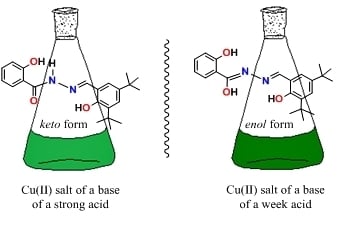
Aroylhydrazone Cu(II) Complexes in keto Form: Structural Characterization and Catalytic Activity towards Cyclohexane Oxidation
Abstract
:1. Introduction
2. Results and Discussion
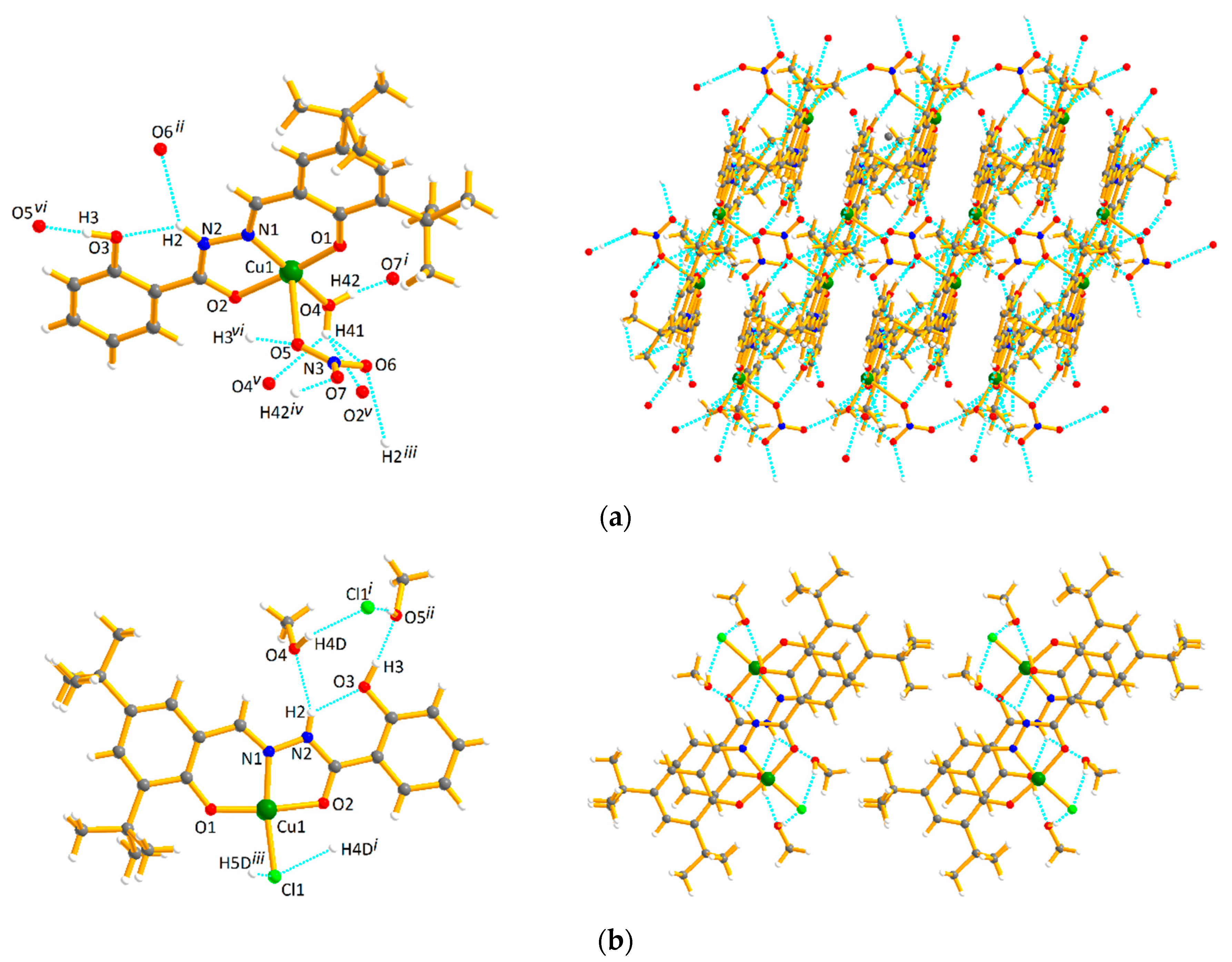
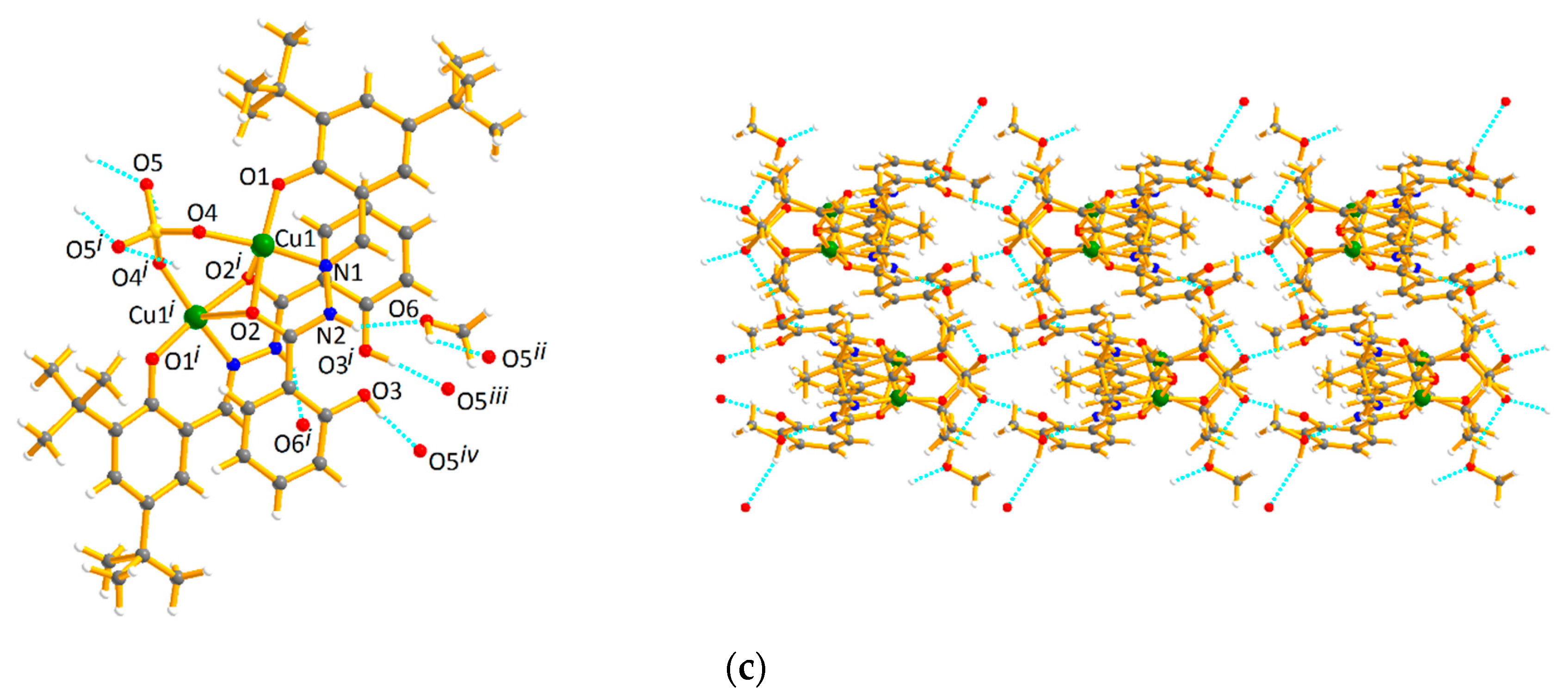
2.1. Crystal Structures
2.2. Catalytic Activity
3. Experimental
3.1. General Materials and Procedures
3.2. Typical Procedures for the Catalytic Oxidation of Cyclohexane and Product Analysis
3.3. Preparations
3.3.1. Synthesis of the Pro-Ligand H3L
3.3.2. Syntheses of the Cu(II) Complexes
[Cu(H2L)(NO3)(H2O)] (1)
[Cu(H2L)Cl]·2MeOH (2)
[{Cu(H2L)}2(µ-SO4)]·2MeOH (3)
3.4. X-ray Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bäckvall, J.-E. Modern Oxidation Methods, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: Weinheim, Germany, 1999–2013.
