We have studied the effects of several plant extracts on four human cell lines, namely MCF-7 (breast cancer), A549 (lung adenocarcinoma), U-937 (histiocytic lymphoma) and TK6 (human B lymphoblastoid cells). The first two cell lines are anchorage-dependent, while the second two grow in suspension. In order to evaluate the in vitro cytotoxic potential of the plant extracts, the effects of different dilutions of each extracts were first analyzed by Trypan Blue exclusion assay, on the adherent cell lines (data not shown). Analysis of these data allowed for the selection of the appropriate dilution of the extracts. In addition, evident cellular effects (partial detachment, floating and changes in morphology) were observed incubating MCF7 and A549 cells with extract from J. communis or C. coggygria. These effects were much less pronounced, when cells were incubated with diluted extracts from E. hyemale or P. scolopendrium. Hence, while focusing our attention on J. communis or C. coggygria, we have used, when needed, the extracts from E. hyemale or P. scolopendrium, as controls.
2.1. Cell Viability and Growth
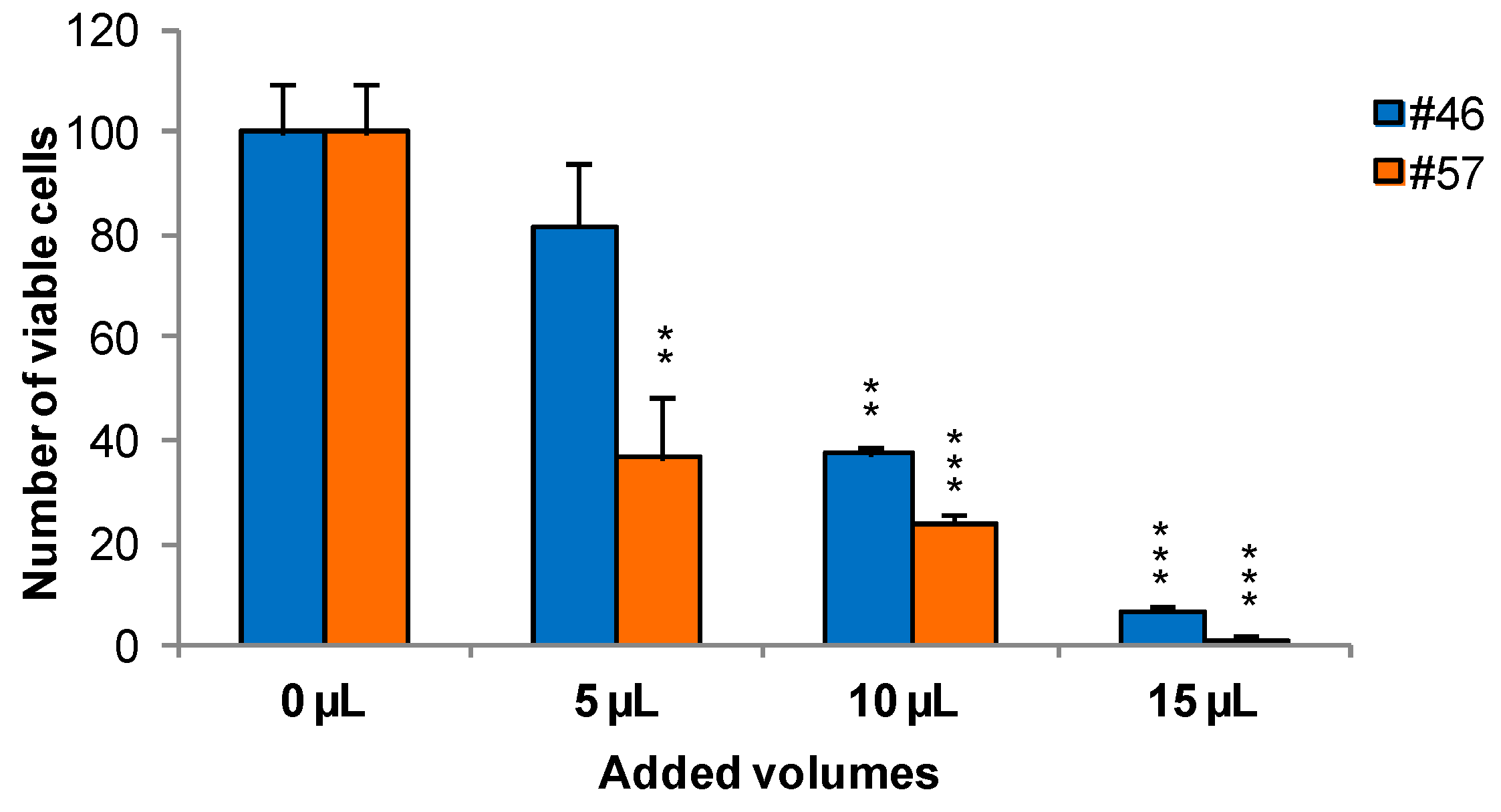
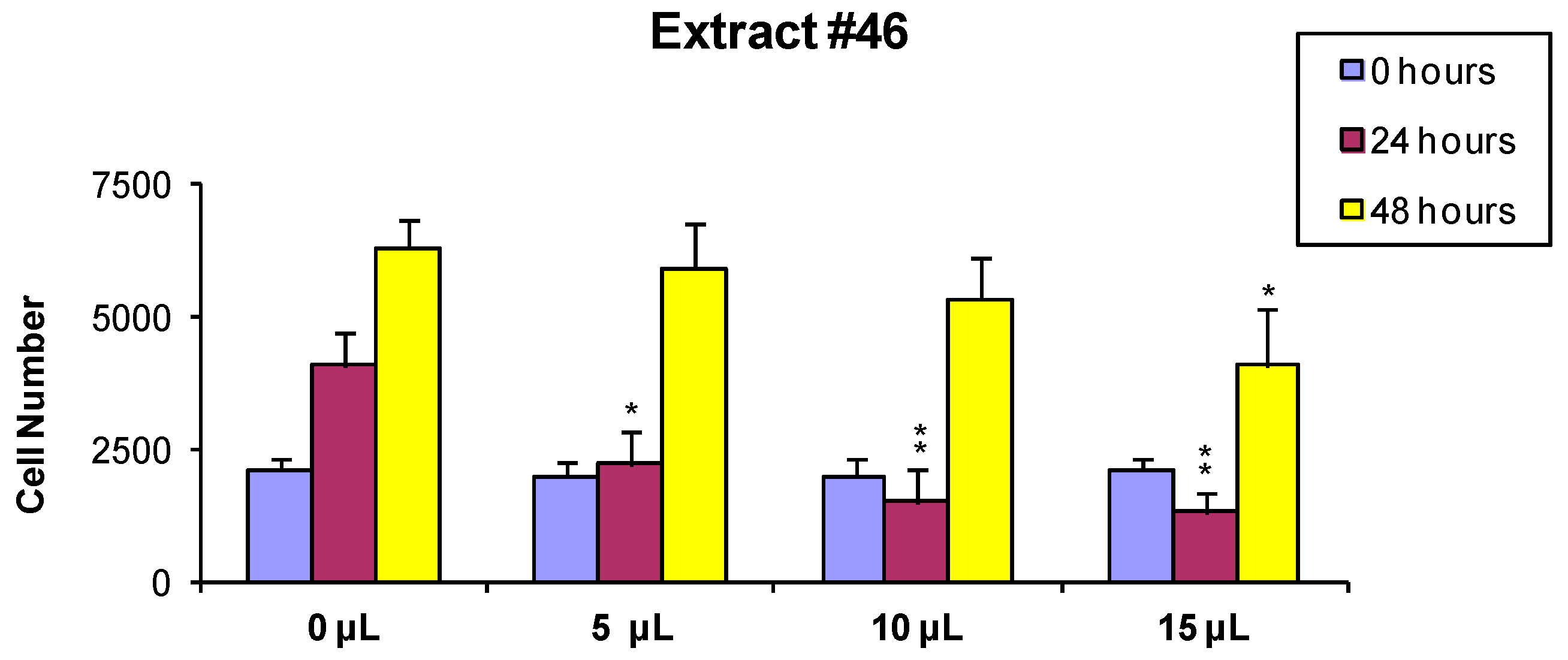
To measure the effects of extracts on cells viability and growth, MCF-7 was selected as an illustrative example cell line. Cells were treated for 24 h with extracts #46 (
J. communis) and #57 (
C. coggygria), at 0.05%, 0.1%, and 0.15%
v/
v; the concentrations are expressed as percent
v/
v of the saturated solutions at room temperature (
i.e., a solution that contains the maximum concentration of solutes. Additional solute will not dissolve in a saturated solution) as also reiterated in
