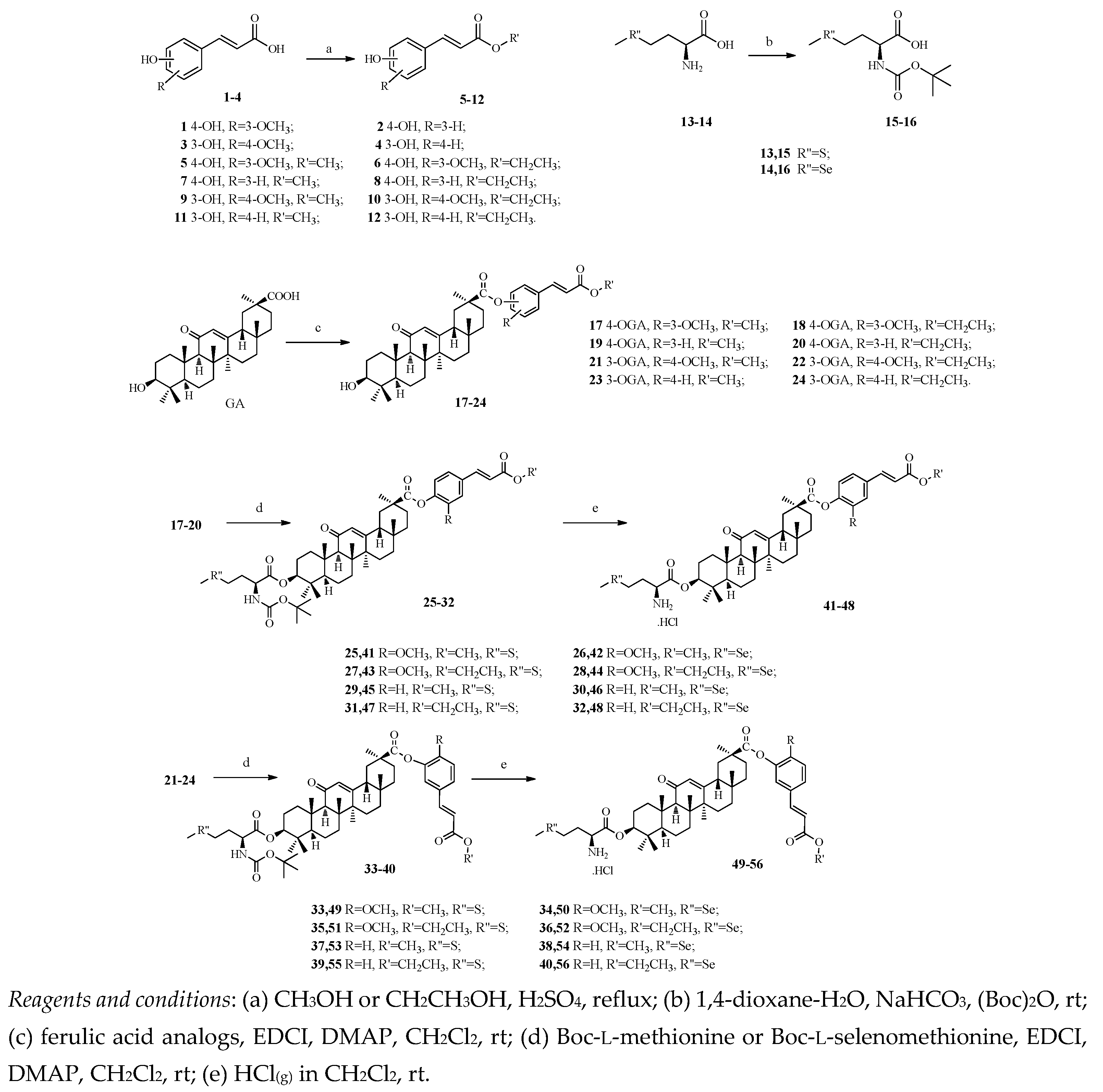
3. Experimental Section
3.1. General Information
All the chemicals and reagents were commercially available and used without further purification. Routine thin-layer chromatography (TLC) was performed on silica gel plates (silica gel GF254 from Qingdao Haiyang Chemical Co., Ltd., Qingdao, China), and visualization was performed using UV. 1H-NMR and 13C-NMR spectra were recorded on a Bruker-400 instrument (Bruker, Billerica, MA, USA) at room temperature with TMS as an internal standard and CDCl3 or DMSO-d6 as solvents. Chemical shifts are expressed in δ (ppm) and coupling constants (J) in Hz. Mass spectra were recorded with a MSQ Plus mass spectrometer (Thermo Scientific, Waltham, MA, USA). Melting points were measured by an SGWX-4 micro melting point apparatus (Shanghai Precision & Scientific Instrument Co. Ltd., Shanghai, China) and are uncorrected.