- Sheldon, R.A.; Arends, I.; Hanefeld, U. Green Chemistry and Catalysis; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Pombeiro, A.J.L. (Ed.) Advances in Organometallic Chemistry and Catalysis, The Silver/Gold Jubilee ICOMC Celebratory Book; J. Wiley & Sons: New York, NY, USA, 2014.
- Shul’pin, G.B. Transition Metals for Organic Synthesis, 2nd ed.; Beller, M., Bolm, C., Eds.; Wiley-VCH: New York, NY, USA, 2004; Volume 2, Chapter 2; pp. 215–242. [Google Scholar]
- Shilov, A.E.; Shul’pin, G.B. Activation of C−H Bonds by Metal Complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Hydrocarbon Oxygenations with Peroxides Catalyzed by Metal Compounds. Mini Rev. Org. Chem. 2009, 6, 95–104. [Google Scholar] [CrossRef]
- Crabtree, R.H. Alkane C–H activation and functionalization with homogeneous transition metal catalysts: A century of progress—A new millennium in prospect. J. Chem. Soc. Dalton Trans. 2001, 2437–2450. [Google Scholar] [CrossRef]
- Que, L., Jr.; Tolman, W.B. Biologically inspired oxidation catalysis. Nature 2008, 455, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Da Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Oxovanadium complexes in catalytic oxidations. Coord. Chem. Rev. 2011, 255, 2232–2248. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Vanadium complexes: Recent progress in oxidation catalysis. Coord. Chem. Rev. 2015, 301, 200–239. [Google Scholar] [CrossRef]
- Sutradhar, M.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. A hexanuclear mixed-valence oxovanadium(IV,V) complex as a highly efficient alkane oxidation catalyst. Inorg. Chem. 2012, 51, 11229–11231. [Google Scholar] [CrossRef] [PubMed]
- Pombeiro, A.J.L. Advances in Organometallic Chemistry and Catalysis; Pombeiro, A.J.L., Ed.; Wiley: Hoboken, NJ, USA, 2013; Chapter 2; pp. 15–25. [Google Scholar]
- Kirillov, A.M.; Kirillova, M.V.; Pombeiro, A.J.L. Multicopper complexes and coordination polymers for mild oxidative functionalization of alkanes. Coord. Chem. Rev. 2012, 256, 2741–2759. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Liu, C.-M.; Pombeiro, A.J.L. Trinuclear Cu(II) structural isomers: Coordination, magnetism, electrochemistry and catalytic activity toward oxidation of alkanes. Eur. J. Inorg. Chem. 2015, 2015, 3959–3969. [Google Scholar] [CrossRef]
- Contaldi, S.; di Nicola, C.; Garau, F.; Karabach, Y.Y.; Martins, L.M.D.R.S.; Monari, M.; Pandolfo, L.; Pettinari, C.; Pombeiro, A.J.L. New coordination polymers based on the triangular [Cu3(μ3-OH)(μ-pz)3]2+ unit and unsaturated carboxylates. Dalton Trans. 2009, 4928–4941. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, C.; Garau, F.; Karabach, Y.Y.; Martins, L.M.D.R.S.; Monari, M.; Pandolfo, L.; Pettinari, C.; Pombeiro, A.J.L. Trinuclear triangular copper(II) clusters—Synthesis, electrochemical studies and catalytic peroxidative oxidation of cycloalkanes. Eur. J. Inorg. Chem. 2009, 2009, 666–676. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Nunes, A.C.C.; Mahmudov, K.T.; Haukka, M.; MacLeod, T.C.O.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Complexes of copper(II) with 3-(ortho-substituted phenylhydrazo)pentane-2,4-diones: Syntheses, properties and catalytic activity for cyclohexane oxidation. Dalton Trans. 