Design, Synthesis and Biological Evaluation of Brain-Targeted Thiamine Disulfide Prodrugs of Ampakine Compound LCX001
Abstract
:1. Introduction
2. Results and Discussion
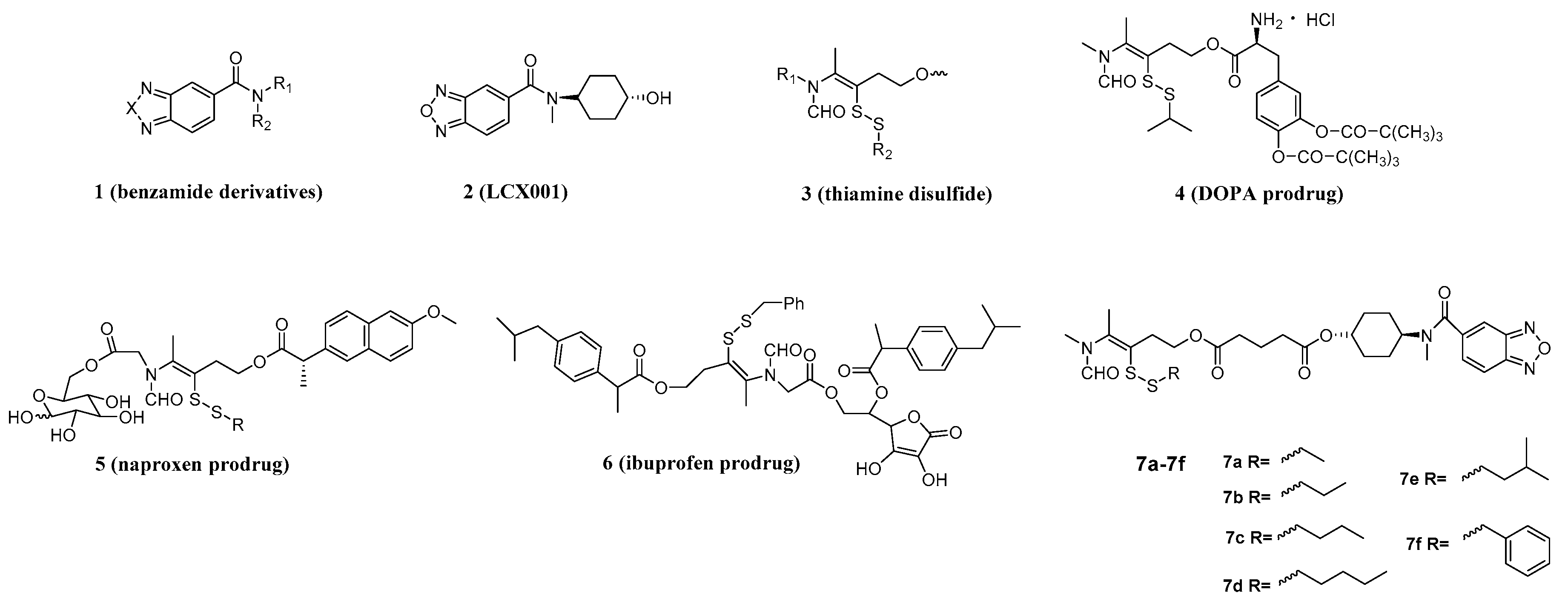
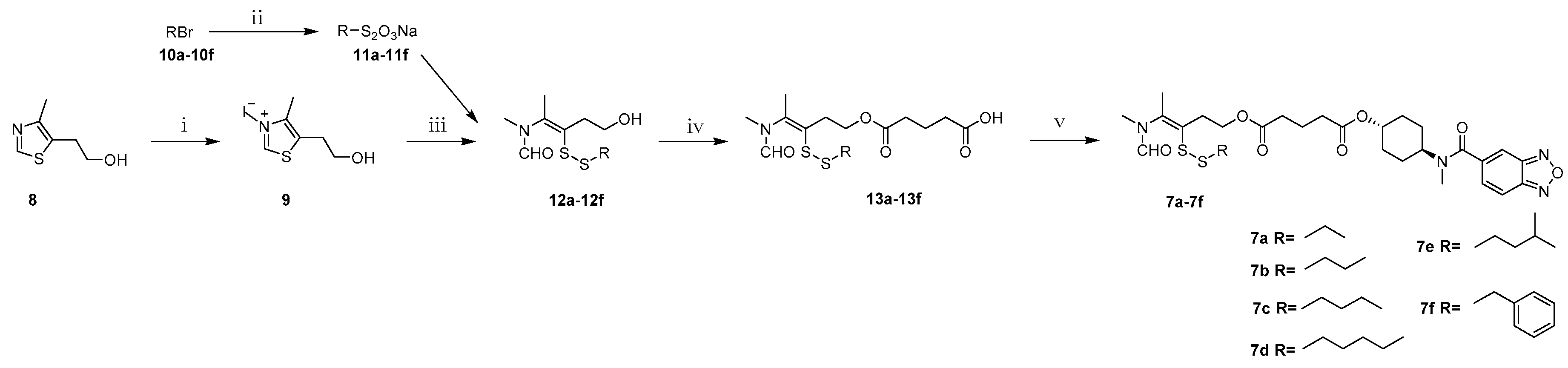
2.1. Chemistry
2.2. Metabolism Studies of the Prodrugs
2.2.1. Metabolic Stability
2.2.2. Release of the Parent Drug by Prodrugs in Brain Homogenate
2.3. In Vivo Studies
2.3.1. Pharmacokinetics in Plasma of the Prodrug 7e
2.3.2. Brain Distribution of Prodrug 7e
2.4. Pharmacodynamic Study of Prodrug 7e
3. Experimental Section
3.1. General Information
3.2. Liquid Chromatographic Conditions
3.3. Mass Spectrometric Conditions
3.4. Stability in Plasma Extract and Brain Homogenate
3.5. Release of the Parent Drug by Prodrugs in Brain Homogenate
3.6. Biodistribution Studies in Vivo
3.6.1. Test Animals
3.6.2. Biodistribution Studies of the Prodrug and LCX001
3.7. Statistical Analysis
3.8. Pharmacodynamic Study of Prodrug 7e
3.8.1. Test Animals
3.8.2. Model of Opiate-Induced Respiratory Depression
3.8.3. Assessment of the Prodrug 7e Effect
3.9. Synthesis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMPA | Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| BBB | Blood-brain barrier |
| EPSP | Excitatory postsynaptic potential |
| FDA | Food and Drug Administration |
| IND | Investigational new drug |
| ADHD | Attention-deficit hyperactivity disorder |
| TDS | Thiamine disulfide system |
| EDCI | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride |
| HOBT | N-Hydroxybenzotriazole |
| DMAP | 4-Dimethylaminopyridine |
| TEA | Triethylamine |
| DCM | Dichloromethane |
| rt | Room temperature |
| MRT | Mean residence times |
| RD | Respiratory depression |
| sc | Subcutaneous injection |
| iv | Intravenous injection |
| NMR | Nuclear Magnetic Resonance |
| LC | Liquid chromatography |
| MS | Mass spectrometry |
| ESI | Electrospray ionization |
| CAD | Collision-activated dissociation |
| CE | Collision energies |
| USA | United States of America |
| TMS | Tetramethylsilane |
References
- Swarm, R.A.; Karanikolas, M.; Kalauokalani, D. Pain treatment in the perioperative period. Curr. Probl. Surg. 2001, 38, 835–920. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, G. When opioid analgesia kills. Perspect. Infirm. 2006, 4, 6–9. [Google Scholar] [PubMed]