3.2. General Method for Synthesizing Compounds 5–12
Ferulic acid (1) or trans-4-hydroxycinnamic acid (2) or isoferulic acid (3) or trans-3-hydroxycinnamic acid (4) and five drops of H2SO4 (95%) were refluxed in methanol or ethanol for 12 h. The reaction mixture was concentrated in vacuo and the residue was dissolved in ethyl acetate. The organic layer was washed with a 5% aqueous NaHCO3 solution and water. After drying over anhydrous Na2SO4, the ethyl acetate was removed in vacuo. The residue was purified by column chromatography on silica gel using ethyl acetate/petroleum ether mixtures as eluents to afford compounds 5–12.
3.3. General Method for Synthesizing Compounds 15–16
The appropriate amino acid (13 or 14, 1 equiv.) and sodium bicarbonate (3 equiv.) was dissolved in a 1:1 mixture of water and 1,4-dioxane. Di-tert-butyl dicarbonate (1.2 equiv.) was added and the mixture was stirred at room temperature for 12 h. The 1,4-dioxane was removed under reduced pressure and the mixture was extracted with ethyl acetate. Then the solution was acidified using 1 M hydrochloric acid solution and extracted three times with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, filtered, and the solvent was evaporated. The crude protected amino acids 15–16 were used without any further purification.
3.4. General Method for Synthesizing Compouds 17–24
GA (485 mg, 1 mmol) was dissolved in dry DCM (30 mL) and stirred at room temperature for 5 min. Then EDCI (230 mg, 1.2 mmol), DMAP (24 mg, 0.2 mmol) and compounds 5–12 (1 mmol) were added to the solution, and then the reaction mixture was stirred at room temperature for 12 h. The organic layer was washed with 1 M HCl solution and concentrated in vacuo. The residue was purified by column chromatography on silica gel with ethyl acetate/petroleum as eluent to yield pure compounds 17–24.
Ferulic acid methyl ester 3β-hydroxy-11-oxo-olean-12-en-30-oate (17). Compound 17 was obtained from GA and ferulic acid methyl ester as a white solid (548.5 mg, 83%); m.p. 229–231 °C; 1H-NMR (CDCl3): δ 0.72–0.74 (m, 1H, H-5), 0.83 (s, 3H, H-28), 0.90 (s, 3H, H-24), 0.96–1.00 (m, 1H, H-1’), 1.03 (s, 3H, H-23), 1.08–1.11 (m, 1H, H-15’), 1.16 (s, 3H, H-26), 1.17 (s, 3H, H-25), 1.23–1.25 (m, 1H, H-16’), 1.27–1.30 (m, 2H, H-22’ and 21’), 1.37 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.44–1.45 (m, 1H, H-22), 1.46–1.48 (m, 1H, H-7’), 1.49–1.51 (m, 1H, H-6’), 1.61–1.63 (m, 1H, H-6), 1.65 (dd, J = 13.2, 4.0 Hz, 1H, H-19’), 1.67–1.68 (m, 1H, H-2’), 1.70–1.73 (m, 1H, H-7), 1.77–1.79 (m, 1H, H-2), 1.89 (ddd, J = 14.4, 