From Nanorods to Nanowires of CdS Synthesized by a Solvothermal Method: Influence of the Morphology on the Photoactivity for Hydrogen Evolution from Water
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Characterization
2.1.1. TXRF and Textural Analyses
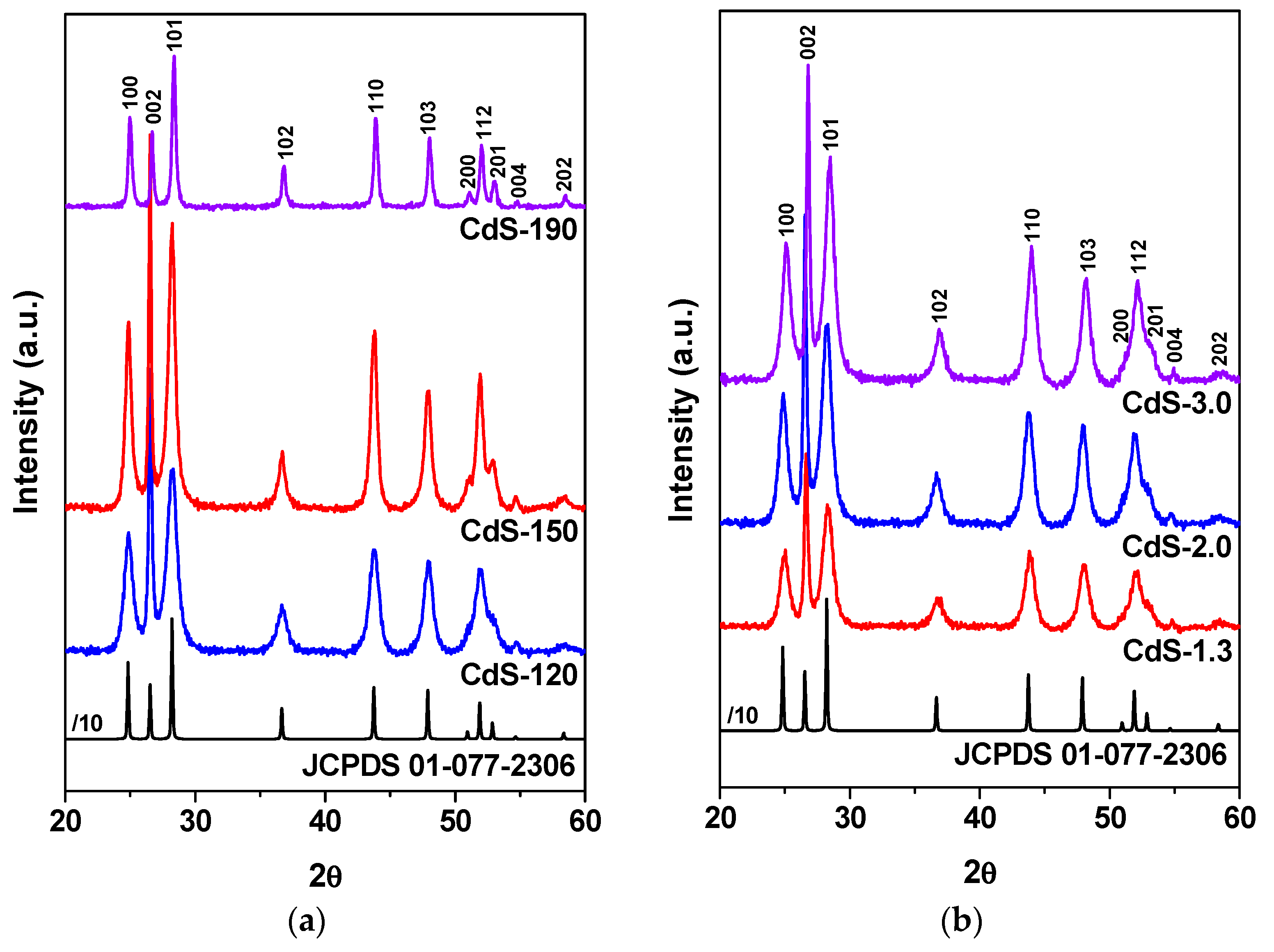
2.1.2. Powder X-ray Diffraction (XRD) Analysis
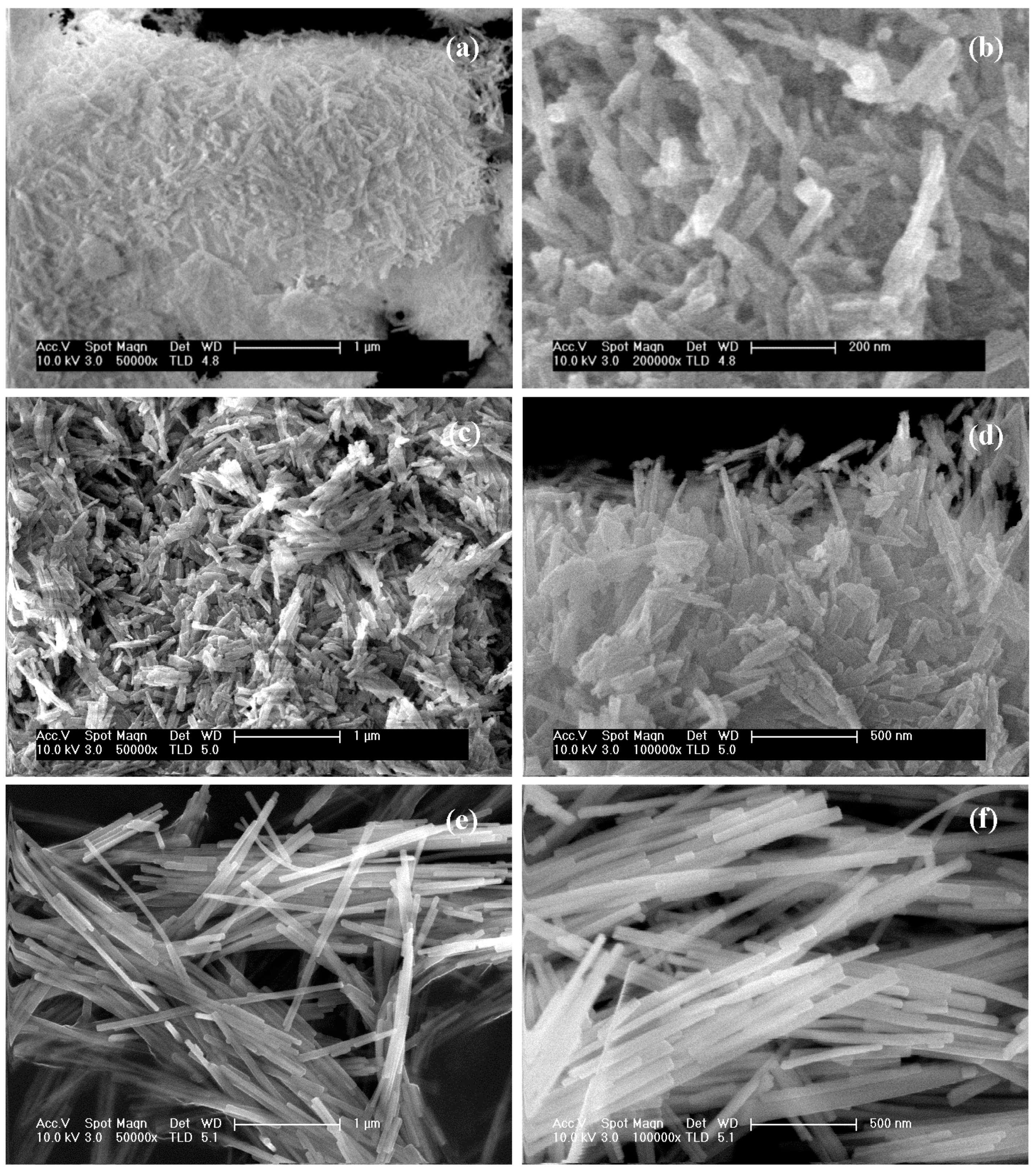
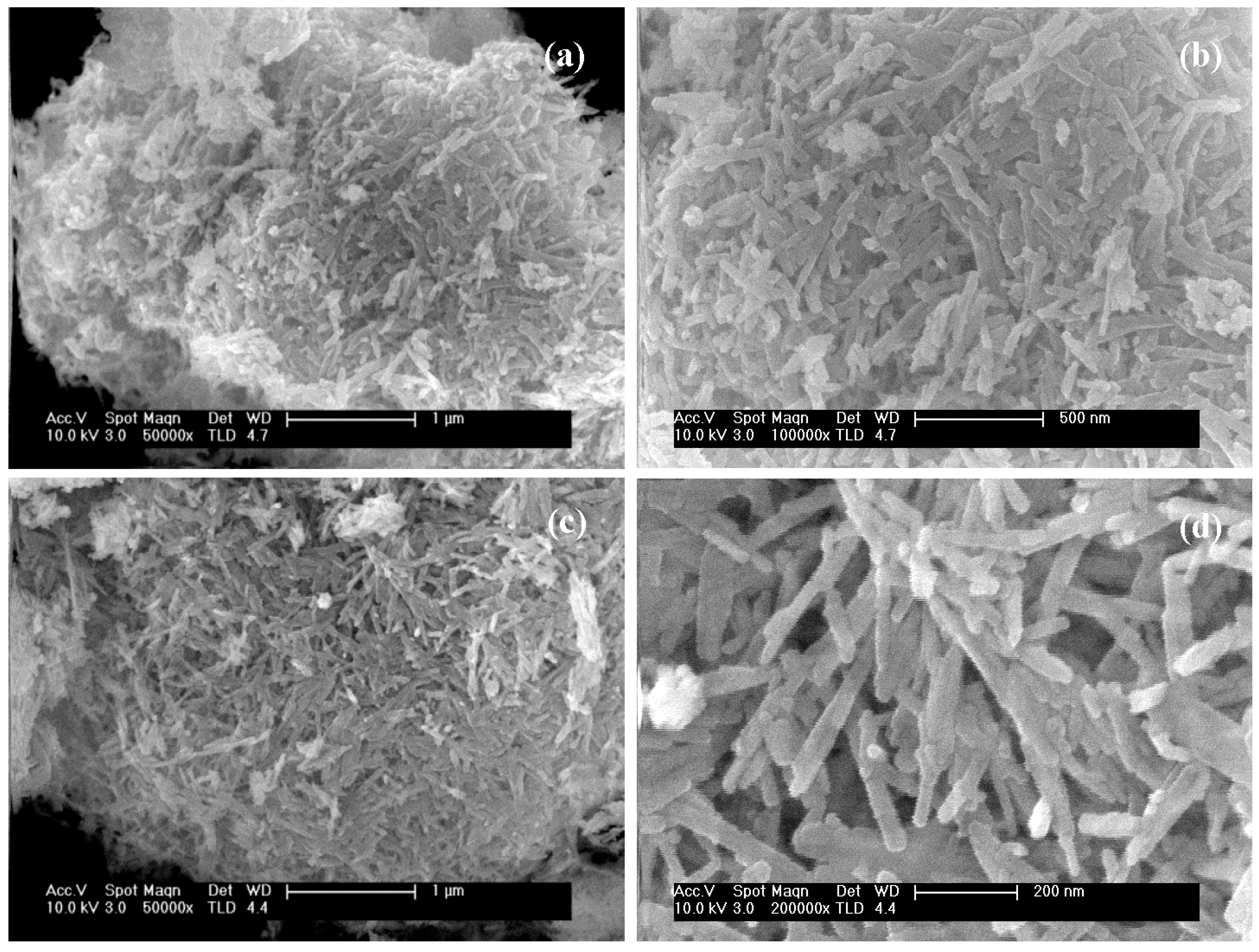
2.1.3. FE-SEM Analysis
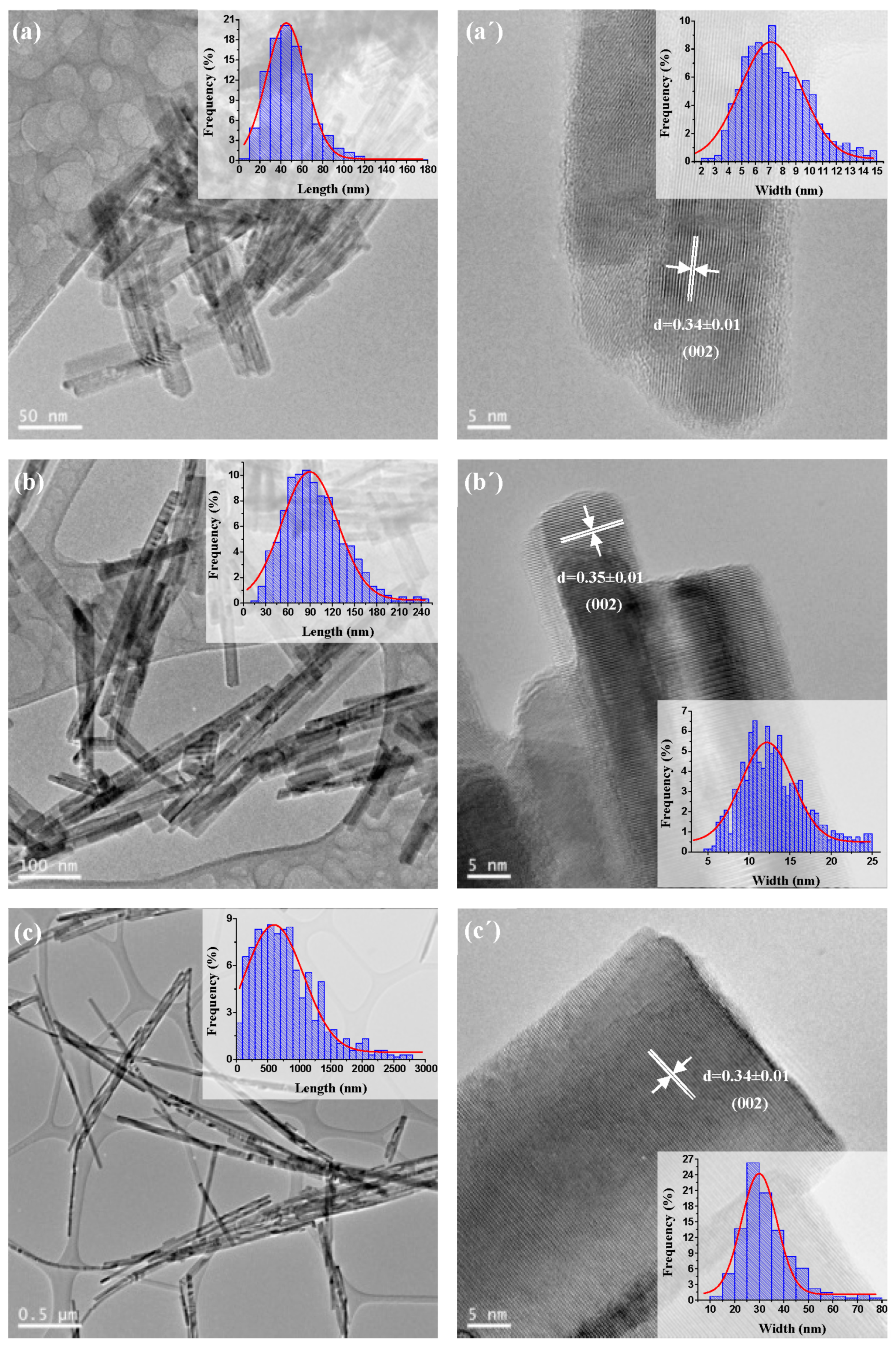
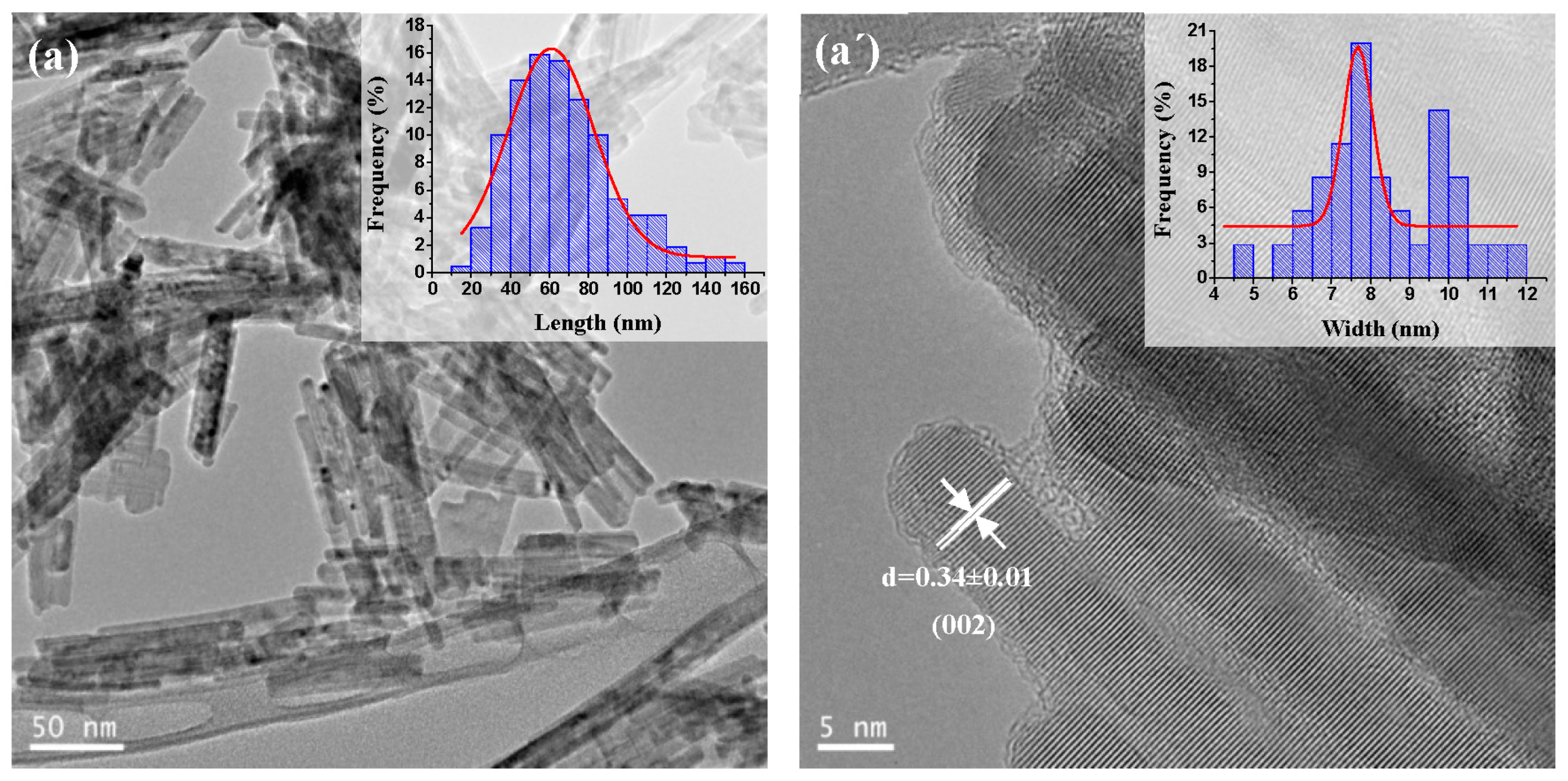
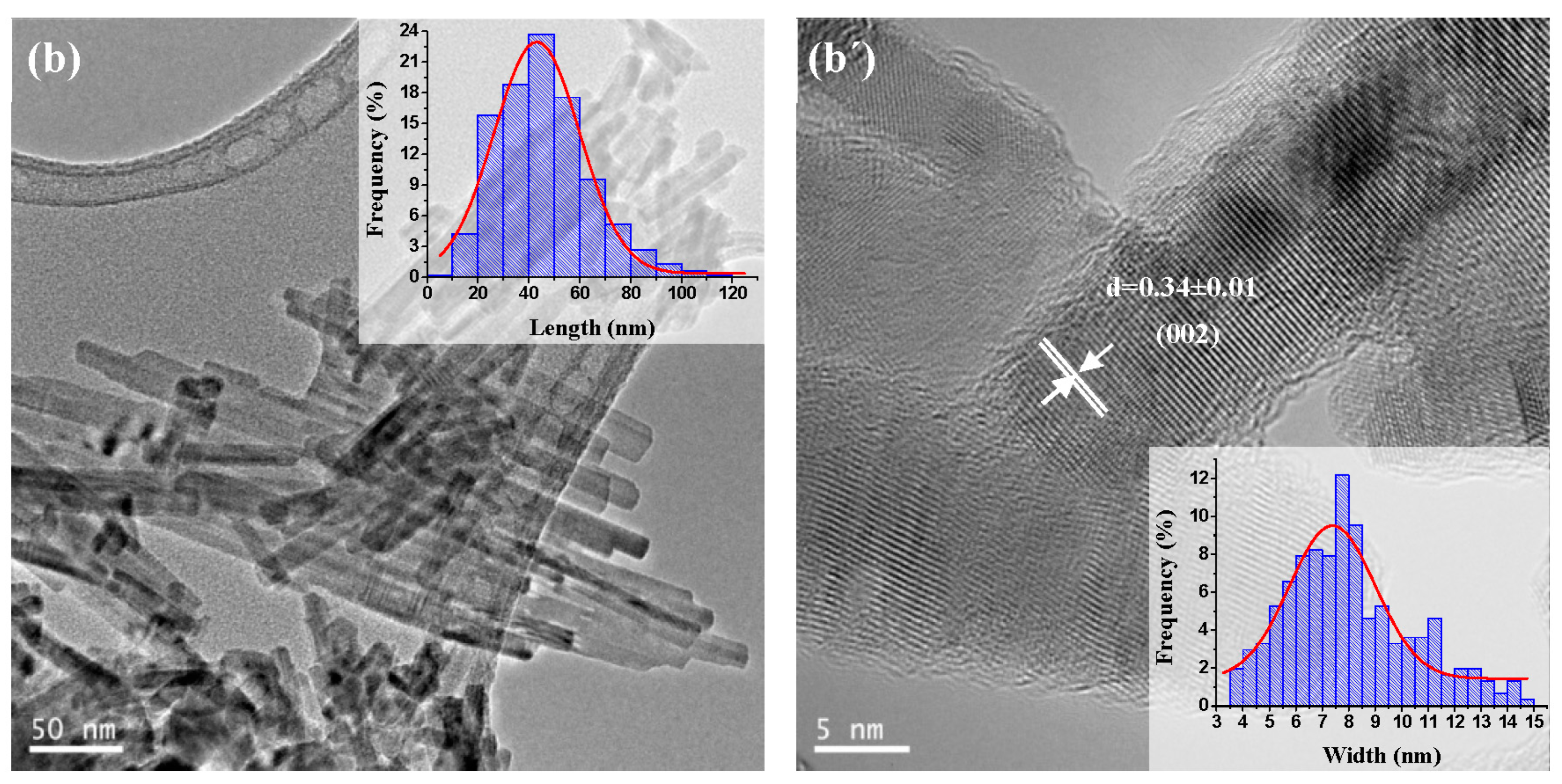
2.1.4. TEM and HRTEM Analysis
2.1.5. UV-Vis Diffuse Reflectance Spectra Analysis
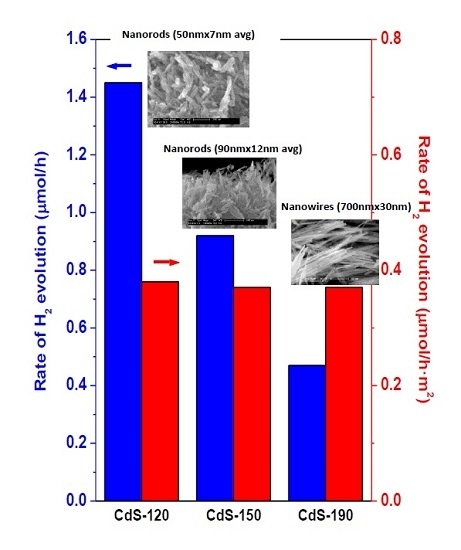
2.2. Photocatalytic Activity
3. Discussion
4. Materials and Methods
4.1. CdS Synthesis
4.2. CdS Characterization
4.3. Photocatalytic Activity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Osterloh, F.E. Inorganic materials as catalysts for photochemical splitting of water. Chem. Mater. 2007, 20, 35–54. [Google Scholar] [CrossRef]
- Rajeshwar, K. Hydrogen generation at irradiated oxide semiconductor-solution interfaces. J. Appl. Electrochem. 2007, 37, 765–787. [Google Scholar] [CrossRef]
- Darwent, J.R.; Porter, G. Photochemical hydrogen production using cadmium sulphide suspensions in aerated water. J. Chem. Soc. Chem. Commun. 1981, 145–146. [Google Scholar] [CrossRef]
- Navarro Yerga, R.M.; Alvarez-Galván, M.C.; Vaquero, F.; Arenales, J.; Fierro, J.L.G. Hydrogen production from water splitting using photo-semiconductor catalysts. In Renewable Hydrogen Technologies, 1st ed.; Gandía, L.M., Arzamendi, G., Diéguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Chapter 3; pp. 43–61. [Google Scholar]
