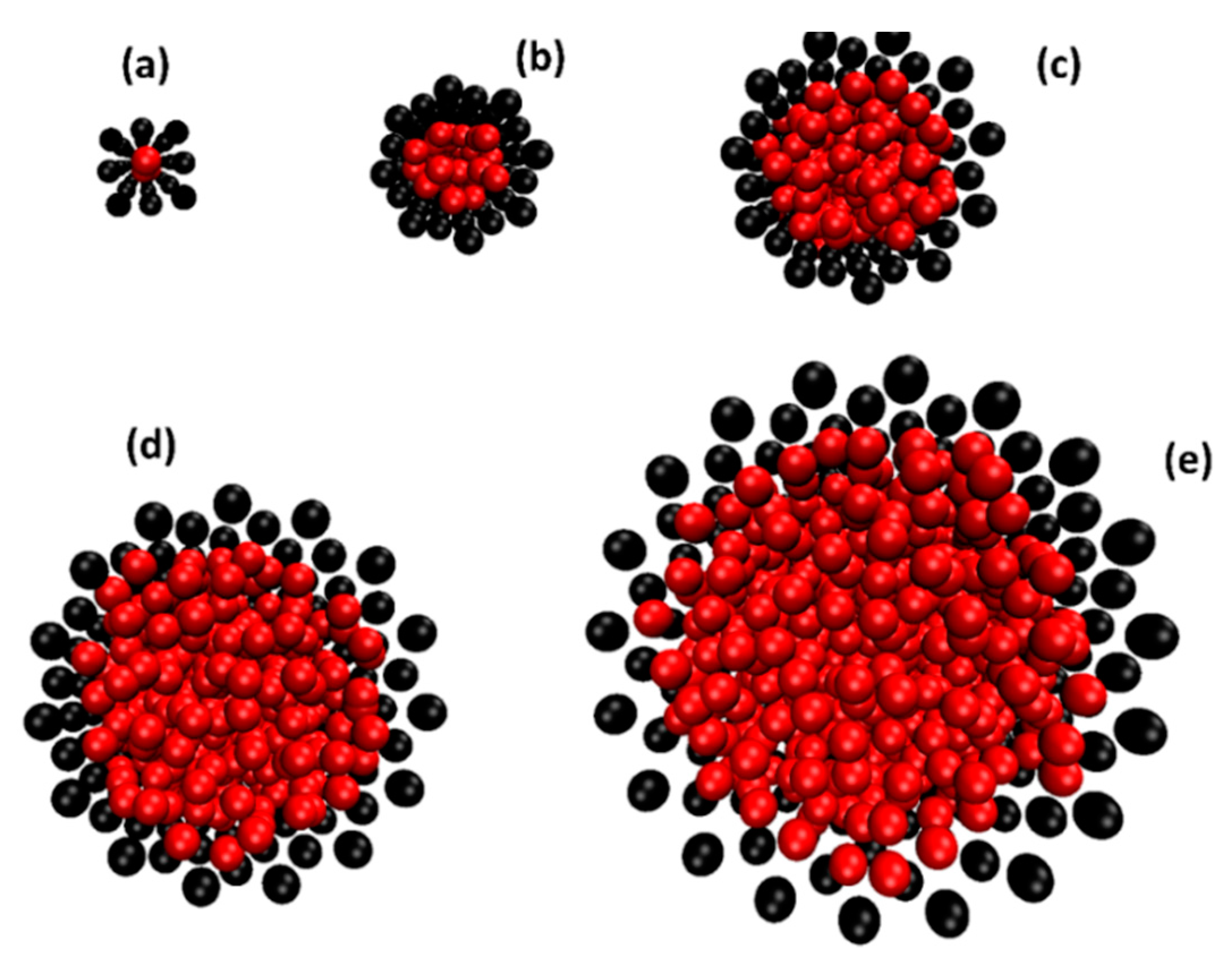
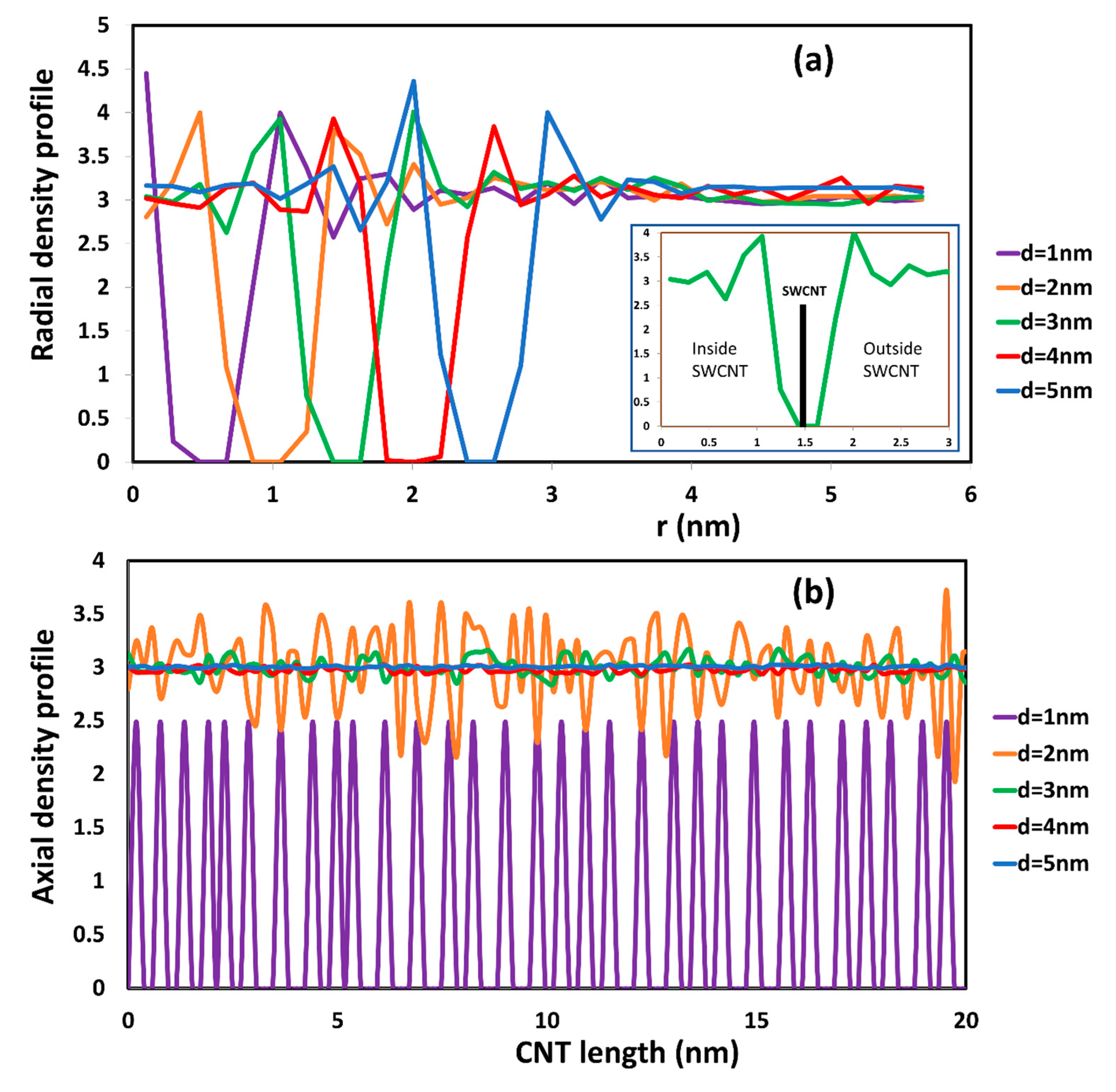
2.1. The Diffusion of Water inside SWCNTs of Different Diameters
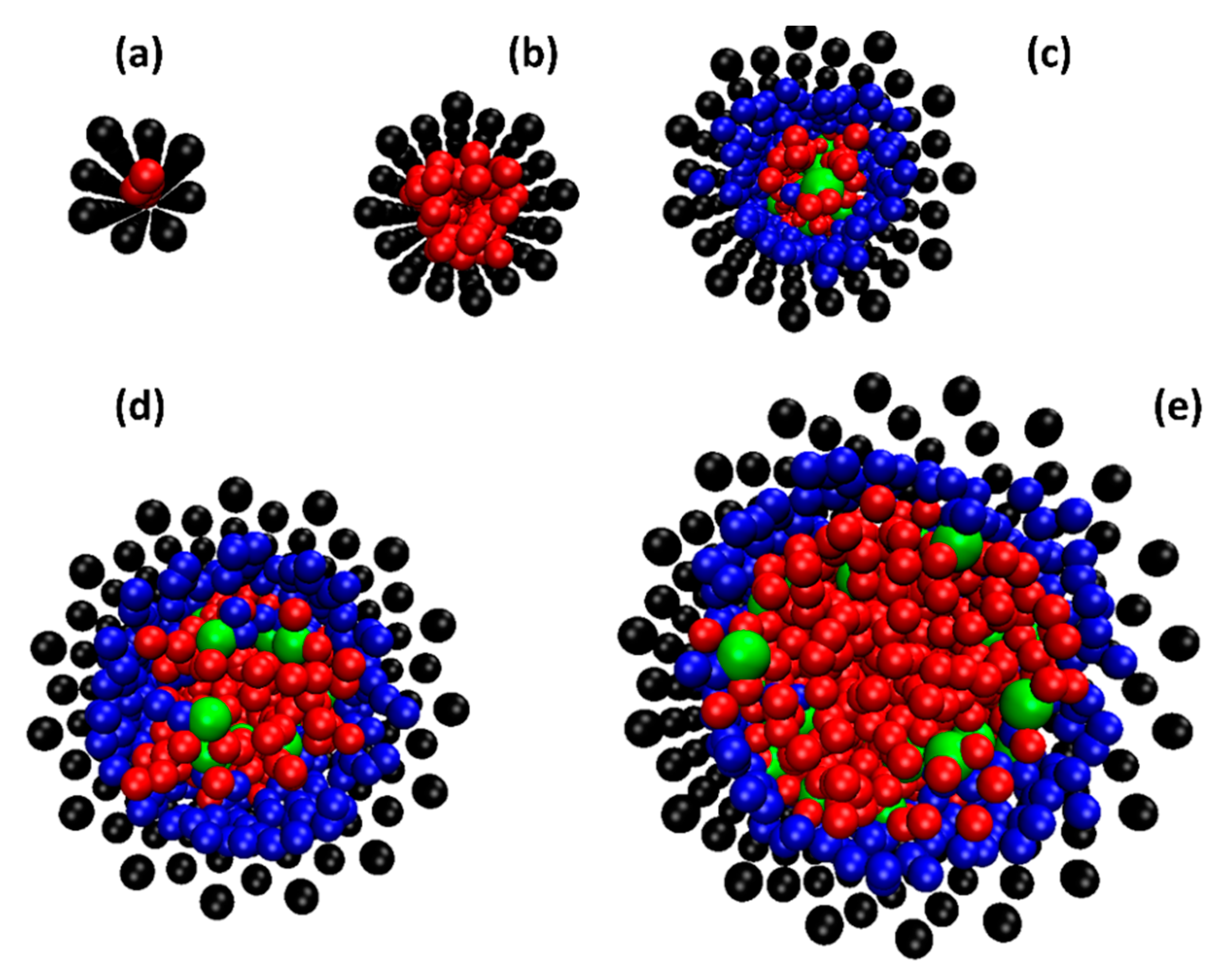
2.2. Can the SDS Molecules Enter the SWCNT?
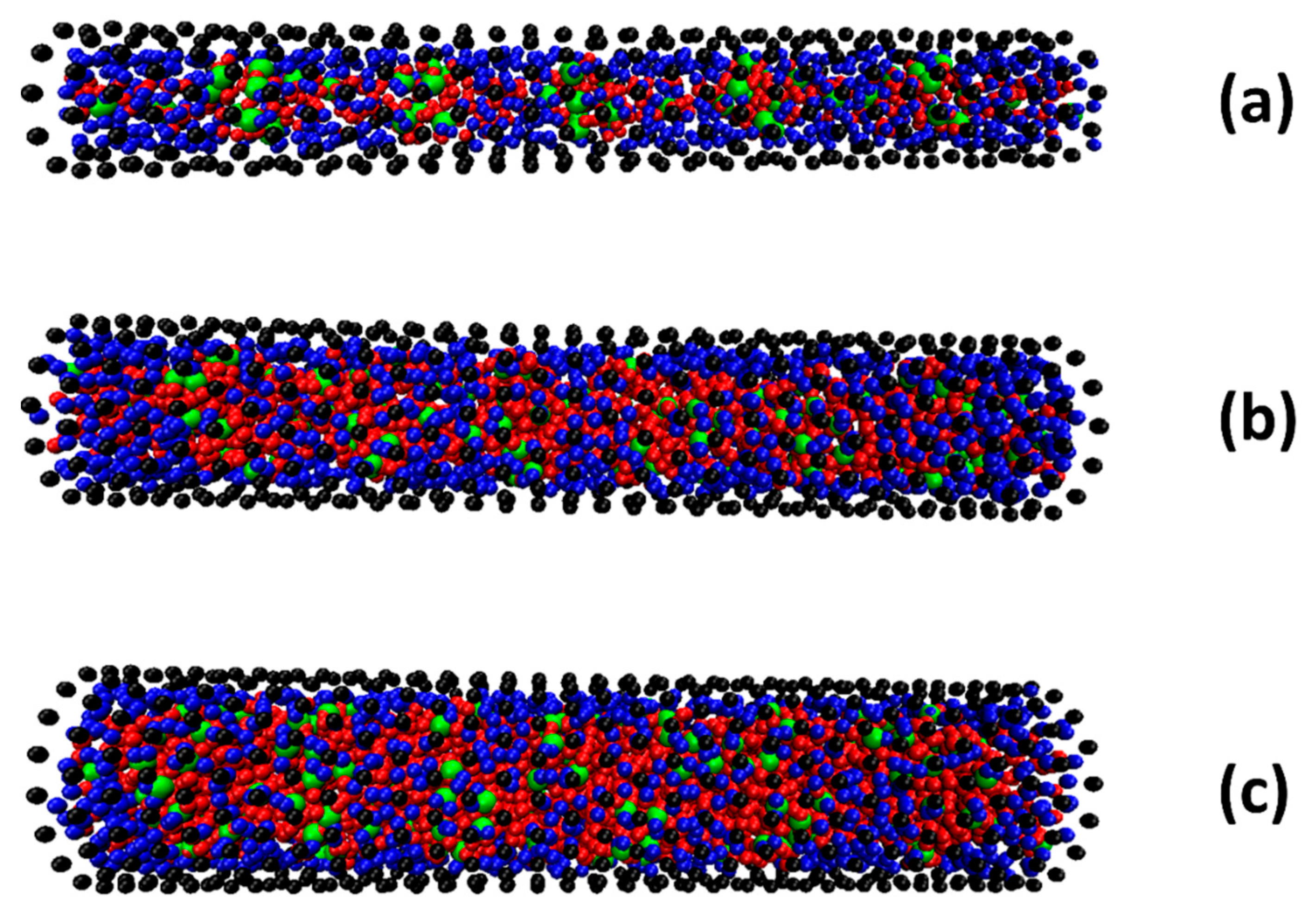
2.3. The Effect of SDS Adsorption on Water Distribution and Diffusion Inside the SWCNTs
3. Computational Details
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baughman, R.H.; Zakhidov, A.A.; Heer, W.A.D. Carbon Nanotubes—The Route Toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Ijima, S.; Ichiashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Jin, Z.; Wang, J.; Li, Y.; Jiang, K.; Fan, S. Fabrication of Ultralong and Electrically Uniform Single-Walled Carbon Nanotubes on Clean Substrates. Nano Lett. 2009, 9, 3137–3141. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, E.; Ebbesen, T.W.; Hiura, H.; Tanigaki, K. Capillarity and Wetting of Carbon Nanotubes. Science 1994, 265, 1850–1852. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.E.; Blackford, M.; Moricca, S.; Webb, N.; Evans, P.J.; Smith, A.M.; Jacobsen, G.; Leung, S.; Day, A.; Hua, Q. The World’s Smallest Gas Cylinders? Science 1997, 277, 933–936. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Yates, J.T., Jr.; Liu, J.; Smalley, R.E. Physical adsorption of xenon in open single walled carbon nanotubes: Observation of a quasi-one-dimensional confined Xe phase. J. Chem. Phys. 2000, 112, 9590–9598. [Google Scholar] [CrossRef]
- Yano, H.; Yoshizaki, S.; Inagaki, S.; Fukushima, Y.; Wada, N. Observation of Superfluid 4He Adsorbed in One-Dimensional Mesopores. J. Low Temp. Phys. 1998, 110, 573–578. [Google Scholar] [CrossRef]
- Gordillo, M.C.; Boronat, J.; Casulleras, J. Quasi-one-dimensional 4He inside carbon nanotubes. Phys. Rev. B 1999, 61, 878–881. [Google Scholar] [CrossRef]
- Pan, X.; Bao, X. The Effects of Confinement inside Carbon Nanotubes on Catalysis. Acc. Chem. Res. 2011, 44, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Baek, Y.; Lee, M.; Jeong, D.H.; Lee, H.H.; Yoon, J.; Kim, Y.H. A carbon nanotube wall membrane for water treatment. Nat. Commun. 2015, 6, 7109. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.J.; Green, M.L. H.; Hill, H.A.O.; Leung, Y.C.; Sadler, P.J.; Sloan, J.; Xavier, A.V.; Tsang, S.C. The immobilisation of proteins in carbon nanotubes. Inorg. Chim. Acta 1998, 272, 261–266. [Google Scholar] [CrossRef]
- Fujiwara, A.; Ishii, K.; Suematsu, H.; Kataura, H.; Maniwa, Y.; Suzuki, S.; Achiba, Y. Gas adsorption in the inside and outside of single-walled carbon nanotubes. Chem. Phys. Lett. 2001, 336, 205–211. [Google Scholar] [CrossRef]