- Lötsch, J.; Dudziak, R.; Freynhagen, R.; Marschner, J.; Geisslinger, G. Fatal respiratory depression after multiple intravenous morphine injections. Clin. Pharmacokinet. 2006, 45, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Aarts, L.; Smith, T.W. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 2010, 112, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Jammal, W.; Gown, G. Opioid prescribing pitfalls: Medicolegal and regulatory issues. Aust. Prescr. 2015, 38, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.S.; Southwell, J.K.; Niehaus, V.R.; Walley, A.Y.; Dailey, M.W. Emergency medical services naloxone access: A national systematic legal review. Acad. Emerg. Med. 2014, 21, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Rekling, J.C.; Feldman, J.L. PreBotzinger complex and pacemaker neurons: Hypothesized site and kernel for respiratory rhythm generation. Annu. Rev. Physiol. 1998, 60, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.M.; Ge, Q.; Feldman, J.L. Modulation of AMPA receptors by cAMP-dependent protein kinase in preBötzinger complex inspiratory neurons regulates respiratory rhythm in the rat. J. Physiol. 2003, 547, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Kc, P.; Martin, R.J. Role of central neurotransmission and chemoreception on airway control. Respir. Physiol. Neurobiol. 2010, 173, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Pace, R.W.; Del Negro, C.A. AMPA and metabotropic glutamate receptors cooperatively generate inspiratory-like depolarization in mouserespiratory neurons in vitro. Eur. J. Neurosci. 2008, 28, 2434–2342. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Poon, B.Y.; Tang, Y.; Funk, G.D.; Greer, J.J. Ampakines alleviate respiratory depression in rats. Am. J. Respir. Crit. Care Med. 2006, 174, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ding, X.; Funk, G.D.; Greer, J.J. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology 2009, 110, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.J.; Ren, J. Ampakine therapy to counter fentanyl-induced respiratory depression. Respir. Physiol. Neurobiol. 2009, 168, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Oertel, B.G.; Felden, L.; Tran, P.V.; Bradshaw, M.H.; Angst, M.S.; Schmidt, H.; Johnson, S.; Greer, J.J.; Geisslinger, G.; Varney, M.A.; et al. Selective antagonism of opioid-induced ventilatory depression by an ampakine molecule in humans without loss of opioid analgesia. Clin. Pharmacol. Ther. 2010, 87, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ding, X.; Greer, J.J. Respiratory depression in rats induced by alcohol and barbiturate and rescue by ampakine CX717. J. Appl. Physiol. 