- Borrell, L.; Cervera-March, S.; Giménez, J.; Simarro, R.; Andújar, J.M. A comparative study of CdS-based semiconductor photocatalysts for solar hydrogen production from sulphide + sulphite substrates. Sol. Energy Mater. Sol. Cells 1992, 25, 25–39. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Matsumura, M.; Saho, Y.; Tsubomura, H. Photocatalytic hydrogen production from solutions of sulfite using platinized cadmium sulfide powder. J. Phys. Chem. 1983, 87, 3807–3808. [Google Scholar] [CrossRef]
- Reber, J.F.; Rusek, M. Photochemical hydrogen production with platinized suspensions of cadmium sulfide and cadmium zinc sulfide modified by silver sulfide. J. Phys. Chem. 1986, 90, 824–834. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P. Alternate synthetic strategy for the preparation of CdS nanoparticles and its exploitation for water splitting. Int. J. Hydrog. Energy 2006, 31, 891–898. [Google Scholar] [CrossRef]
- Sinha, A.S.K.; Sahu, N.; Arora, M.K.; Upadhyay, S.N. Preparation of egg-shell type Al2O3-supported CdS photocatalysts for reduction of H2O to H2. Catal. Today 2001, 69, 297–305. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Pal, B.; Torimoto, T.; Iwasaki, K.; Shibayama, T.; Takahashi, H.; Ohtani, B. Size and structure-dependent photocatalytic activity of jingle-bell-shaped silica-coated cadmium sulfide nanoparticles for methanol dehydrogenation. J. Phys. Chem. B 2004, 108, 18670–18674. [Google Scholar] [CrossRef]
- Young-Jin, C.; Kyung-Soo, P.; Jae-Gwan, P. Network-bridge structure of CdSxSe1−x nanowire-based optical sensors. Nanotechnology 2010, 21, 505605. [Google Scholar]
- Mahdi, M.A.; Hassan, J.J.; Ng, S.S.; Hassan, Z.; Ahmed, N.M. Synthesis and characterization of single-crystal CdS nanosheet for high-speed photodetection. Phys. E Low-Dimens. Syst. Nanostruct. 2012, 44, 1716–1721. [Google Scholar] [CrossRef]
- Rai, P.; Song, H.-M.; Kim, Y.-S.; Song, M.-K.; Oh, P.-R.; Yoon, J.-M.; Yu, Y.-T. Microwave assisted hydrothermal synthesis of single crystalline ZnO nanorods for gas sensor application. Mater. Lett. 2012, 68, 90–93. [Google Scholar] [CrossRef]