- Pan, X.; Fan, Z.; Chen, W.; Ding, Y.; Luo, H.; Bao, X. Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles. Nat. Mater. 2007, 6, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.Z.; Duan, W.H.; Wang, Q.; Dowman, M.; Kodikara, J. Driving Forces and Transportation Efficiency in Water Transportation Through Single-Walled Carbon Nanotubes. J. Nanotechnol. Eng. Med. 2012, 3, 020904. [Google Scholar] [CrossRef]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Walther, J.H.; Ritos, K.; Cruz-Chu, E.R.; Megaridis, C.M.; Koumoutsakos, P. Barriers to Superfast Water Transport in Carbon Nanotube Membranes. Nano Lett. 2013, 13, 1910–1914. [Google Scholar] [CrossRef] [PubMed]
- Anastassiou, A.; Karahaliou, E.K.; Alexiadis, O.; Mavrantzas, V.G. Detailed atomistic simulation of the nano-sorption and nano-diffusivity of water, tyrosol, vanillic acid, and p-coumaric acid in single wall carbon nanotubes. J. Chem. Phys. 2013, 139, 164711. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Yasuoka, K.; Zeng, X.C. Self-Assembly of Surfactants and Polymorphic Transition in Nanotubes. J. Am. Chem. Soc. 2008, 130, 7916–7920. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; McGaughey, A.J.H. Water Flow in Carbon Nanotubes: Transition to Subcontinuum Transport. Phys. Rev. Lett. 2009, 102, 184502. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; McBride, C.; Sanz, E.; Abascal, J.L. Radial distribution functions and densities for the SPC/E, TIP4Pand TIP5P models for liquid water and ices Ih,Ic, II, III, IV, V,VI, VII, VIII, IX, XI and XII. Phys. Chem. Chem. Phys. 2005, 7, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Farimani, A.B.; Aluru, N.R. Spatial Diffusion of Water in Carbon Nanotubes: From Fickian to Ballistic Motion. J. Phys. Chem. B 2011, 115, 12145–12149. [Google Scholar] [CrossRef] [PubMed]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Duan, W.H.; Wang, Q.; Collins, F. Dispersion of carbon nanotubes with SDS surfactants: A study from a binding energy perspective. Chem. Sci. 2011, 2, 1407–1413. [Google Scholar] [CrossRef]
- Calvaresi, M.; Dallavalle, M.; Zerbetto, F. Wrapping Nanotubes with Micelles, Hemimicelles, and Cylindrical Micelles. Small 2009, 5, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Tummala, N.R.; Striolo, A. SDS Surfactants on Carbon Nanotubes: Aggregate Morphology. ACS Nano 2009, 3, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Vo, M.D.; Shiau, B.; Harwell, J.H.; Papavassiliou, D.V. Adsorption of anionic and non-ionic surfactants on Carbon nanotubes in water with Dissipative Particle Dynamics simulation. J. Chem. Phys. 2016. submitted. [Google Scholar]