2011, 40, 2822–2836. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Mukherjee, S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. A cyclic tetranuclear cuboid type copper(II) complex doubly supported by cyclohexane-1,4-dicarboxylate: Molecular and supramolecular structure and cyclohexane oxidation activity. RSC Adv. 2014, 4, 48449–48457. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Pombeiro, A.J.L. Coordination chemistry of arylhydrazones of methylene active compounds. Coord. Chem. Rev. 2013, 257, 1244–1281. [Google Scholar] [CrossRef]
- Sutradhar, M.; Pombeiro, A.J.L. Coordination chemistry of non-oxido, oxido and dioxidovanadium(IV/V) complexes with azine fragment ligands. Coord. Chem. Rev. 2014, 265, 89–24. [Google Scholar] [CrossRef]
- Sutradhar, M.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Liu, C-M.; Pombeiro, A.J.L. Tautomeric effect of hydrazone Schiff bases in tetranuclear Cu(II) complexes: Magnetism and catalytic activity towards mild hydrocarboxylation of alkanes. Dalton Trans. 2013, 42, 16578–16587. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Alegria, E.C.B.A.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Iron(III) and cobalt(III) complexes with both tautomeric (keto and enol) forms of aroylhydrazone ligands: Catalysts for the microwave assisted oxidation of alcohols. RSC Adv. 2016. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Sutradhar, M.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Ribera, A.; Nunes, A.V.M.; Gahramanova, S.I.; Marchetti, F.; Pombeiro, A.J.L. MnII and CuII complexes with arylhydrazones of active methylene compounds as effective heterogeneous catalysts for solvent- and additive-free microwave-assisted peroxidative oxidation of alcohols. RSC Adv. 2015, 5, 25979–25987. [Google Scholar] [CrossRef]
- Nasani, R.; Saha, M.; Mobin, S.M.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; Kirillov, A.M.; Mukhopadhyay, S. Copper–organic frameworks assembled from in situ generated 5-(4-pyridyl)tetrazole building blocks: Synthesis, structural features, topological analysis and catalytic oxidation of alcohols. Dalton Trans. 2014, 43, 9944–9954. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Roy Barman, T.; Klanke, J.; Drew, M.G.B.; Rentschler, E. A novel Cu(II) dimer containing oxime-hydrazone Schiff base ligands with an unusual mode of coordination: Study of magnetic, autoreduction and solution properties. Polyhedron 2013, 53, 48–55. [Google Scholar] [CrossRef]
- Faggi, E.; Gavara, R.; Bolte, M.; Fajarí, L.; Juliá, L.; Rodríguezb, L.; Alfonso, I. Copper(II) complexes of macrocyclic and open-chain pseudopeptidic ligands: Synthesis, characterization and interaction with dicarboxylates. Dalton Trans. 2015, 44, 12700–12710. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(ii) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[l,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Kuznetsov, M.L.; Figiel, P.J.; Karabach, Y.Y.; Luzyanin, K.V.; Pombeiro, A.J.L. Ortho-Hydroxyphenylhydrazo-β-Diketones: Tautomery, Coordination Ability, and Catalytic Activity of Their Copper(II) Complexes toward Oxidation of Cyclohexane and Benzylic Alcohols. Inorg. Chem. 2011, 50, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, M.V.; Kirillov, A.M.; Mandelli, D.; Carvalho, W.A.; Pombeiro, A.J.L.; Shul’pin, G.B. Mild homogeneous oxidation of alkanes and alcohols including glycerol with tert-butyl hydroperoxide catalyzed by a tetracopper(II) complex. J. Catal. 