- Aboulaich, A.; Billaud, D.; Abyan, M.; Balan, L.; Gaumet, J.-J.; Medjadhi, G.; Ghanbaja, J.; Schneider, R. One-pot noninjection route to CdS quantum dots via hydrothermal synthesis. ACS Appl. Mater. Interfaces 2012, 4, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Einevoll, G.T. Confinement of excitons in quantum dots. Phys. Rev. B 1992, 45, 3410–3417. [Google Scholar] [CrossRef]
- Dalvand, P.; Mohammadi, M.R. Controlling morphology and structure of nanocrystalline cadmium sulfide (CdS) by tailoring solvothermal processing parameters. J. Nanopart. Res. 2011, 13, 3011–3018. [Google Scholar] [CrossRef]
- Demazeau, G. Solvothermal processes: New trends in materials chemistry. J. Phys. Conf. Ser. 2008, 121, 082003. [Google Scholar] [CrossRef]
- Mahdi, M.A.; Hassan, J.J.; Ng, S.S.; Hassan, Z. Growth of CdS nanosheets and nanowires through the solvothermal method. J. Cryst. Growth 2012, 359, 43–48. [Google Scholar] [CrossRef]
- Yan, S.; Sun, L.; Qu, P.; Huang, N.; Song, Y.; Xiao, Z. Synthesis of uniform CdS nanowires in high yield and its single nanowire electrical property. J. Solid State Chem. 2009, 182, 2941–2945. [Google Scholar] [CrossRef]
- Li, Y.; Huang, F.; Zhang, Q.; Gu, Z. Solvothermal synthesis of nanocrystalline cadmium sulfide. J. Mater. Sci. 2000, 35, 5933–5937. [Google Scholar] [CrossRef]
- Yu, J.; Yu, Y.; Zhou, P.; Xiao, W.; Cheng, B. Morphology-dependent photocatalytic H2-production activity of CdS. Appl. Catal. B Environ. 2014, 156–157, 184–191. [Google Scholar] [CrossRef]
- Lang, D.; Xiang, Q.; Qiu, G.; Feng, X.; Liu, F. Effects of crystalline phase and morphology on the visible light photocatalytic H2-production activity of CdS nanocrystals. Dalton Trans. 2014, 43, 7245–7253. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Joshi, U.A.; Lee, J.S. Solvothermal synthesis of CdS nanowires for photocatalytic hydrogen and electricity production. J. Phys. Chem. C 2007, 111, 13280–13287. [Google Scholar] [CrossRef]