- Español, P.; Warren, P.B. Statistical Mechanics of Dissipative Particle Dynamics. Europhys. Lett. 1995, 30, 191–196. [Google Scholar] [CrossRef]
- Lu, J.R.; Li, Z.X.; Thomas, R.K.; Staples, E.J.; Tucker, I.; Penfold, J. Neutron Reflection from a Layer of Monododecyl Hexaethylene Glycol Adsorbed at the Air-Liquid Interface: The Configuration of the Ethylene Glycol Chain. J. Phys. Chem. 1993, 97, 8012–8020. [Google Scholar] [CrossRef]
- Nagarajan, R.; Wang, C.C. Theory of Surfactant Aggregation in Water/Ethylene Glycol Mixed Solvents. Langmuir 2004, 16, 5242–5251. [Google Scholar] [CrossRef]
- Plimpton, S.J. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Groot, R.D.; Rabone, K.L. Mesoscopic simulation of cell membrane damage, morphology change and rupture by nonionic surfactants. Biophys. J. 2001, 81, 725–736. [Google Scholar] [CrossRef]
- Wua, H.; Xu, J.; He, X.; Zhao, Y.; Wen, H. Mesoscopic simulation of self-assembly in surfactant oligomers by dissipative particle dynamics. Coll. Surfaces A Physicochem. Eng. Asp. 2006, 290, 239–246. [Google Scholar] [CrossRef]