Section 3.2. At the end of this incubation, Trypan blue solution was added and the cells were counted using a hemocytometer. Data indicate that extracts affect cell viability in a dose-dependent manner. In addition, it appears that extract #57 is more toxic to cells than extract #46 (
Figure 1).
In order to evaluate if the observed cytotoxic effects were reversible or irreversible, MCF7 cells were incubated for 24 h with the extracts, as described above, and the cells surviving the treatment were washed and released in fresh medium, and further analyzed 24 and 48 h later As shown in
Figure 2 and
Figure 3, the effect of extract #46 on cell proliferation was reversible, while that of extract #57 was irreversible at higher concentration.
These data, on the whole, demonstrate that both J. communis and C. coggygria extracts affect cell viability in a dose- and time-dependent manner. The extract #57, in addition, when used at elevated concentration (≥15 µL/mL) induces an irreversible growth arrest and cell death.
2.2. Effects of the Extracts on Cell Cycle
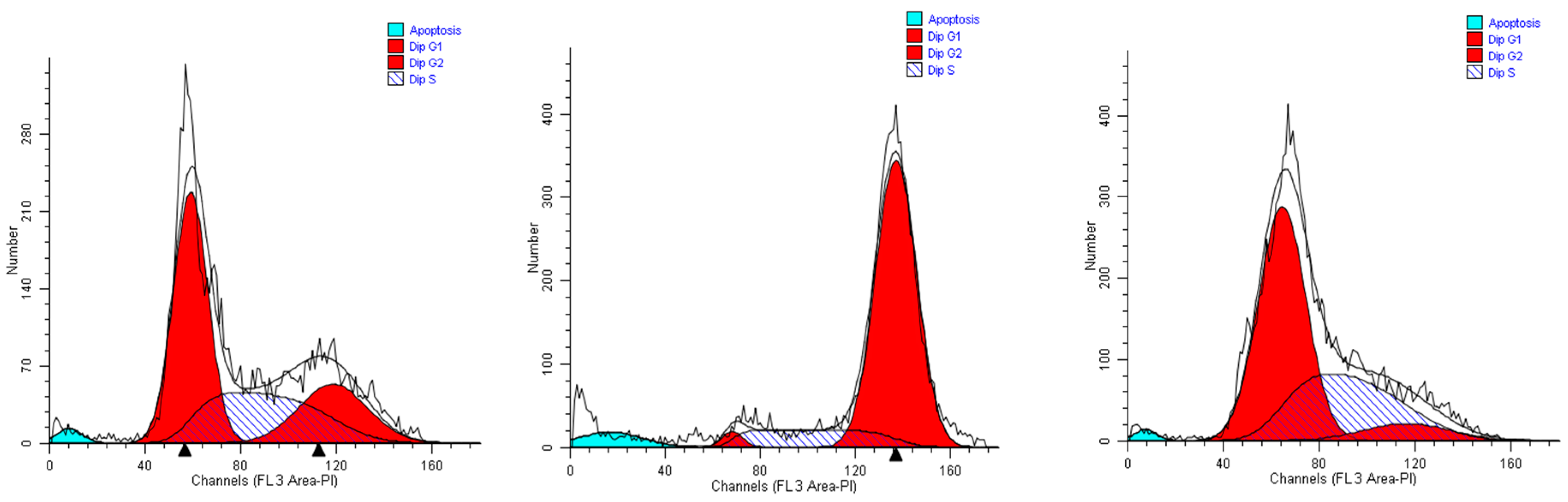
We first studied the alterations in the cell cycle profile of A549 cells treated with the plant extracts for 24 h. We show data obtained with the highest life-compatible concentration explored (0.15% v/v). In such conditions, the cell cycle profiles displayed significant alterations.
When A549 cells were incubated with the extract #46 for 24 h, a significant number of cells detached, while the others changed morphology assuming a rounded shape. Accordingly, cell cycle analyses showed an accumulation of cells in the G2/M phase (from <8% in normal proliferating cells to about 90% in extract #46-treated cells) (
Figure 4, middle panel) This effect was reversible as, replacing the cells media with fresh media (not containing the extracts), cells regain a normal phenotype (morphology) and then appear to regain their grow potential.