- Qingqing, W.; Gang, X.; Gaorong, H. Solvothermal synthesis and characterization of uniform CdS nanowires in high yield. J. Solid State Chem. 2005, 178, 2680–2685. [Google Scholar] [CrossRef]
- Zou, G.; Li, H.; Zhang, Y.; Xiong, K.; Qian, Y. Solvothermal/hydrothermal route to semiconductor nanowires. Nanotechnology 2006, 17, S313. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark Alexander, V.; Olivier James, P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing Kenneth, S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Nie, L.; Meng, A.; Yu, J.; Jaroniec, M. Hierarchically macro-mesoporous Pt/γ-Al2O3 composite microspheres for efficient formaldehyde oxidation at room temperature. Sci. Rep. 2013, 3, 3215. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, D.; Guo, L.; Fu, F.; Zhang, Z.; Wei, Q. Template-free hydrothermal synthesis of novel three-dimensional dendritic CdS nanoarchitectures. J. Phys. Chem. C 2009, 113, 5984–5990. [Google Scholar] [CrossRef]
- Jianxi, Y.; Gaoling, Z.; Dan, W.; Gaorong, H. Solvothermal synthesis and characterization of CdS nanowires/PVA composite films. Mater. Lett. 2005, 59, 3652–3655. [Google Scholar] [CrossRef]
- Mo, D.; Liu, J.; Yao, H.J.; Duan, J.L.; Hou, M.D.; Sun, Y.M.; Chen, Y.F.; Xue, Z.H.; Zhang, L. Preparation and characterization of CdS nanotubes and nanowires by electrochemical synthesis in ion-track templates. J. Cryst. Growth 2008, 310, 612–616. [Google Scholar] [CrossRef]
- Fan, Y.; Deng, M.; Chen, G.; Zhang, Q.; Luo, Y.; Li, D.; Meng, Q. Effect of calcination on the photocatalytic performance of CdS under visible light irradiation. J. Alloys Compd. 2011, 509, 1477–1481. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Ding, L.; Ge, W.K.; Chen, H.Y.; Gwo, S. Green, yellow, and orange defect emission from ZnO nanostructures: Influence of excitation wavelength. Appl. Phys. Lett. 2006, 88, 103107. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, J.-H.; Yu, S.-H.; Yang, L.; Zhou, G.-E.; Qian, Y.-T. Formation process of CdS nanorods via solvothermal route. Chem. Mater. 2000, 12, 3259–3263. [Google Scholar] [CrossRef]