- Duan, B.; Zhang, X.; Qiao, B.; Kong, B.; Yang, X. Description of Ionic Surfactant/Water System by Adjusting Mesoscopic Parameters. J. Phys. Chem. B 2009, 113, 8854–8859. [Google Scholar] [CrossRef] [PubMed]
- Vo, M.; Papavassiliou, D.V. Interaction parameters between carbon nanotubes and water in Dissipative Particle Dynamics. Mol. Simul. 2016, 42, 737–744. [Google Scholar] [CrossRef]
- Cifuentes, A.; Bernal, J.L.; Diez-Masa, J.C. Determination of Critical Micelle Concentration Values Using Capillary Electrophoresis Instrumentation. Anal. Chem. 1997, 69, 4271–4274. [Google Scholar] [CrossRef]
- Sample Availability: Not available.

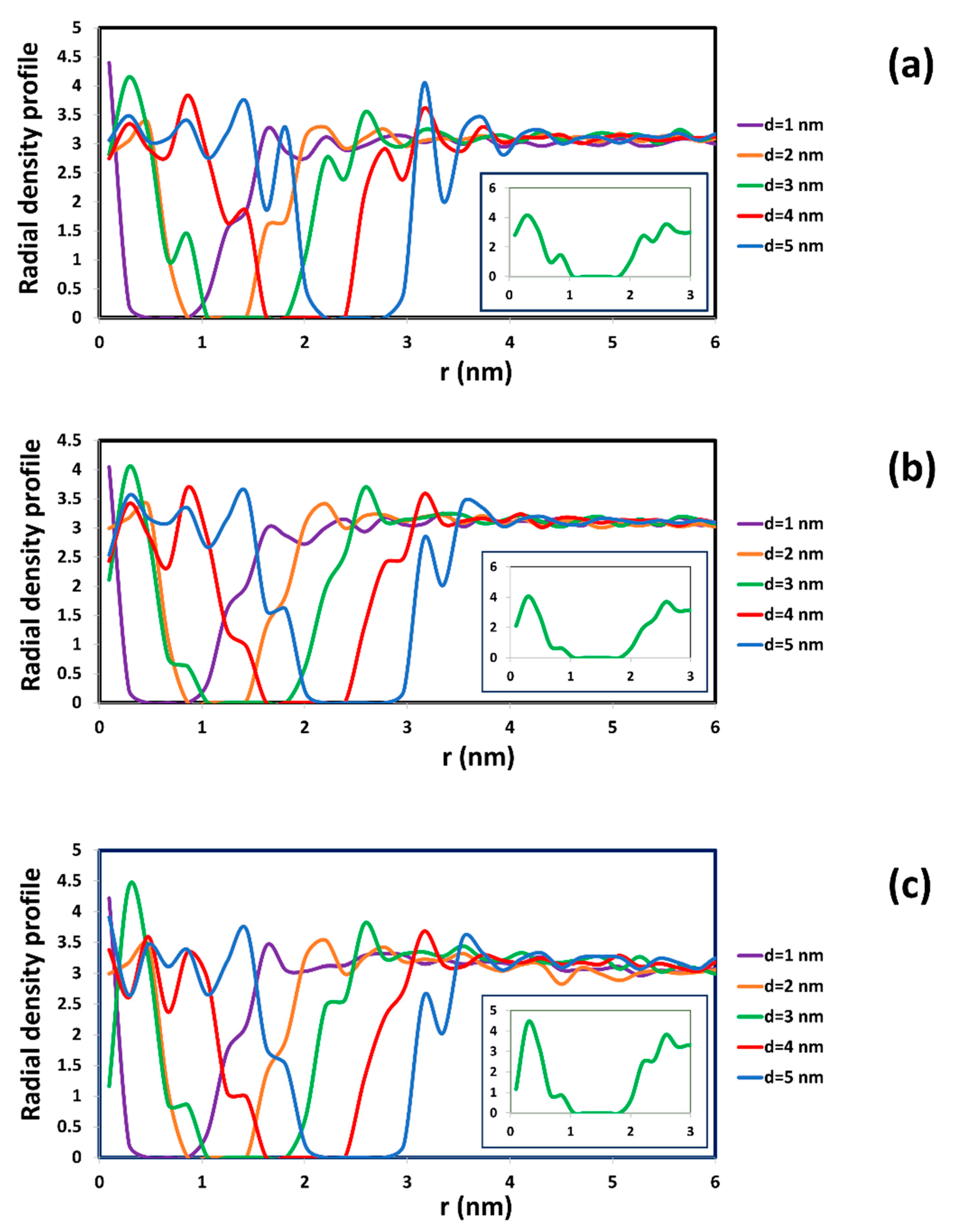
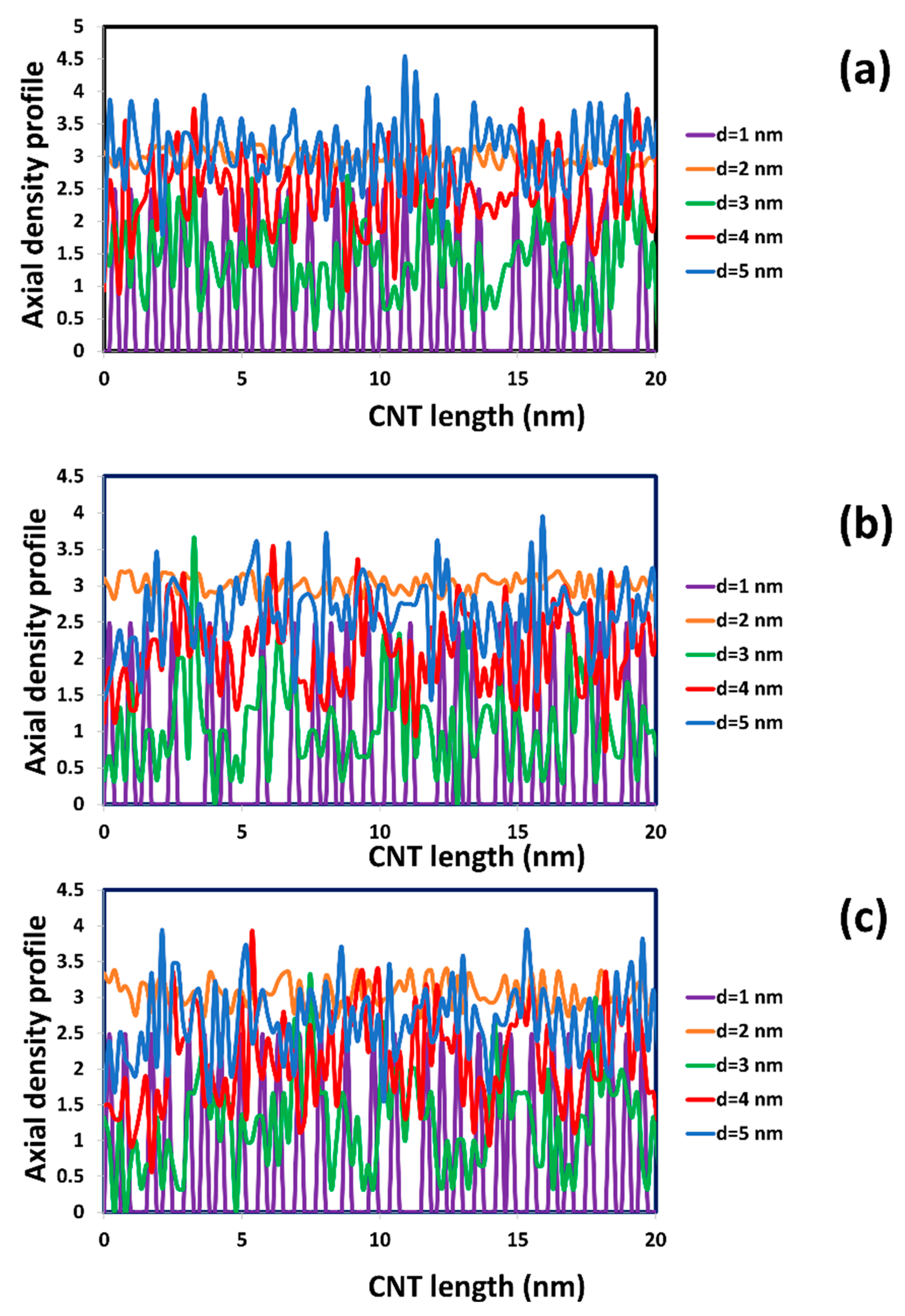
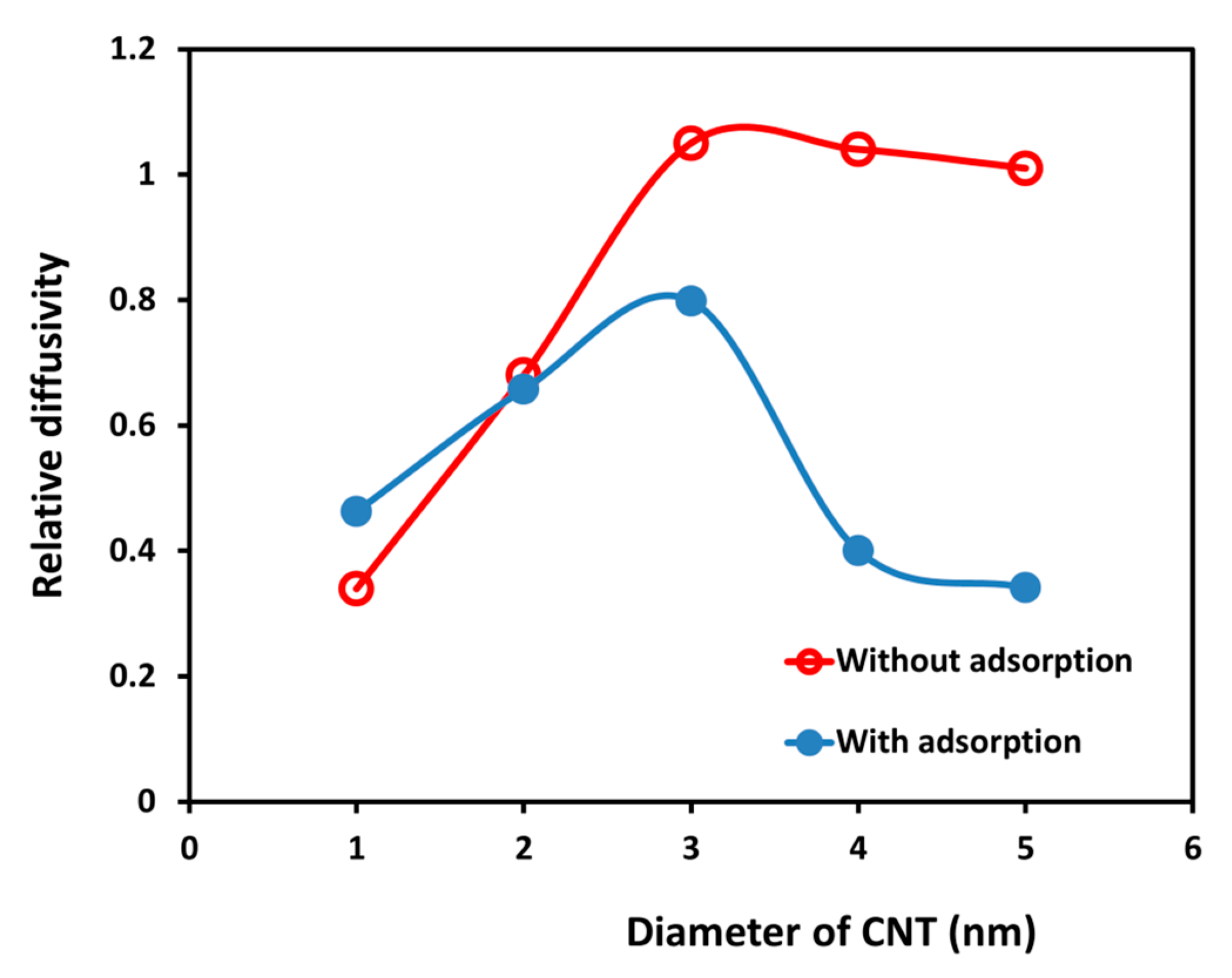


| Diameter of CNT and Chirality | Average Residence Time (ns) | Diffusivity of Water inside SWCNT (cm2/s) | Diffusivity Ratio between Water inside SWCNT and in Bulk Phase (from Our DPD Simulation) | Diffusivity Ratio between Water inside SWCNT and in Bulk Phase (from MD Simulation) [21] |
|---|---|---|---|---|
| 1 nm (8, 8) | 57.1 | 1.27 × 10−5 | 0.34 | 0.21 |
| 2 nm (15, 15) | 26.3 | 2.53 × 10−5 | 0.68 | 0.95 |
| 3 nm (22, 22) | 18.6 | 3.91 × 10−5 | 1.05 | 1.15 |
| 4 nm (30, 30) | 17.9 | 3.86 × 10−5 | 1.04 | 1.06 |
| 5 nm (37, 37) | 18.3 | 3.77 × 10−5 | 1.01 | 1.02 |