2010, 272, 9–16. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Gradinaru, J.; Kozlov, Y.N. Alkane hydroperoxidation with peroxides catalysed by copper complexes. Org. Biomol. Chem. 2003, 1, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, M.V.; Kozlov, Y.N.; Shul’pina, L.S.; Lyakin, O.Y.; Kirillov, A.M.; Talsi, E.P.; Pombeiro, A.J.L.; Shul’pin, G.B. Remarkably fast oxidation of alkanes by hydrogen peroxide catalyzed by a tetracopper(II) triethanolaminate complex: Promoting effects of acid co-catalysts and water, kinetic and mechanistic features. J. Catal. 2009, 268, 26–38. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kirillova, M.V.; Shul’pina, L.S.; Figiel, P.J.; Gruenwald, K.R.; Guedes da Silva, M.F.C.; Haukka, M.; Pombeiro, A.J.L.; Shul’pin, G.B. Mild oxidative functionalization of alkanes and alcohols catalyzed by new mono- and dicopper(II) aminopolyalcoholates. J. Mol. Catal. A Chem. 2011, 350, 26–34. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kuznetsov, M.L.; Kozlov, Y.N.; Shul’pina, L.S.; Kitaygorodskiy, A.; Pombeiro, A.J.L.; Shul’pin, G.B. Participation of Oligovanadates in Alkane Oxidation with H2O2 Catalyzed by Vanadate Anion in Acidified Acetonitrile: Kinetic and DFT Studies. ACS Catal. 2011, 1, 1511–1520. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A: Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Kudinov, A.R.; Mandelli, D. Extremely Efficient Alkane Oxidation by a New Catalytic Reagent H2O2/Os3(CO)12/Pyridine. Inorg. Chem. 2009, 48, 10480–10482. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Petrovskiy, P.V. Oxidation of alkanes and alcohols with hydrogen peroxide catalyzed by complex Os3(CO)10(µ-H)2. Appl. Organomet. Chem. 2010, 24, 464–472. [Google Scholar] [CrossRef]
- Fernandes, R.R.; Lasri, J.; Kirillov, A.M.; Guedes da Silva, M.F.C.; Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. New FeII and CuII Complexes Bearing Azathia Macrocycles-Catalyst Precursors for Mild Peroxidative Oxidation of Cyclohexane and 1-Phenylethanol. Eur. J. Inorg. Chem. 2011, 3781–3790. [Google Scholar] [CrossRef]
- Milunovic, M.N.M.; Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Pombeiro, A.J.L.; Krachler, R.; Trettenhahn, G.; Turta, C.; Shova, S.; Arion, V.B. Hexanuclear and Undecanuclear Iron(III) Carboxylates as Catalyst Precursors for Cyclohexane Oxidation. Dalton Trans. 2013, 42, 14388–14401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mishra, G.S.; Alegria, E.C.B.; Martins, L.M.D.R.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Cyclohexane oxidation with dioxygen catalyzed by supported pyrazole rhenium complexes. J. Mol. Catal. A 2008, 285, 92–100. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Martins, A.; Alegria, E.C.B.A.; Carvalho, A.P.; Pombeiro, A.J.L. Efficient cyclohexane oxidation with hydrogen peroxide catalysed by a C-scorpionate iron(II) complex immobilized on desilicated MOR zeolite. Appl. Catal. A. 2013, 464, 43–50. [Google Scholar] [CrossRef]