- Shanmugapriya, T.; Vinayakan, R.; Thomas, K.G.; Ramamurthy, P. Synthesis of CdS nanorods and nanospheres: Shape tuning by the controlled addition of a sulfide precursor at room temperature. CrystEngComm 2011, 13, 2340–2345. [Google Scholar] [CrossRef]
- Navarro, R.M.; del Valle, F.; Villoria de la Mano, J.A.; Álvarez-Galván, M.C.; Fierro, J.L.G. Photocatalytic water splitting under visible light: Concept and catalysts development. In Advances in Chemical Engineering; Hugo, I.D.L., Benito Serrano, R., Eds.; Academic Press: Burlington, MA, USA, 2009; Volume 36, pp. 111–143. [Google Scholar]
- Navarro Yerga, R.M.; Alvarez Galvan, M.C.; del Valle, F.; Villoria de la Mano, J.A.; Fierro, J.L. Water splitting on semiconductor catalysts under visible-light irradiation. ChemSusChem 2009, 2, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Ji, S.M.; Bae, S.W.; Son, H.C.; Lee, J.S. Optimization of CdS/TiO2 nano-bulk composite photocatalysts for hydrogen production from Na2S/Na2SO3 aqueous electrolyte solution under visible light (λ ≥ 420 nm). J. Photochem. Photobiol. A Chem. 2007, 188, 112–119. [Google Scholar] [CrossRef]
- Thiruvengadathan, R.; Regev, O. Hierarchically ordered cadmium sulfide nanowires dispersed in aqueous solution. Chem. Mater. 2005, 17, 3281–3287. [Google Scholar] [CrossRef]
- Tsuji, I.; Kato, H.; Kobayashi, H.; Kudo, A. Photocatalytic H2 evolution reaction from aqueous solutions over band structure-controlled (AgIn)xZn2(1−x)S2 solid solution photocatalysts with visible-light response and their surface nanostructures. J. Am. Chem. Soc. 2004, 126, 13406–13413. [Google Scholar] [CrossRef] [PubMed]
- Reber, J.F.; Meier, K. Photochemical production of hydrogen with zinc sulfide suspensions. J. Phys. Chem. 1984, 88, 5903–5913. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds CdS-120, CdS-150 and CdS-190 are available from the authors.