14.4, 5.2 Hz, 1H, H-16), 2.02–2.04 (m, 1H, H-21), 2.08–2.10 (m, 1H, H-15), 2.12 (dd, J = 13.2, 4.0 Hz, 1H, H-18), 2.37 (s, 1H, H-9), 2.43–2.45 (m, 1H, H-19), 2.80 (ddd, J = 13.2, 3.2, 3.2 Hz, 1H, H-1), 3.25 (dd, J = 10.8, 5.6 Hz, 1H, H-3), 3.84 (s, 3H, COOCH3), 3.89 (s, 3H, OCH3), 5.73 (s, 1H, H-12), 6.42 (d, Jtrans = 12.8 Hz, 1H, H-β), 7.02 (d, J = 6.8 Hz, 1H, Bn-H-5), 7.13 (s, 1H, Bn-H-3), 7.15 (d, J = 6.8 Hz, 1H, Bn-H-6), 7.68 (d, Jtrans = 12.8 Hz, 1H, H-α); 13C-NMR (CDCl3): δ 200.10, 174.43, 168.44, 166.88, 151.40, 149.95, 147.92, 128.43, 123.12, 121.17, 118.34, 113.48, 109.11, 78.66, 61.63, 55.68, 54.84, 51.64, 48.00, 45.33, 44.37, 43.11, 41.21, 38.04, 38.02, 37.42, 37.06, 32.75, 31.81, 31.13, 28.47, 28.05, 28.04, 27.28, 26.40, 26.35, 23.38, 18.59, 17.44, 16.30, 15.57; ESI-MS: m/z = 661.26 [M + H]+. Anal. Calcd. for C41H56O7 (660.40): C, 74.51; H, 8.54%. Found: C, 74.48; H, 8.56%.
Ferulic acid ethyl ester 3β-hydroxy-11-oxo-olean-12-en-30-oate (18). Compound 18 was obtained from GA and ferulic acid ethyl ester as a white solid (573.7 mg, 85%); m.p. 224–226 °C; 1H-NMR (CDCl3): δ 0.72–0.74 (m, 1H, H-5), 0.83 (s, 3H, H-28), 0.90 (s, 3H, H-24), 0.96–1.00 (m, 1H, H-1’), 1.03 (s, 3H, H-23), 1.08–1.11 (m, 1H, H-15’), 1.16 (s, 3H, H-26), 1.17 (s, 3H, H-25), 1.23–1.25 (m, 1H, H-16’), 1.27–1.30 (m, 2H, H-22’ and H-21’), 1.37 (t, J = 5.6 Hz, 3H, CH3), 1.38 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.43–1.46 (m, 1H, H-22), 1.47–1.48 (m, 1H, H-7’), 1.49–1.51 (m, 1H, H-6’), 1.62–1.64 (m, 1H, H-6), 1.65 (dd, J = 13.2, 4.0 Hz, 1H, H-19’), 1.67–1.68 (m, 1H, H-2’), 1.70–1.72 (m, 1H, H-7), 1.76–1.78 (m, 1H, H-2), 1.89 (ddd, J = 14.0, 14.0, 4.8 Hz, 1H, H-16), 2.03–2.05 (m, 1H, H-21), 2.08–2.10 (m, 1H, H-15), 2.12 (dd, J = 12.8, 3.6 Hz, 1H, H-18), 2.37 (s, 1H, H-9), 2.43–2.45 (m, 1H, H-19), 2.80 (ddd, J = 13.6, 3.6, 3.6 Hz, 1H, H-1), 3.25 (dd, J = 11.2, 5.2 Hz, 1H, H-3), 3.88 (s, 3H, OCH3), 4.30 (q, J = 5.6 Hz, 2H, CH2CH3), 5.73 (s, 1H, H-12), 6.41 (d, Jtrans = 12.8 Hz, 1H, H-β), 7.02 (d, J = 6.8 Hz, 1H, Bn-H-5), 7.13 (s, 1H, Bn-H-3), 7.15 (d, J = 6.8 Hz, 1H, Bn-H-6), 7.67 (d, Jtrans = 12.8 Hz, 1H, H-α); 13C-NMR (CDCl3): δ 200.02, 174.30, 168.32, 166.80, 151.34, 149.95, 147.90, 128.45, 123.01, 121.12, 118.24, 113.47, 109.10, 78.63, 61.75, 60.63, 55.70, 54.87, 47.98, 45.32, 44.36, 43.13, 41.14, 38.00, 37.94, 37.56, 36.93, 32.65, 31.91, 31.15, 28.48, 28.06, 28.04, 27.25, 26.45, 26.38, 23.33, 18.63, 17.39, 16.32, 15.52, 14.28; ESI-MS: m/z = 675.23 [M + H]+. Anal. Calcd. for C42H58O7 (674.42): C, 74.74; H, 8.66%. Found: C, 74.70; H, 8.69%.