| Diameter of CNT | Total Concentration of SDS (wt %) | Percent Adsorption Inside CNT | Percent Adsorption Outside CNT | Total SDS Adsorption (wt %) |
|---|---|---|---|---|
| 1 nm | 1 | 0 | 100 | 0.45 |
| 2 | 0 | 100 | 0.44 | |
| 3 | 0 | 100 | 0.47 | |
| 2 nm | 1 | 0 | 100 | 0.64 |
| 2 | 0 | 100 | 0.68 | |
| 3 | 0 | 100 | 0.67 | |
| 3 nm | 1 | 1.3 | 98.7 | 0.82 |
| 2 | 1.5 | 98.5 | 1.05 | |
| 3 | 2.3 | 97.7 | 0.98 | |
| 4 nm | 1 | 12.9 | 87.1 | 0.88 |
| 2 | 8.7 | 91.3 | 1.25 | |
| 3 | 10.1 | 89.9 | 1.27 | |
| 5 nm | 1 | 12.3 | 87.7 | 0.90 |
| 2 | 13.3 | 86.7 | 1.48 | |
| 3 | 13.1 | 86.9 | 1.50 |
| Diameter of CNT | Total Concentration of SDS (wt %) | Average Residence Time (ns) | Diffusivity of Water Inside SWCNT (cm2/s) | Ratio between Diffusivity of Water Inside SWCNT with and without SDS Adsorption |
|---|---|---|---|---|
| 1 nm | 1 | 58.1 | 1.80 × 10−5 | 1.42 |
| 2 | 63.0 | 1.61 × 10−5 | 1.28 | |
| 3 | 61.7 | 1.78 × 10−5 | 1.41 | |
| 2 nm | 1 | 25.4 | 2.49 × 10−5 | 0.98 |
| 2 | 24.5 | 2.59 × 10−5 | 1.02 | |
| 3 | 27.7 | 2.29 × 10−5 | 0.90 | |
| 3 nm | 1 | 20.6 | 3.07 × 10−5 | 0.79 |
| 2 | 20.8 | 3.04 × 10−5 | 0.78 | |
| 3 | 22.3 | 2.84 × 10−5 | 0.73 | |
| 4 nm | 1 | 43.8 | 1.45 × 10−5 | 0.37 |
| 2 | 40.6 | 1.56 × 10−5 | 0.40 | |
| 3 | 42.8 | 1.48 × 10−5 | 0.38 | |
| 5 nm | 1 | 47.6 | 1.33 × 10−5 | 0.35 |
| 2 | 51.7 | 1.22 × 10−5 | 0.32 | |