- Zaltariov, M.-F.; Alexandru, M.; Cazacu, M.; Shova, S.; Novitchi, G.; Train, C.; Dobrov, A.; Kirillova, M.V.; Alegria, E.C.B.A.; Pombeiro, A.J.L.; Arion, V.B. Tetranuclear copper(II) complexes with macrocyclic and open-chain disiloxane ligands as catalyst precursors for hydrocarboxylation and oxidation of alkanes and 1-phenylethanol. Eur. J. Inorg. Chem. 2014, 29, 4946–4956. [Google Scholar] [CrossRef]
- Weissermel, K.; Arpe, H.J. Industrial Organic Chemistry, 2nd ed.; VCH Verlagsgesellschaft: Weinheim, Germany, 1993. [Google Scholar]
- Kopylovich, M.N.; Gajewska, M.J.; Mahmudov, K.T.; Kirillova, M.V.; Figiel, P.J.; Guedes da Silva, M.F.C.; Gil-Hernández, B.; Sanchizd, J.; Pombeiro, A.J.L. Copper(II) complexes with a new carboxylic-functionalized arylhydrazone of β-diketone as effective catalysts for acid-free oxidations. New J. Chem. 2012, 36, 1646–1654. [Google Scholar] [CrossRef]
- Palmucci, J.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Marchetti, F.; Pettinari, C.; Pombeiro, A.J.L. Arylhydrazones of barbituric acid: Synthesis, coordination ability and catalytic activity of their CoII, CoII/III and CuII complexes toward peroxidative oxidation of alkanes. RSC Adv. 2015, 5, 84142–84152. [Google Scholar] [CrossRef]
- Sheldon, R.A. E factors, green chemistry and catalysis: An odyssey. Chem. Commun. 2008, 29, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.; Belanzoni, P.; Gamez, P.; Reedijk, J.; Baerends, E.J. Activation of the C-H Bond by Electrophilic Attack: Theoretical Study of the Reaction Mechanism of the Aerobic Oxidation of Alcohols to Aldehydes by the Cu(bipy)2+/2,2,6,6-Tetramethylpiperidinyl-1-oxy Cocatalyst System. Inorg. Chem. 2009, 48, 11909–11920. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, I.N.; Gekham, A.E.; Minin, V.V.; Larin, G.M.; Bashtanov, M.E.; Krasnovskii, A.A.; Moiseev, I.I. Free radical/singlet dioxygen system under the conditions of catalytic hydrogen peroxide decomposition. Kinet. Catal. 2000, 41, 170–177. [Google Scholar] [CrossRef]
- Mattalia, J.M.; Vacher, B.; Samat, A.; Chanon, M.J. Mechanistic investigation of the reaction between α-sulfonyl carbanions and polyhalogenmethanes. Electron transfer versus polar pathways. J. Am. Chem. Soc. 1992, 114, 4111–4119. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Pombeiro, A.J.L. Radical Formation in the [MeReO3]-Catalyzed Aqueous Peroxidative Oxidation of Alkanes: A Theoretical Mechanistic Study. Inorg. Chem. 2009, 48, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B.; Süss-Fink, G. Oxidations by the reagent “H2O2-vanadium complex-pyrazine-2-carboxylic acid”. Part 4. Oxidation of alkanes, benzene and alcohols by an adduct of H2O, with urea. J. Chem. Soc. Perkin Trans. 1995, 2, 1459–1463. [Google Scholar] [CrossRef]
- Shul’pin, G.B. C-H functionalization: Thoroughly tuning ligands at a metal ion, a chemist can greatly enhance catalyst’s activity and selectivity. Dalton Trans. 2013, 42, 12794–12818. [Google Scholar] [CrossRef] [PubMed]
- APEX2; Bruker AXS Inc.: Madison, WI, USA, 2004.
- SAINT; Bruker AXS Inc.: Madison, WI, USA, 2004.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.