In contrast, when the same cells were treated with extract #57 at low concentrations (
i.e., 0.15% (
v/
v), an accumulation of cells in G1 phase of cell cycle was observed (+20% as compared to controls), associated with a reduction of cells in S phase (
Figure 4, right panel). Even in this case, replacing the media, the cells return cycling normally.
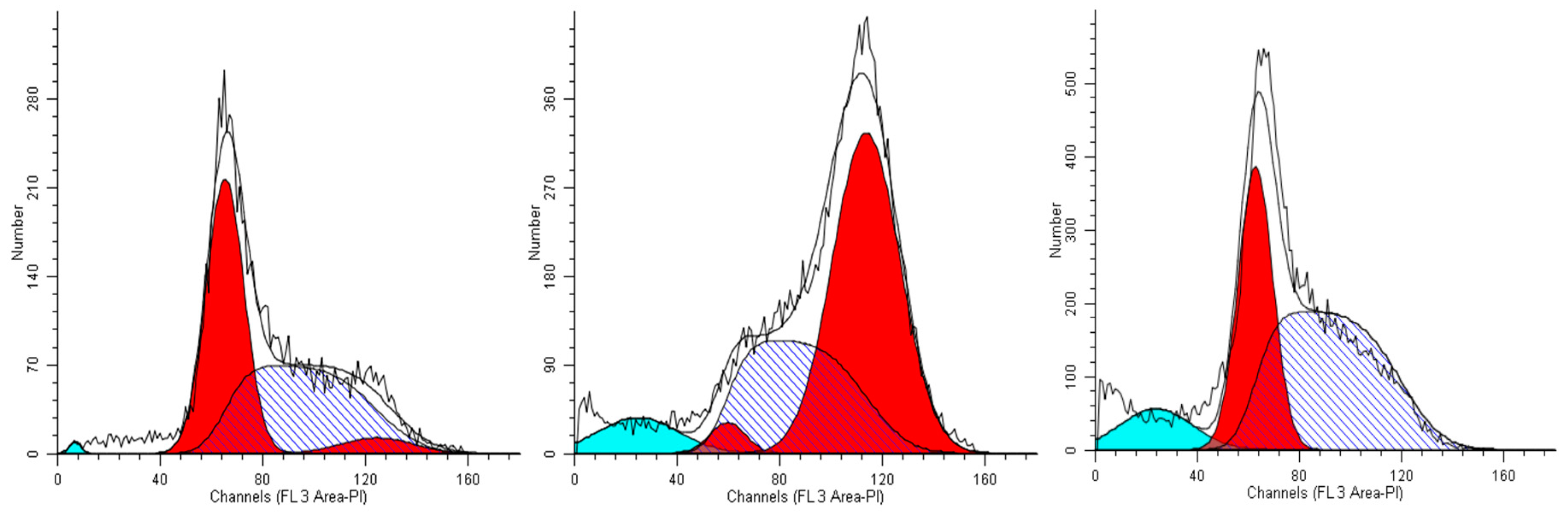
As the MCF-7 cells are concerned, we obtained results quite completely superimposable to those gained with A549 cells when incubated with extract #46 (0.15%,
v/
v). Most of the cells accrual in the G2M phase, reaching 82% of the total. Considering that in control cells (
Figure 5, left panel) the population in this phase does not exceed 30%, extract #46 induces an increase superior to 50% (
Figure 5, middle panel). The addition of extract #57 to cell medium for 24 h induced a substantial increase in the percentage of resting cells (G0/G1), as the increase amounts to near 25% (
Figure 5, right panel).
Overall, the cytometric data presented above refer principally to two different epithelial cancer lines that, although transformed are p53 positive and grow adhering to the surface of culture plates. Both sets of data, indeed, suggest that the extracts #46 and #57, individually, cause in the two cell lines similar and coherent effects. In particular, it appears that extract #46 arrests the cycle in the G2/M phase, while the extract #57 blocks the cells in the G1 phase.
These observations prompted us to verify if the specific effects produced by the two extracts on adherent cells could be reproduced also on cells growing in suspension. Indeed, most of the data obtained in this regard are concerned with the U937 cell line, but these findings are also supported by some experiments on TK-6 cells (not shown).
Twenty-four hours of incubation of U937 cells in a medium containing 0.15% (
v/
v) of extract #46 determined a remarkable accumulation of cells in the G2/M phase. The percentage of cells populating this phase in the controls amounted to about 5% of the total (
Figure 6, left panel), while after the incubation the same population was increased to more than 60% (
Figure 6, middle panel).
The addition of extract #57 (0.15%,
v/
v) to cell medium for 24 h induced a visible increase in the percentage of G0/G1 cells (compare the left and right panels of
Figure 6). The addition of both extracts #46 and #57, however, also causes the formation of debris (non-specific cell fragments and possibly apoptosis).
Also TK-6 cells were incubated with extracts #46 and #57 producing the same type of effect as observed in the other cell strains (not shown).
The treatment of the various cell lines for 24 h with other extracts, namely #29 (
E. hyemale) and #32 (
P. scolopendrium), did not affect the cell cycle profiles (
Figure S1–3) as well as their morphology and viability. This demonstrates that the active principles interfering with the cell cycle of various cells are specific of the particular plant extract.
Until now, only limited data about molecular events underlying the cell cycle perturbation caused by exposure of cells to
C. coggygria and
J. communis extracts were available. Most of the findings reported are concerned with the effects on cell counting and residual cell viability [
5,
6].
2.3. Protein Expression
Even less information on the changes in the expression of specific proteins by cells treated with the extracts from the two plants has been reported.
Changes in the proliferation rate of cells is a controlled process in which some proteins play pivotal roles arresting or allowing cell cycle progression between successive phases. It is known, in fact, that the inhibition of cell proliferation, in general caused by the extracts observed should be echoed in the expression of some proteins involved in cell cycle progression and/or arrest and cell final fate. These proteins may also mediate the manifestation of premature cellular senescence. This process is an ensemble of complex biological processes some of which have apparently opposing effects (repair, tumor suppression/promotion, and aging).
For this reason, we were aimed to study the changes in the expression levels of a panel of these proteins when cells were exposed to non-lethal doses of the extracts. Only a limited number of such proteins, namely Cyclin B, Cdk1, Cdk2, p53, p21, p27, Caspase 9, Bcl-XL and Bcl-2, were actually analyzed and principally (but not exclusively) in MCF-7 and A549 cells.