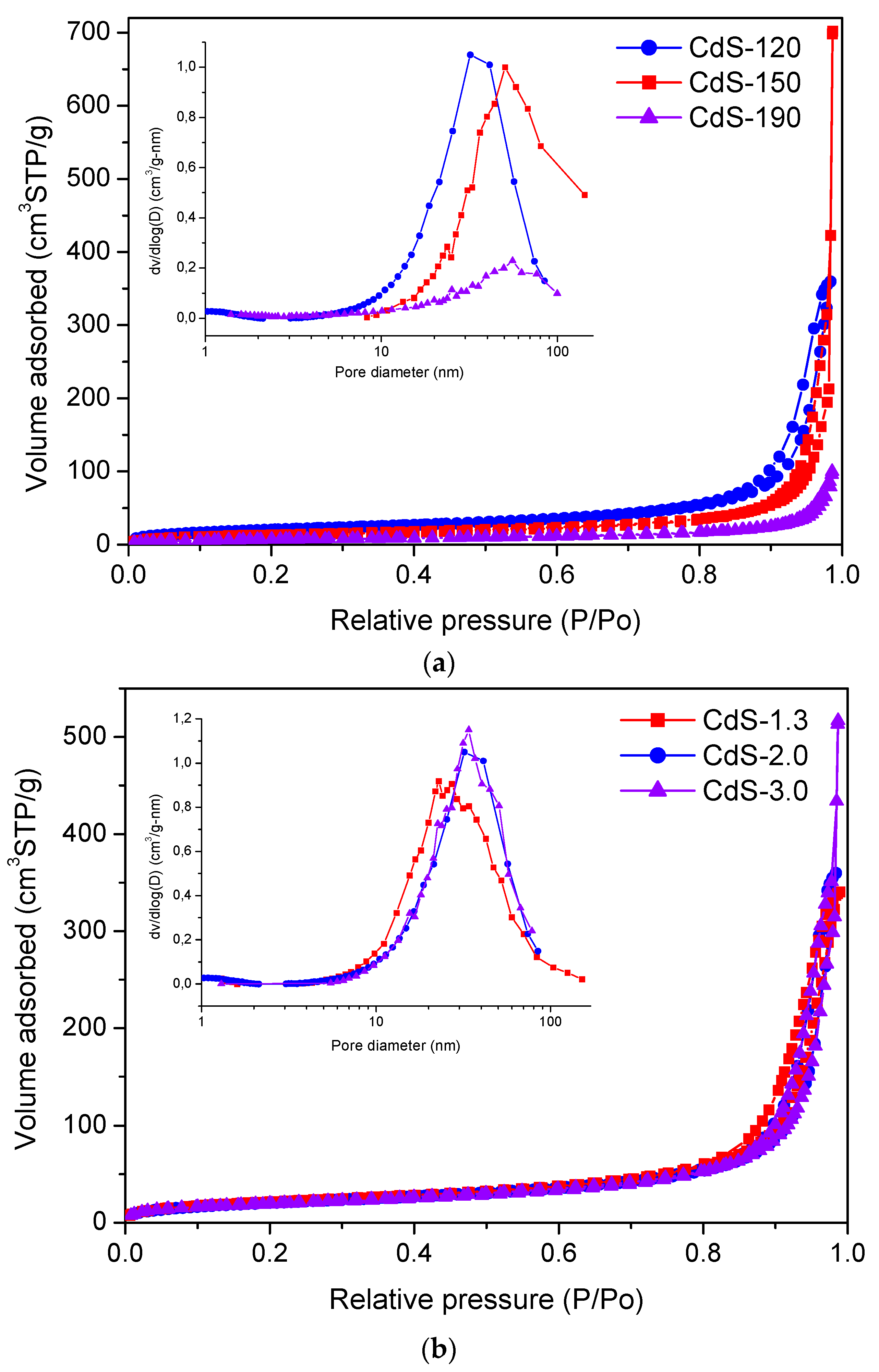
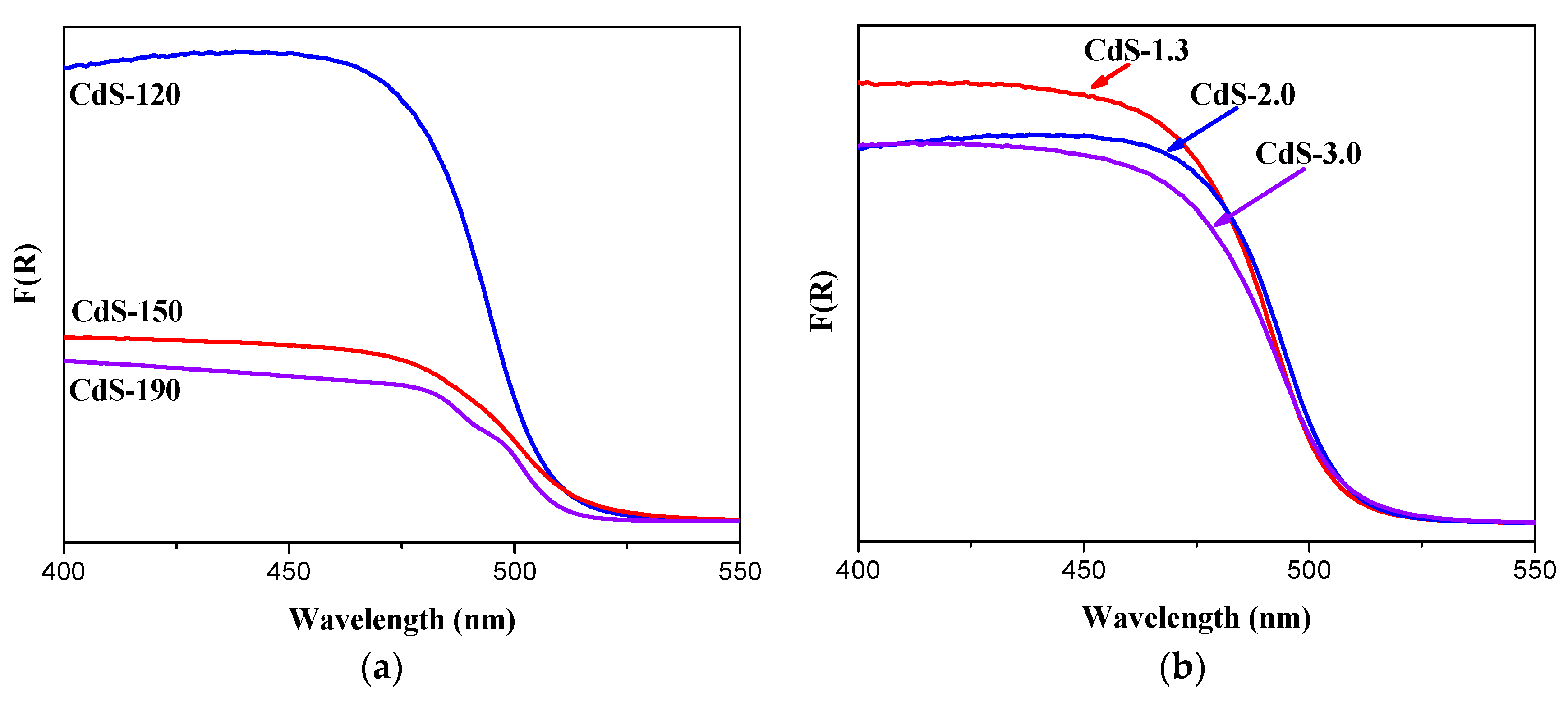
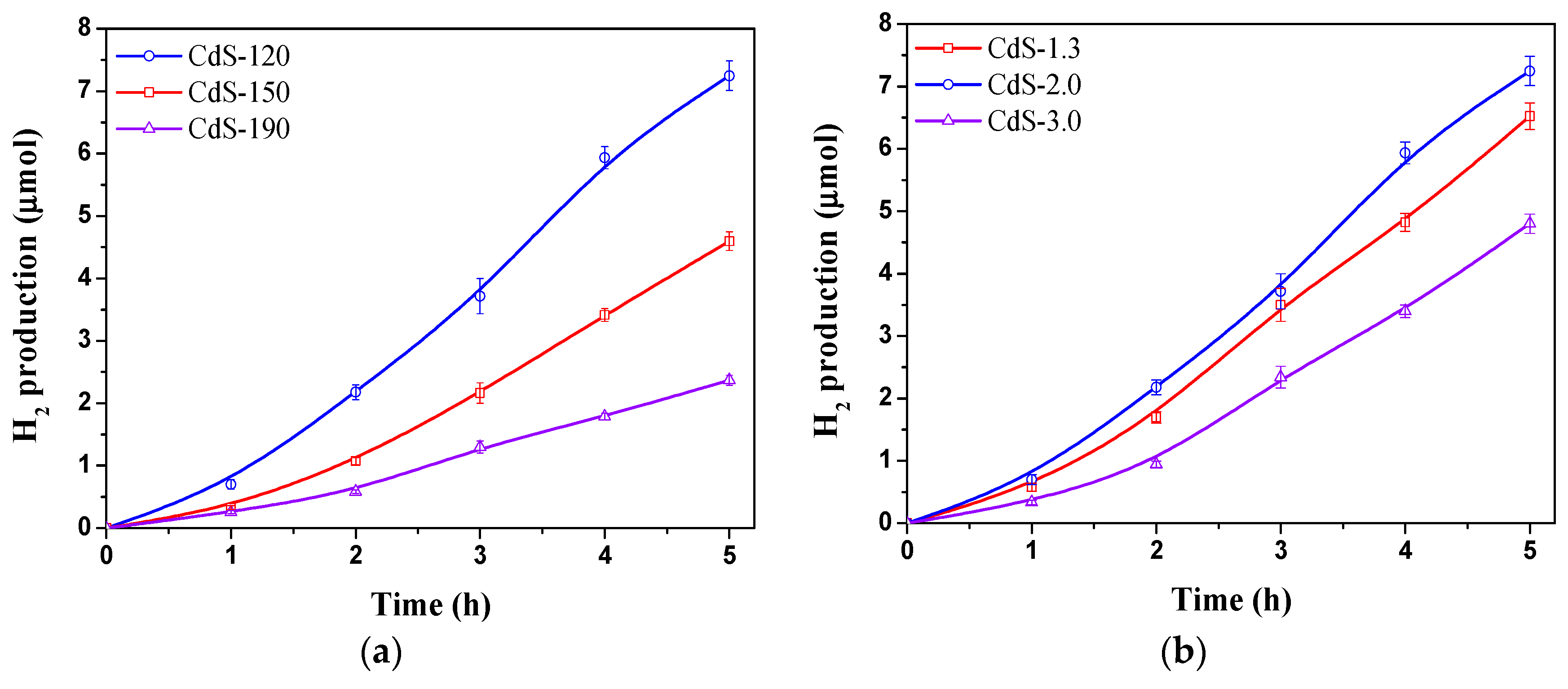
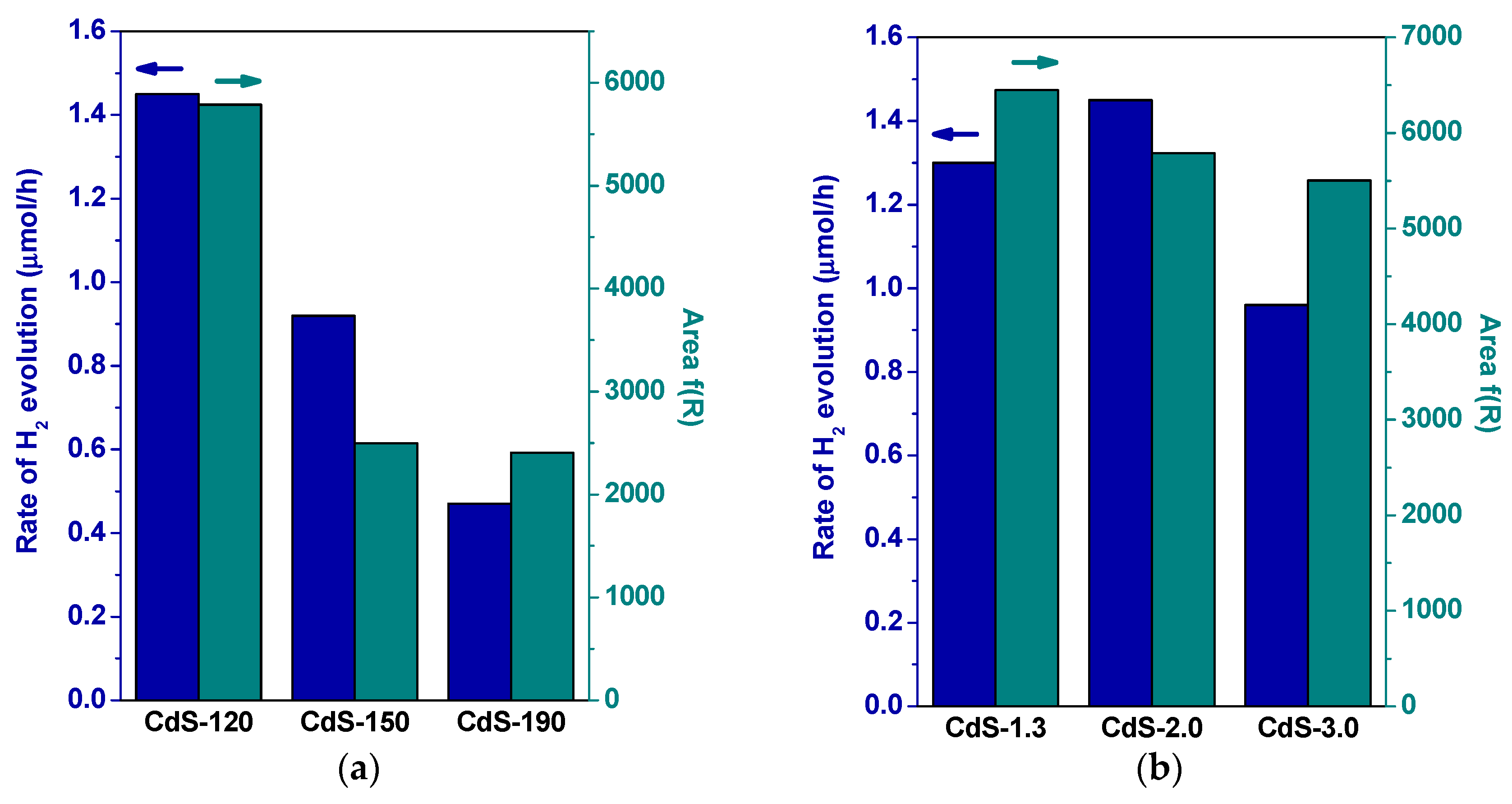

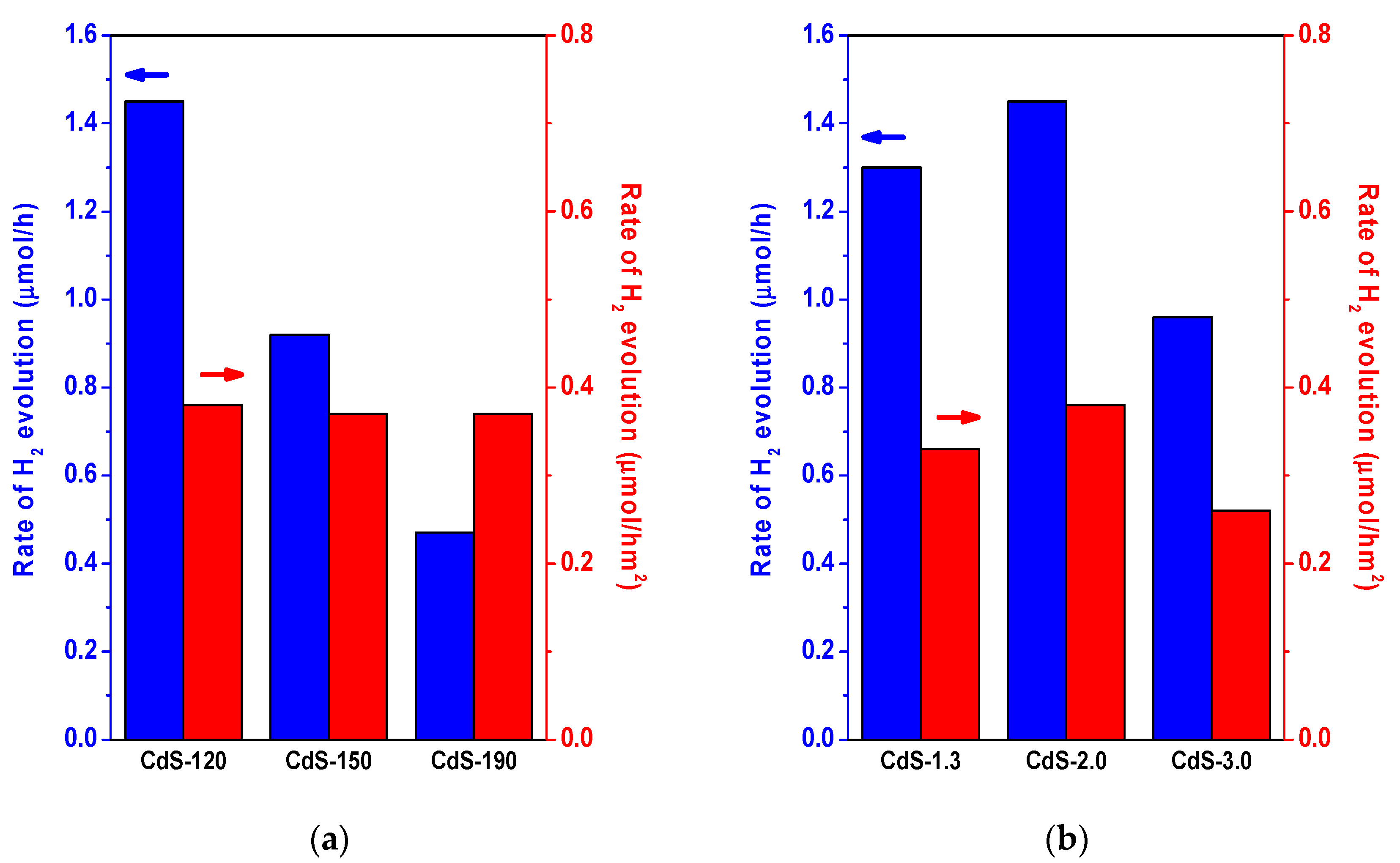
| CdS Sample | Surface Composition | BET (m2/g) | Band Gap (eV) | |
|---|---|---|---|---|
| Cd (%) | S (%) | |||
| CdS-120 | 46.1 | 53.9 | 75.5 | 2.49 |
| CdS-150 | 48.2 | 51.8 | 49.7 | 2.45 |
| CdS-190 | 46.7 | 53.3 | 25.7 | 2.48 |
| CdS-1.3 | 45.8 | 54.2 | 78.9 | 2.50 |
| CdS-2.0 | 46.1 | 53.9 | 75.5 | 2.49 |
| CdS-3.0 | 46.8 | 53.2 | 72.4 | 2.49 |
| CdS Sample | Crystal Structure | Relative Intensity | Dp (nm) | |
|---|---|---|---|---|
| I(100)/I(002) | I(101)/I(002) | |||
| CdS-120 | Hexagonal | 0.44 | 0.65 | 43.5 |
| CdS-150 | Hexagonal | 0.53 | 0.78 | 61.0 |
| CdS-190 | Hexagonal | 1.04 | 1.58 | 98.3 |
| CdS-1.3 | Hexagonal | 0.40 | 0.67 | 38.3 |
| CdS-2.0 | Hexagonal | 0.44 | 0.65 | 43.5 |
| CdS-3.0 | Hexagonal | 0.42 | 0.70 | 45.2 |
| CdS Sample | Morphology | Size (µm) |