2012, 113, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Lenal, F.; Yang, M.; Ding, X.; Greer, J.J. Coadministration of the AMPAKINE CX717 with propofol reduces respiratory depression and fatal apneas. Anesthesiology 2013, 118, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- ElMallah, M.K.; Pagliardini, S.; Turner, S.M.; Cerreta, A.J.; Falk, D.J.; Byrne, B.J.; Greer, J.J.; Fuller, D.D. Stimulation of Respiratory Motor Output and Ventilation in a Murine Model of Pompe Disease by Ampakines. Am. J. Respir. Cell Mol. Biol. 2015, 53, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ding, X.; Greer, J.J. Ampakines enhance weak endogenous respiratory drive and alleviate apnea in perinatal rats. Am. J. Respir. Crit. Care Med. 2015, 191, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Partin, K.M. AMPA receptor potentiators: From drug design to cognitive enhancement. Curr. Opin. Pharmacol. 2015, 20, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lippa Arnold, S. “Strategic Initiatives in Respiratory Disorders and Key Objectives”. In Proceedings of the Rodman and Renshaw 16th Annual Global Investment Conference, New York, NY, USA, 8–11 September 2014.
- Street, L.; Mueller, R.; Lee, S. Bicyclic Amide Derivatives for the Treatment of Respiratory Depression. International Patent 2008143963, 27 November 2008. [Google Scholar]
- Mueller, R.; Street, L. Bicyclic Amides for Enhancing Glutamatergic Synaptic Responses. U.S. Patent 8263591, 11 September 2012. [Google Scholar]
- Toyoaki, I.; Takashi, S.; Hiroshi, I.; Takashi, K.; Teruomi, I. Drug delivery to the brain. DOPA prodrugs based on a ring-closure reaction to quaternary thiazolium compounds. Int. J. Pharm. 1995, 116, 51–63. [Google Scholar]
- Fanm, W.; Wu, Y.; Li, X.K.; Yao, N.; Li, X.; Yu, Y.G.; Hai, L. Design, synthesis and biological evaluation of brain-specific glucosyl thiamine disulfide prodrugs of naproxen. Eur. J. Med. Chem. 2011, 46, 3651–3661. [Google Scholar]
- Zhao, Y.; Qu, B.; Wu, X.; Li, X.; Liu, Q.; Jin, X.; Guo, L.; Hai, L.; Wu, Y. Design, synthesis and biological evaluation of brain targeting L-ascorbic acid prodrugs of ibuprofen with “lock-in” function. Eur. J. Med. Chem. 2014, 82, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Quan, W. Investigation on Receptor Mechanisms Underlying Powerful Antinociception and Low Addiction of Novel. Ph.D. thesis, Academy of Military Medical Sciences, Beijing, China, 2011. Available online: http://www.cnki.net/ (accessed on 8 April 2016). [Google Scholar]
- Sample Availability: Samples of the compounds 7–13 are available from the authors.