2.3.1. Cyclin B, Cdk 1
Cyclic B and Cdk1 are proteins involved in the cell cycle, generally acknowledged as pivotal regulators of cell cycle during the transition from the G2 to M phase.
Figure 7 illustrates the electrophoretic expression profiles of Cyclin B and Cdk1 of MCF-7 cells exposed for 24 h to extract #46 (0.15%,
v/
v). The clear augment of expression of both Cyclin B and CDK1 is in accord with the experimentally observed arrest of cell cycle.
In the
Figure 7A is also shown the expression profile of Cyclin B of MCF-7 cells exposed to extracts #29 (
E. hyemale) and #32 (
P. scolopendrium) that do not alter the cell cycle of all cells studied and are used as negative controls.
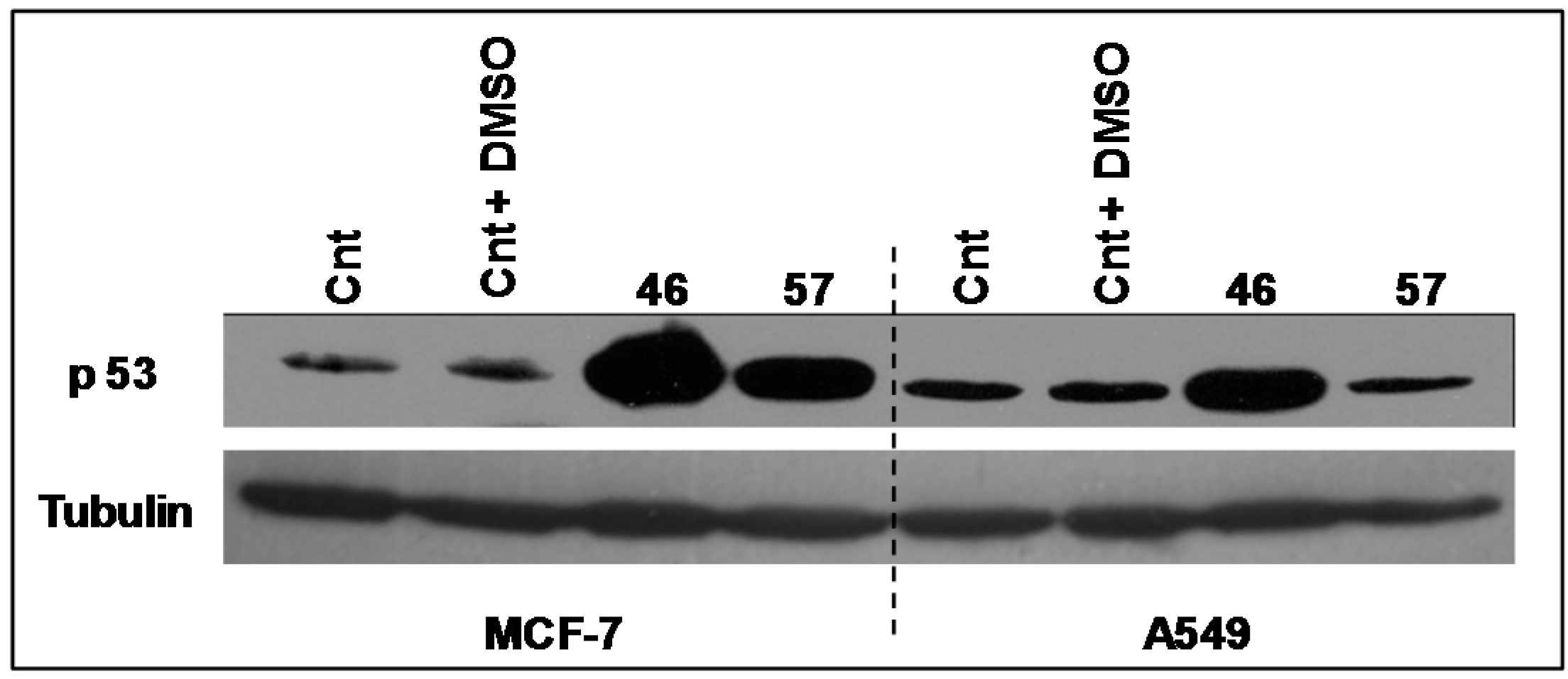
2.3.2. p53
The expression levels of the transcriptional factor p53 (
Figure 8) have been analyzed in A549 and MCF-7 incubated with extracts #46 and #57. It appears that the arrest in the G2/M phase induced by extract #46 is accompanied by an increase in p53 expression in both A549 and MCF-7 cells. Differently, extract #57 induces the expression of p53 exclusively in MCF-7 cells.
2.3.3. Cdk2, p21 and p27
The protein Cdk2 is indispensable for the G1/S transition exerting its action through its association with specific regulatory cyclins. When required by specific conditions, Cdk2 function may be reduced or abolished by interacting with the protein p21, a specific inhibitor [
7]. p27 might also play different roles in the cell cycle. Its over expression, for instance, regulates growth and differentiation, while moderate expression induces cell cycle arrest [
7,
8,
9,
10].
The expression of Cdk2 by A549 (
Figure 9A, left) and MCF-7 cells (
Figure 9, right) treated with extracts #46 and #57 changes as the electrophoretic doublet, present in controls, tends to fade following a decrease in the kinase activity.
As p21 is concerned, we have analyzed its expression in both MCF-7 and A549 cells (
Figure 9B). In this case, it appears that, compared to controls, p21 is strongly expressed in MCF-7 cells, while no evident changes have been observed in A549 cells.
Similarly we have analyzed the expression profiles of p27, a protein that may also block the cycle progression at the level of the G1/S transition.
Figure 9C depicts the expression of p27 in A549 and MCF7 treated with extracts #46 and #57. It appears that A549 cells are more sensitive to extracts than MCF-7 as A549 cells do not express appreciable basal levels of p27. On the contrary, the addition of either extracts #46 or #57 (the latter in particular) results in substantial increase of expression levels of this protein. This effect was not observed in MCF-7 cells, whose p27 basal level is already sustained. Then, the addition of extract #57 does not cause any appreciable augment of p27 expression.