|---|---|---|
| CdS-120 | Sheets with emerging irregular filaments | 0.4–6.0 |
| CdS-150 | Irregular clusters of filaments | 0.7–12.0 |
| CdS-190 | Defined filaments | 4.0–30.0 |
| CdS-1.3 | Sheets with emerging irregular filaments | 0.3–4.0 |
| CdS-2.0 | Sheets with emerging irregular filaments | 0.4–6.0 |
| CdS-3.0 | Sheets with defined filaments | 0.7–8.0 |
| CdS Samples | Average Length (nm) | Average Width (nm) | Aspect Ratio (Length/Width) | Morphology |
|---|---|---|---|---|
| CdS-120 | 50 | 7.0 | 7.1 | nanorods |
| CdS-150 | 90 | 12.0 | 7.5 | nanorods |
| CdS-190 | 700 | 30.0 | 23.3 | nanowires |
| CdS-1.3 | 60 | 7.5 | 8.0 | nanorods |
| CdS-2.0 | 50 | 7.0 | 7.1 | nanorods |
| CdS-3.0 | 45 | 7.2 | 6.2 | nanorods |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaquero, F.; G. Fierro, J.L.; Navarro Yerga, R.M. From Nanorods to Nanowires of CdS Synthesized by a Solvothermal Method: Influence of the Morphology on the Photoactivity for Hydrogen Evolution from Water. Molecules 2016, 21, 401. https://doi.org/10.3390/molecules21040401
Vaquero F, G. Fierro JL, Navarro Yerga RM. From Nanorods to Nanowires of CdS Synthesized by a Solvothermal Method: Influence of the Morphology on the Photoactivity for Hydrogen Evolution from Water. Molecules. 2016; 21(4):401. https://doi.org/10.3390/molecules21040401
Chicago/Turabian StyleVaquero, Fernando, José Luis G. Fierro, and Rufino M. Navarro Yerga. 2016. "From Nanorods to Nanowires of CdS Synthesized by a Solvothermal Method: Influence of the Morphology on the Photoactivity for Hydrogen Evolution from Water" Molecules 21, no. 4: 401. https://doi.org/10.3390/molecules21040401
APA StyleVaquero, F., G. Fierro, J. L., & Navarro Yerga, R. M. (2016). From Nanorods to Nanowires of CdS Synthesized by a Solvothermal Method: Influence of the Morphology on the Photoactivity for Hydrogen Evolution from Water. Molecules, 21(4), 401. https://doi.org/10.3390/molecules21040401