| 3 | 49.4 | 1.28 × 10−5 | 0.34 |
| W | CNT | T | H | |
|---|---|---|---|---|
| W | 25 | 60 | 80 | 15 |
| CNT | 25 | 25 | 40 | |
| T | 15 | 80 | ||
| H | 35 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, M.D.; Papavassiliou, D.V. Effect of Sodium Dodecyl Sulfate Adsorption on the Behavior of Water inside Single Walled Carbon Nanotubes with Dissipative Particle Dynamics Simulation. Molecules 2016, 21, 500. https://doi.org/10.3390/molecules21040500
Vo MD, Papavassiliou DV. Effect of Sodium Dodecyl Sulfate Adsorption on the Behavior of Water inside Single Walled Carbon Nanotubes with Dissipative Particle Dynamics Simulation. Molecules. 2016; 21(4):500. https://doi.org/10.3390/molecules21040500
Chicago/Turabian StyleVo, Minh D., and Dimitrios V. Papavassiliou. 2016. "Effect of Sodium Dodecyl Sulfate Adsorption on the Behavior of Water inside Single Walled Carbon Nanotubes with Dissipative Particle Dynamics Simulation" Molecules 21, no. 4: 500. https://doi.org/10.3390/molecules21040500
APA StyleVo, M. D., & Papavassiliou, D. V. (2016). Effect of Sodium Dodecyl Sulfate Adsorption on the Behavior of Water inside Single Walled Carbon Nanotubes with Dissipative Particle Dynamics Simulation. Molecules, 21(4), 500. https://doi.org/10.3390/molecules21040500