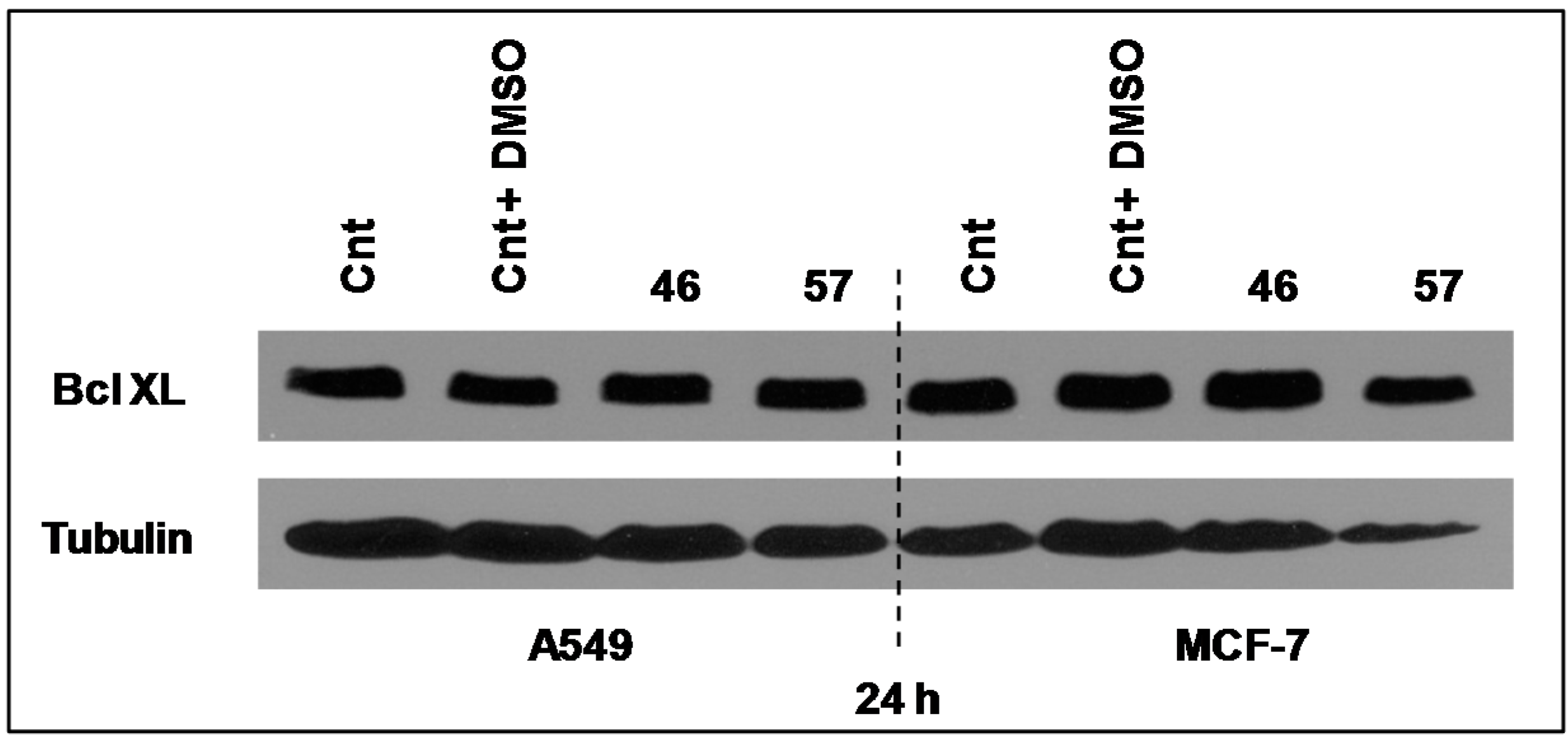
2.3.4. Bcl-XL
Bcl-XL is a member of Bcl2 family proteins that is located within the mitochondrial membrane. Its pro-survival action is founded on its capacity to prevent Cytochrome C release and the associated caspases activation [
11]. However, its expression level in both MCF-7 and A549 cells after 24 h of incubation with extracts #46 and #57 did not appear to sensibly change (
Figure 10).
We did not investigate in detail the potential anti-/pro-apoptotic effects exerted by the extracts on cell lines. Our observations are limited to changes in Bcl-2 expression and Caspase 9 activation in the MCF-7 cells only by extract #46. These data, while indicating the absence of anti-apoptotic effects (
Figure S4), suggest that the expression of Caspase 9 was scarcely altered within 24 h. Indeed, only at the highest extract concentration (
i.e., 15 μg/mL), the expression of Caspase 9 appears to be visibly reduced indicating a possible initiation of an apoptotic process. This observation is reported in
Figure S5.
2.4. Evaluation of Senescence in Cells
The cell cycle arrest in G1 phase, determined by the action of some agents including chemotherapy may induce in cells premature senescence [