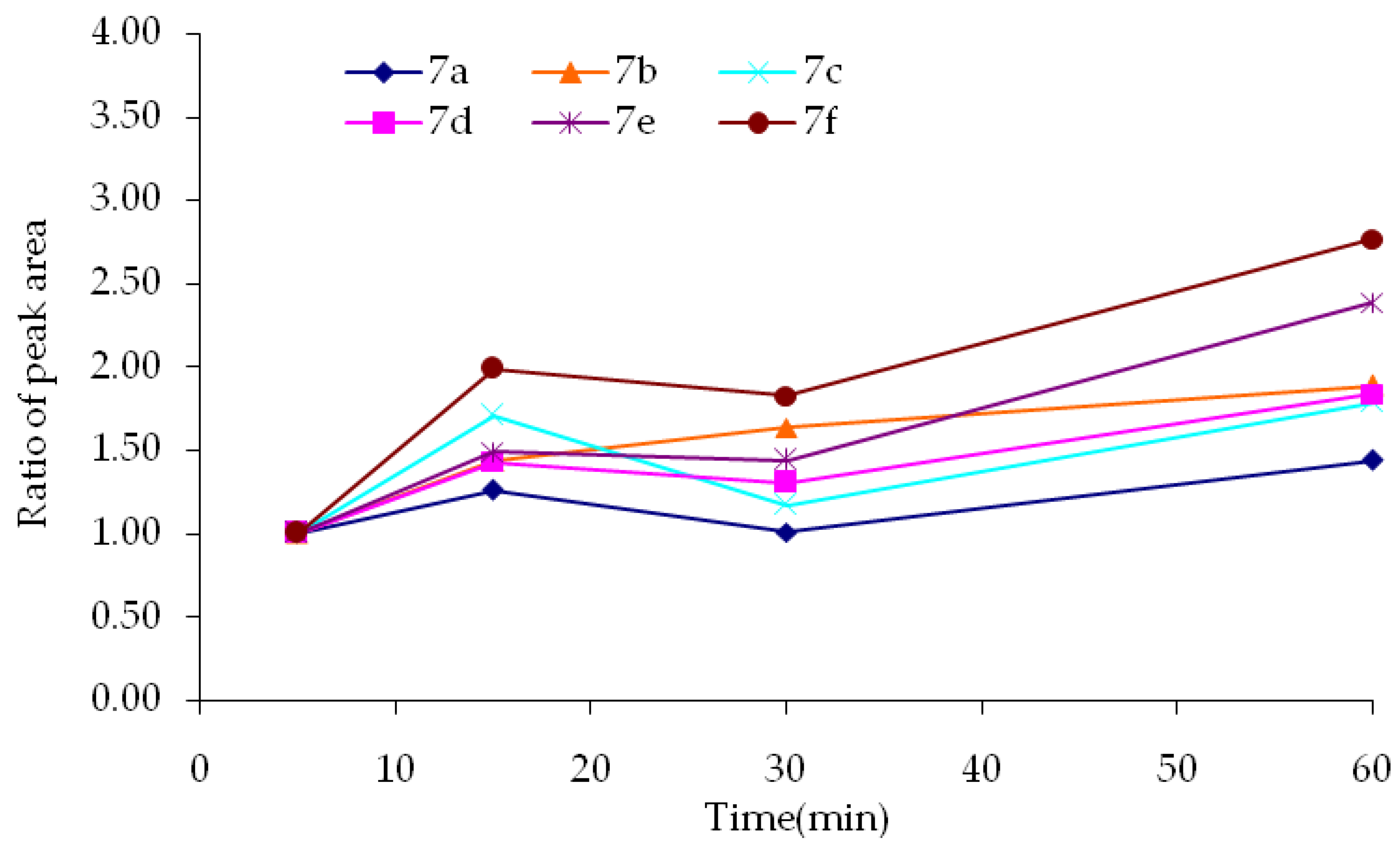



| Compound | R | Biological Matrix | Kinetic Constants | |
|---|---|---|---|---|
| Ke (min−1) (×10−2) | t1/2 (min) | |||
| 7a |  | Plasma | 31.1 | 2.23 |
| Brain | 19.9 | 3.48 | ||
| 7b |  | Plasma | 7.06 | 9.82 |
| Brain | 6.82 | 10.17 | ||
| 7c |  | Plasma | 6.77 | 10.24 |
| Brain | 4.05 | 17.10 | ||
| 7d |  | Plasma | 4.50 | 15.41 |
| Brain | 6.34 | 10.93 | ||
| 7e |  | Plasma | 4.34 | 15.97 |
| Brain | 3.69 | 18.78 | ||
| 7f |  | Plasma | 4.48 | 15.47 |
| Brain | 5.78 | 11.99 | ||
| Compound | Cmax (ng/mL) | AUC0-t (h·ng/mL) | Tmax (h) | t1/2 (h) | MRT0-t (h) |
|---|---|---|---|---|---|
| LCX001 | 833.67 ± 79.85 | 951.66 ± 115.52 | 0.08 | 0.53 ± 0.17 | 1.08 ± 0.04 |
| 7e | 590.36 ± 99.90 * | 384.16 ± 39.21 * | 0.08 | 0.48 ± 0.20 | 0.83 ± 0.03 * |
| Compound | Cmax (ng/g) | AUC0-t (h·ng/g) | Tmax (h) | t1/2 (h) | MRT0-t (h) |
|---|---|---|---|---|---|
| LCX001 | 609.40 ± 73.66 | 504.32 ± 204.71 | 0.08 | 0.59 ± 0.08 | 0.70 ± 0.22 |
| 7e | 454.53 ± 74.69 | 1125.49 ± 336.57 * | 0.17 | 2.50 ± 0.42 * | 2.30 ± 0.73 * |
| Experiment | Treatment | Test Material | n | ROUTE | Dose (mg/kg) | Death Number (n) | Death Rate |
|---|---|---|---|---|---|---|---|
| RD | 030418, 15 mg/kg, sc | Saline | 10 | iv | 8 | 80% | |
| RD | 030418, 15 mg/kg, sc | LCX001 | 10 | iv | 10 | 2 | 20% |
| RD | 030418, 15 mg/kg, sc | 7e | 10 | iv | 30 | 0 | 0% |
| RD | 030418, 15 mg/kg, sc | 7e | 10 | iv | 10 | 0 | 0% |
| RD | 030418, 15 mg/kg, sc | 7e | 20 | iv | 3 | 4 | 20% |