| 1 | 2 | 3 | |
|---|---|---|---|
| Empirical formula | C22H29Cu∙N3O7 | C24H35ClCu N2O5 | C46H62Cu2N4O12S |
| Formula Weight | 511.02 | 530.53 | 1022.13 |
| Crystal system | Triclinic | Triclinic | Monoclinic |
| Space group | P-1 | P-1 | C2/c |
| Temperature/K | 296 (2) | 150 (2) | 296 (2) |
| a/Å | 6.8442 (8) | 6.856 (2) | 37.902 (19) |
| b/Å | 8.8397 (10) | 13.166 (5) | 10.738 (4) |
| c/Å | 20.419 (2) | 14.628 (5) | 12.920 (6) |
| α/° | 77.915 (4) | 77.747 (16) | 90 |
| β/° | 88.361 (4) | 86.071 (17) | 109.210 (16) |
| γ/° | 83.059 (3) | 89.369 (17) | 90 |
| V (Å3) | 1199.1 (2) | 1287.2 (8) | 4965 (4) |
| Z | 2 | 2 | 4 |
| Dcalc (g cm−3) | 1.415 | 1.369 | 1.367 |
| µ(Mo Kα) (mm−1) | 0.957 | 0.988 | 0.961 |
| Rfls. collected/unique/observed | 8926/4197/3191 | 33,721/4900/2698 | 19,819/4647/2866 |
| Rint | 0.0401 | 0.1921 | 0.0995 |
| Final R1 a, wR2 b (I ≥ 2σ) | 0.0487, 0.1045 | 0.0564, 0.0949 | 0.0487, 0.1106 |
| Goodness-of-fit on F2 | 1.061 | 0.974 | 1.032 |
| 1 | 2 | 3 | |
|---|---|---|---|
| Cu1—O1 | 1.854 (3) | 1.870 (3) | 1.848 (3) |
| Cu1—O2 | 1.967 (3) | 1.965 (3) | 1.983 (3) |
| Cu1—O4 | 1.964 (3) | - | 1.914 (3) |
| Cu1—N1 | 1.916 (3) | 1.917 (4) | 1.898 (3) |
| Cu1—Cl1 | - | 2.2354 (14) | - |
| C16—O1 | |||
| O1—Cu1—N1 | 93.11 (12) | 92.57 (13) | 93.20 (13) |
| O1—Cu1—O2 | 173.82 (11) | 172.54 (13) | 173.34 (11) |
| O1—Cu1—O4 | 94.30 (12) | - | 95.17 (13) |
| O1—Cu1—Cl1 | - | 95.06 (10) | - |
| O2—Cu1—Cl1 | - | 92.04 (10) | - |
| O4—Cu1—O2 | 91.85 (11) | 91.18 (12) | |
| N1—Cu1—O2 | 80.94 (11) | 80.79 (13) | 80.92 (13) |
| N1—Cu1—O4 | 167.48 (12) | 166.71 (13) | |
| N1—Cu1—Cl1 | 168.18 (11) |
| D—H···A | H···A | D···A | D—H···A |
|---|---|---|---|
| 1 | |||
| N2—H2···O3 | 1.96 (9) | 2.622 (4) | 128 (4) |
| N2—H2···O6 ii | 2.37 (9) | 3.130 (4) | 141 (8) |
| O4—H42···O7 i | 1.94 (3) | 2.797 (4) | 166 (9) |
| O3—H3···O5 vi | 1.69 (9) | 2.600 (4) | 167 (9) |
| O4—H41···O2 v | 2.39 (6) | 2.986 (4) | 126 (8) |
| O4—H41···O4 v | 2.36 (7) | 2.977 (6) | 129 (7) |
| O4—H41···O6 | 2.52 (8) | 2.998 (4) | 116 (6) |
| 2 | |||
| O3—H3···O5 ii | 1.74 (2) | 2.598 (5) | 169 (4) |
| O4—H4D···Cl1 i | 2.52 (2) | 3.360 (3) | 164 (4) |
| O5 ii—H5D ii···Cl1 i | 2.28 (2) | 3.148 (3) | 171 (4) |
| N2—H2···O4 | 2.26 (5) | 2.999 (5) | 151 (4) |
| N2—H2···O3 | 2.11 (4) | 2.643 (5) | 123 (4) |
| 3 | |||
| O6—H6D···O5 ii | 2.43 (6) | 3.166 (5) | 139 (8) |
| N2—H2···O3 | 2.15 (8) | 2.620 (4) | 114 (7) |
| N2—H2···O6 | 2.06 (8) | 2.839 (5) | 151 (8) |