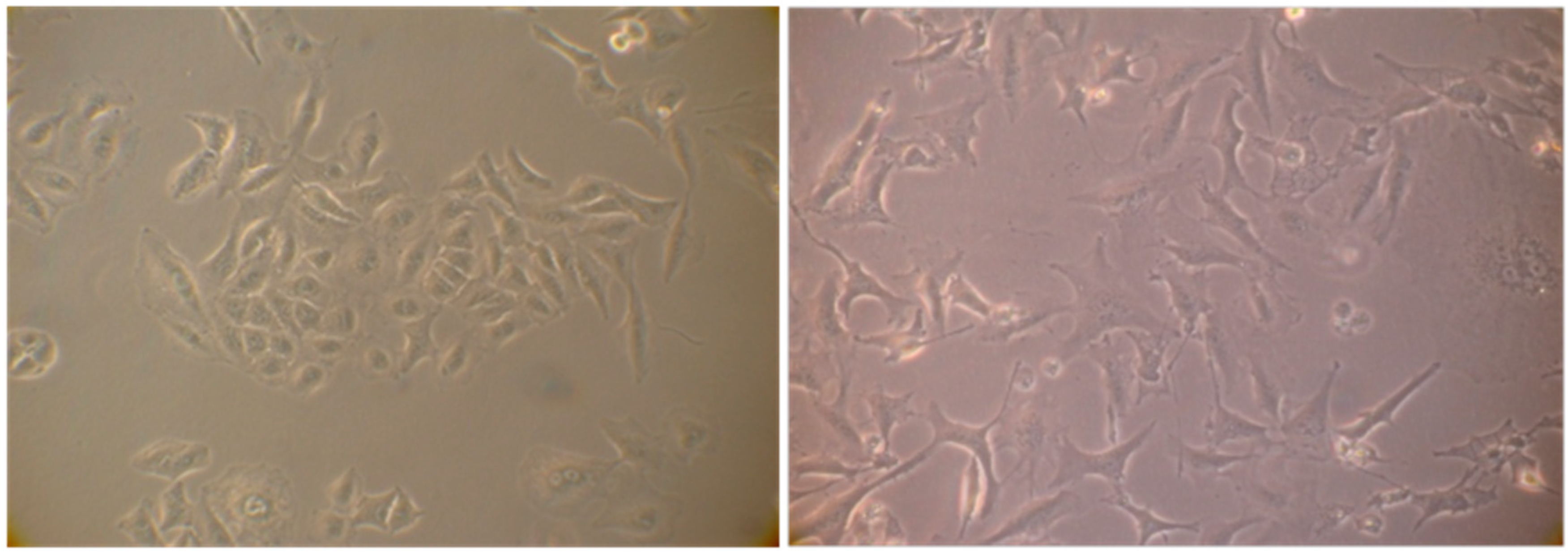
12] an ensemble of complex biological processes characterized by cells specific morphological and biochemical changes. The effects produced by the extract #57, which compels most cells into G1 phase, prompted us to verify if it would induce such condition in A549 cells that are prone to become senescent in stressing condition. To this purpose, A549 cells were incubated for six days in the presence of 5 µL/mL of extract #57 of medium (every two days, the medium was replaced with fresh medium containing the same amount of extract). Cells were then incubated for additional 72 h with fresh medium without the extract. At the end of this treatment, cells were analyzed for morphological changes (
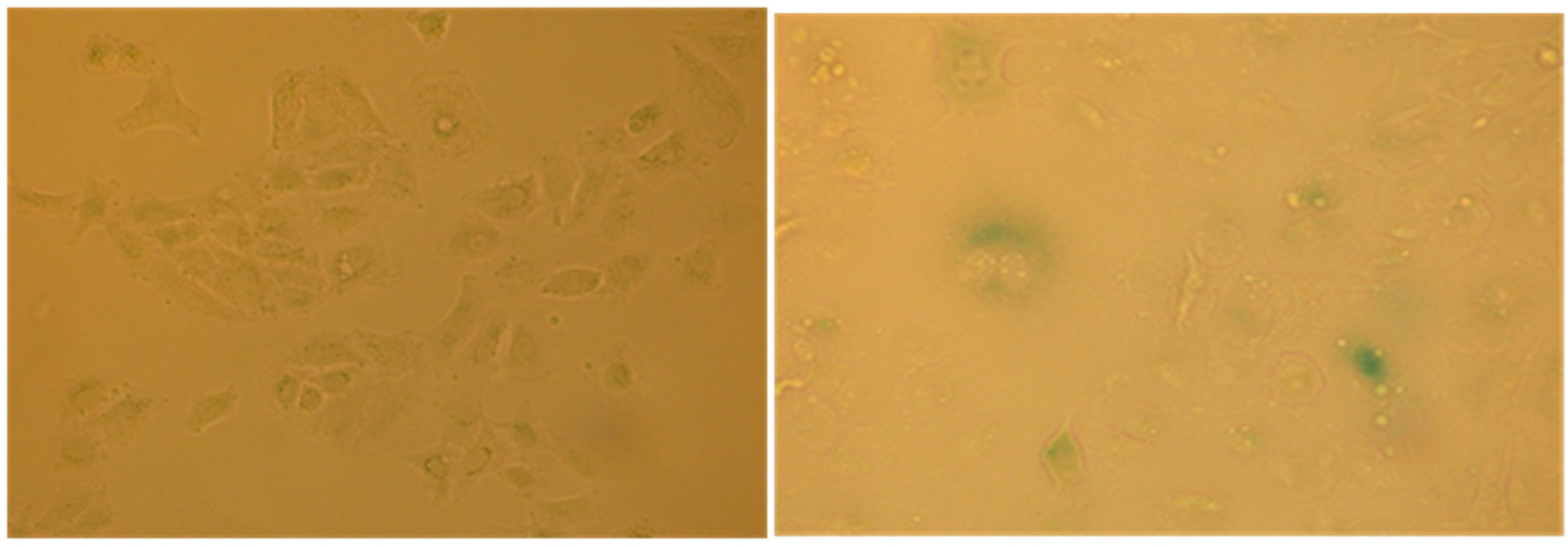
Figure 11) and for the acquired capacity to express SA-β-galactosidase activity (
Figure 12), through the appearance of the characteristic green-blue spots in the cytoplasm.
This finding indicates that the extract #57 at a non-lethal concentration promotes specific G1 accumulation and induce premature senescence, a characteristic effects associated to anticancer drugs when used at low concentrations (doxorubicin, for example).