| RD | 030418, 15 mg/kg, sc | 7e | 10 | iv | 1 | 3 | 30% |
| RD | 030418, 15 mg/kg, sc | 7e | 10 | iv | 0.5 | 5 | 50% |
| [M + H]+ | Precursor ion | Product ion | CE (V) |
|---|---|---|---|
| LCX001 | 276.2 | 178.2 | 20 |
| Glipizide | 446.0 | 321.0 | 20 |
| 7a | 607.5 | 258.2 | 25 |
| 7b | 621.5 | 258.2 | 26 |
| 7c | 635.5 | 258.2 | 27 |
| 7d | 649.5 | 258.2 | 29 |
| 7e | 649.5 | 258.2 | 29 |
| 7f | 669.5 | 258.2 | 29 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, D.; Meng, F.-H.; Dai, W.; Yong, Z.; Liu, J.-Q.; Zhou, X.-B.; Li, S. Design, Synthesis and Biological Evaluation of Brain-Targeted Thiamine Disulfide Prodrugs of Ampakine Compound LCX001. Molecules 2016, 21, 488. https://doi.org/10.3390/molecules21040488
Xiao D, Meng F-H, Dai W, Yong Z, Liu J-Q, Zhou X-B, Li S. Design, Synthesis and Biological Evaluation of Brain-Targeted Thiamine Disulfide Prodrugs of Ampakine Compound LCX001. Molecules. 2016; 21(4):488. https://doi.org/10.3390/molecules21040488
Chicago/Turabian StyleXiao, Dian, Fan-Hua Meng, Wei Dai, Zheng Yong, Jin-Qiu Liu, Xin-Bo Zhou, and Song Li. 2016. "Design, Synthesis and Biological Evaluation of Brain-Targeted Thiamine Disulfide Prodrugs of Ampakine Compound LCX001" Molecules 21, no. 4: 488. https://doi.org/10.3390/molecules21040488
APA StyleXiao, D., Meng, F.-H., Dai, W., Yong, Z., Liu, J.-Q., Zhou, X.-B., & Li, S. (2016). Design, Synthesis and Biological Evaluation of Brain-Targeted Thiamine Disulfide Prodrugs of Ampakine Compound LCX001. Molecules, 21(4), 488. https://doi.org/10.3390/molecules21040488