| O3—H3···O5 iv | 1.79 (8) | 2.620 (4) | 177 (10) |
| Entry | Precatalyst | Acid Co-Catalyst b | Reaction Time (h) | Yield (%) c | Total TON [TOF (h−1)] d | ||
|---|---|---|---|---|---|---|---|
| CyOH | CyO | Total | |||||
| 1 | 1 | - | 6 | 10.8 | 0.6 | 11.4 | 114 (19) |
| 2 | - | 24 | 27.9 | 1.5 | 29.4 | 294 (12) | |
| 3 e | - | 24 | 11.0 | 8.3 | 19.3 | 193 (8) | |
| 4 | HNO3 (25) | 6 | 10.4 | 0.9 | 12.3 | 123 (21) | |
| 5 | Hpca (25) | 6 | 12.5 | 1.1 | 13.6 | 136 (23) | |
| 6 | TFA (10) | 6 | 13.4 | 1.1 | 14.5 | 145 (24) | |
| 7 | TFA (25) | 6 | 17.3 | 0.9 | 18.2 | 182 (30) | |
| 8 | 2 | - | 6 | 7.8 | 1.3 | 9.1 | 91 (15) |
| 9 | - | 24 | 18.7 | 2.6 | 21.3 | 213 (9) | |
| 10 e | - | 24 | 6.2 | 4.9 | 11.1 | 111 (5) | |
| 11 | HNO3 (25) | 6 | 13.0 | 1.6 | 14.6 | 146 (24) | |
| 12 | TFA (25) | 6 | 14.2 | 2.0 | 16.2 | 162 (27) | |
| 13 | 3 | - | 6 | 16.4 | 1.6 | 18.0 | 180 (30) |
| 14 | - | 24 | 37.6 | 1.6 | 39.2 | 392 (16) | |
| 15 e | - | 24 | 18.7 | 3.3 | 22.0 | 220 (9) | |
| 16 | HNO3 (25) | 6 | 18.7 | 1.6 | 20.3 | 203 (34) | |
| 17 | TFA (25) | 6 | 22.9 | 2.3 | 25.2 | 252 (42) | |
| 18 f | - | 6 | 1.2 | 0.4 | 1.6 | 16 (3) | |
| 19 g | - | 6 | 0.5 | 0.4 | 0.9 | 9 (2) | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutradhar, M.; Alegria, E.C.B.A.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Aroylhydrazone Cu(II) Complexes in keto Form: Structural Characterization and Catalytic Activity towards Cyclohexane Oxidation. Molecules 2016, 21, 425. https://doi.org/10.3390/molecules21040425
Sutradhar M, Alegria ECBA, Guedes da Silva MFC, Martins LMDRS, Pombeiro AJL. Aroylhydrazone Cu(II) Complexes in keto Form: Structural Characterization and Catalytic Activity towards Cyclohexane Oxidation. Molecules. 2016; 21(4):425. https://doi.org/10.3390/molecules21040425
Chicago/Turabian StyleSutradhar, Manas, Elisabete C. B. A. Alegria, M. Fátima C. Guedes da Silva, Luísa M. D. R. S. Martins, and Armando J. L. Pombeiro. 2016. "Aroylhydrazone Cu(II) Complexes in keto Form: Structural Characterization and Catalytic Activity towards Cyclohexane Oxidation" Molecules 21, no. 4: 425. https://doi.org/10.3390/molecules21040425
APA StyleSutradhar, M., Alegria, E. C. B. A., Guedes da Silva, M. F. C., Martins, L. M. D. R. S., & Pombeiro, A. J. L. (2016). Aroylhydrazone Cu(II) Complexes in keto Form: Structural Characterization and Catalytic Activity towards Cyclohexane Oxidation. Molecules, 21(4), 425. https://doi.org/10.3390/molecules21040425