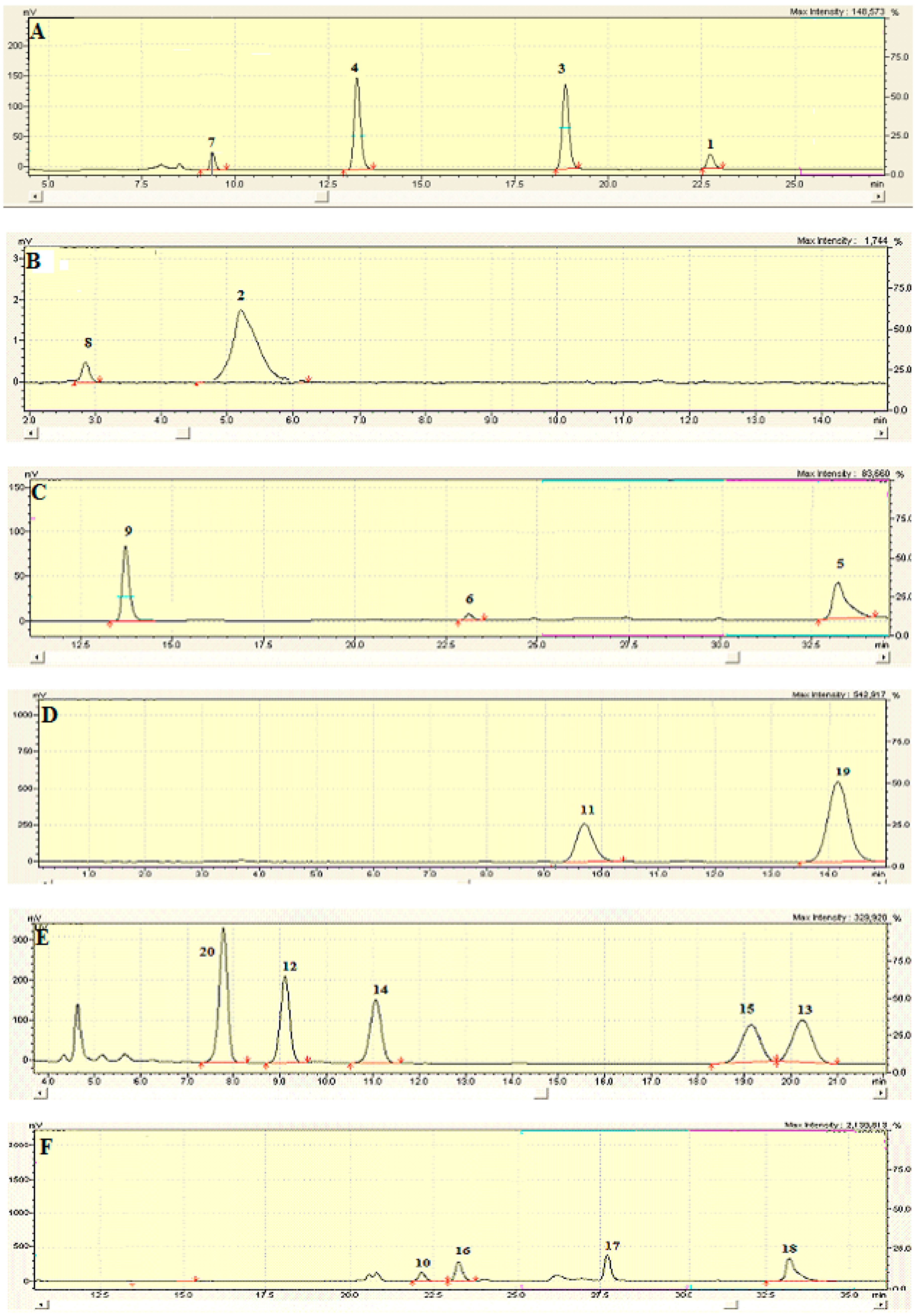
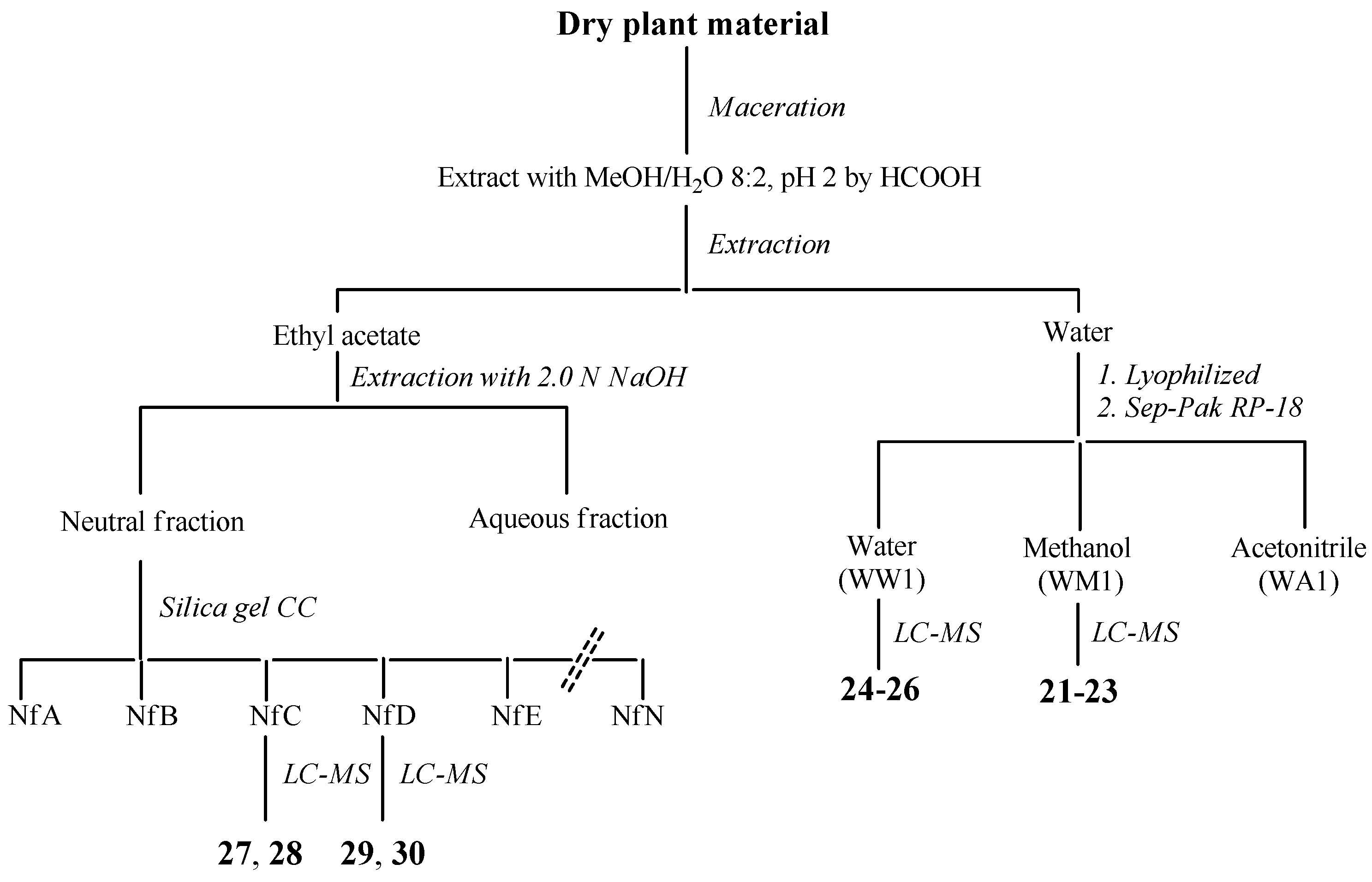
2.6. Identification and Characterization of Chemical Components of the Plant Extracts
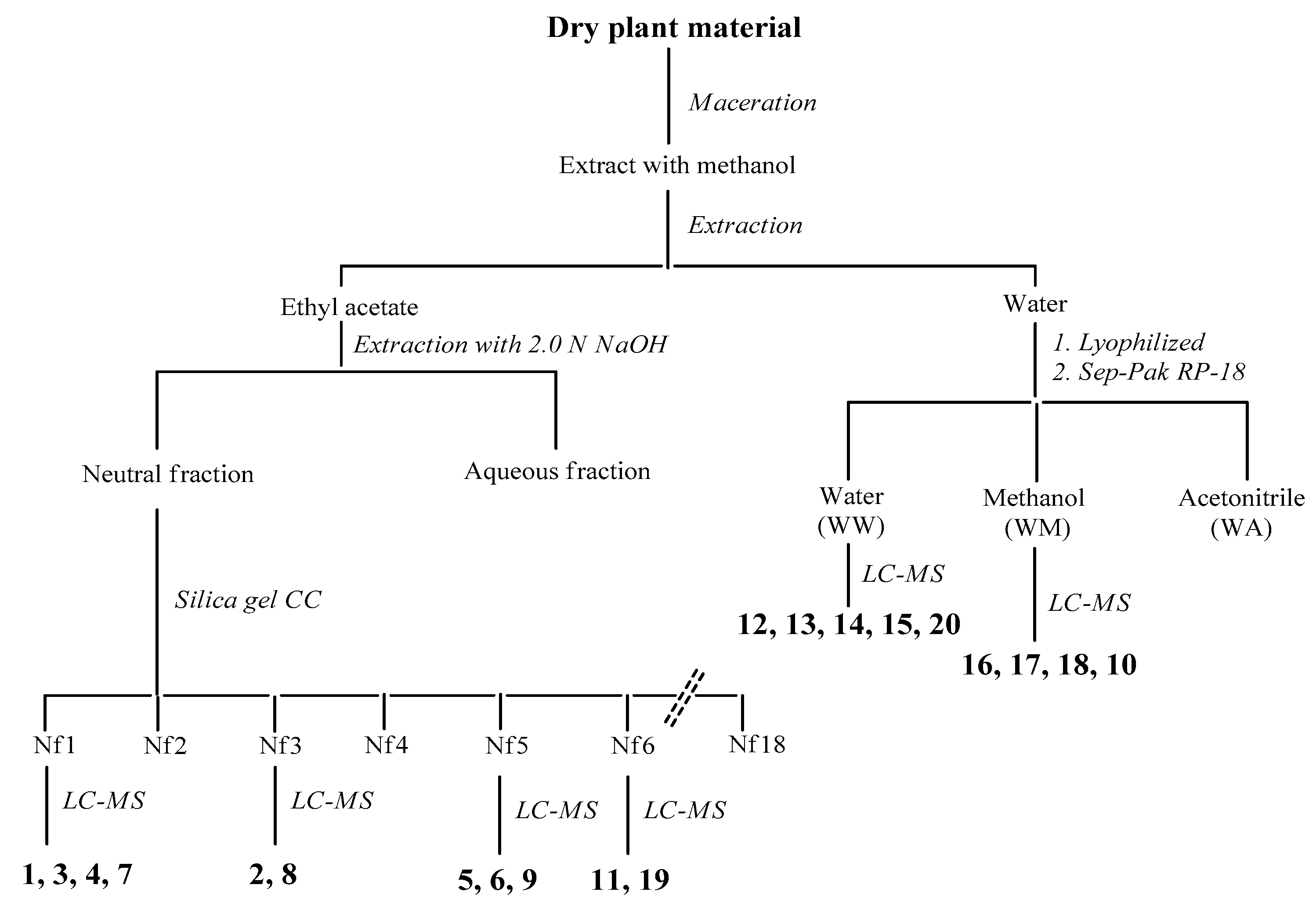
The last aspect that we have considered was the identification of the major natural components of the plant extracts. The
Table 1 summarizes the array of phenolic compounds that we have identified in methanolic extracts of
C. coggygria and
J. communis by liquid chromatography–mass spectrometry (LC-MS) detection. In particular, LC-MS investigation of methanol extract of
C. coggygria led to the identification of flavonoids
1–
11 and
19 and polyphenols derivatives
12–
18 and
20, while in
J. communis methanol extract the flavonoids
21–
28, the α-ionone glycoside
29 and the lignin
30 were found. All isolated compounds were identified for comparison with the data reported in the literature and in the database of the instrument. Their chemical structures are shown in
Figure A1 and
Figure A2 whereas the IUPAC names are indicated in
Table A1 and
Table A2 of the
Appendix A.
Most of them certainly hold biological properties that may interfere with cell proliferation as suggested by Kuntz
et al. [
13]. For instance, as far as flavonoids are concerned, fisetin, sulfuretin, isoliquiritigenin, quercetin, apigetrin, and amentoflavone [
14,
15,
16,
17,
18,
19,
20] have all been considered active against breast cancer cells acting at various levels.
Similarly, polyphenol derivatives isolated from
C. coggygria and
J. communis have shown cytotoxic activity against several cancer cell lines: caffeic, coumaric and ferulic acids can act against lung and colon cancer cell line growth, controlling adhesion and migration steps of tumor progression [
21], and penta-
O-galloylglucose [
22], inhibit the proliferation of human breast cancer cells. The anti-proliferative action is not limited to simple phenolics: also the lignan matairesinol evidenced a cytostatic activity against breast and colon cancers [
23].
Polyphenols derivatives may cause cell cycle arrest and induce apoptosis; resveratrol was shown to activate p53 by phosphorylation, and significantly increases the pro-apoptotic signals such as Bak and p21 genes [
24,
25], whereas Gallic acid stimulated the synthesis of Bax and Bad protein and induced a decrease of Bcl-2 and Bcl-xL levels in NCI-H460 lung cancer cells [
26]. But also flavonoids as quercetin affect the cell cycle of different cancer cell lines, inhibiting the activity of CDK2 [
27], activating caspases and downregulating the expression of anti-apoptotic Bcl-2 and tumor suppressing p53 proteins [
28]. In the same way, apigenin and kampferol can induce a strong down-regulation of CDK1 and a relatively small up-regulation of p27 [
29]. Both extracts that we studied possess mild to weak antioxidant properties. This may result by the concomitant presence of several natural substances whose individual characteristics may differ from those of the blend that we find in the extract. Natural products, and particularly those possessing considerable variation of phenolic composition certainly have a steric complexity larger than any synthetic drugs [
30]. This implies that plant crude extracts may present unpredictable therapeutic effects that are different, in type and intensity, from those exhibited by individual components [
31].
Undoubtedly, the extracts own attractive and unique properties that may find interesting applications for in vitro studies and even as potential drugs. This, however, deserves further attention and requires more targeted studies.