trans-4-Hydroxycinnamic acid methyl ester 3β-hydroxy-11-oxo-olean-12-en-30-oate (19). Compound 19 was obtained from GA and trans-4-hydroxycinnamic acid methyl ester as a white solid (530 mg, 84%); m.p. 202–205 °C; 1H-NMR (CDCl3): δ 0.69–0.72 (m, 1H, H-5), 0.81 (s, 3H, H-28), 0.86 (s, 3H, H-24), 0.94–0.98 (m, 1H, H-1’), 1.01 (s, 3H, H-23), 1.06–1.09 (m, 1H, H-15’), 1.14 (s, 3H, H-26), 1.14 (s, 3H, H-25), 1.19–1.21 (m, 1H, H-16’), 1.24–1.26 (m, 2H, H-22’ and H-21’), 1.35 (s, 3H, H-29), 1.40 (s, 3H, H-27), 1.43–1.45 (m, 1H, H-22), 1.47–1.48 (m, 1H, H-7’), 1.50–1.53 (m, 1H, H-6’), 1.60–1.62 (m, 1H, H-6), 1.64 (dd, J = 13.2, 4.0 Hz, 1H, H-19’), 1.67–1.69 (m, 1H, H-2’), 1.72–1.74 (m, 1H, H-7), 1.75–1.78 (m, 1H, H-2), 1.87 (ddd, J = 14.8, 14.8, 5.6 Hz, 1H, H-16), 2.04–2.06 (m, 1H, H-21), 2.11–2.13 (m, 1H, H-15), 2.24 (dd, J = 13.6, 4.4 Hz, 1H, H-18), 2.35 (s, 1H, H-9), 2.43–2.46 (m, 1H, H-19), 2.78 (ddd, J = 12.8, 2.8, 2.8 Hz, 1H, H-1), 3.23 (dd, J = 10.4, 6.0 Hz, 1H, H-3), 3.81 (s, 3H, COOCH3), 5.67 (s, 1H, H-12), 6.41 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.08 (d, J = 8.8 Hz, 2H, Bn-H-2 and 6), 7.55 (d, J = 8.8 Hz, 2H, Bn-H-3 and 5), 7.68 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (CDCl3): δ 200.03, 174.86, 168.65, 166.93, 162.26, 145.61, 132.27, 129.25, 128.70, 122.12, 118.41, 78.74, 61.82, 54.94, 51.86, 48.43, 45.36, 44.39, 43.21, 41.11, 39.16, 39.09, 37.75, 37.10, 32.69, 31.88, 31.13, 28.54, 28.09, 28.06, 27.32, 26.41, 26.38, 23.49, 18.65, 17.49, 16.47, 15.62; ESI-MS: m/z = 631.21 [M + H]+. Anal. Calcd. for C40H54O6 (630.39): C, 76.16; H, 8.63%. Found: C, 76.13; H, 8.67%.
trans-4-Hydroxycinnamic acid ethyl ester 3β-hydroxy-11-oxo-olean-12-en-30-oate (20). Compound 20 was obtained from GA and trans-4-hydroxycinnamic acid ethyl ester as a white solid (548.1 mg, 85%); m.p. 195–196 °C; 1H-NMR (CDCl3): δ 0.69–0.72 (m, 1H, H-5), 0.81 (s, 3H, H-28), 0.86 (s, 3H, H-24), 0.94–0.98 (m, 1H, H-1’), 1.01 (s, 3H, H-23), 1.06–1.09 (m, 1H, H-15’), 1.14 (s, 3H, H-26), 1.14 (s, 3H, H-25), 1.19–1.21 (m, 1H, H-16’), 1.24–1.26 (m, 2H, H-22’ and H-21’), 1.34 (t, J = 7.2 Hz, 3H, CH3), 1.35 (s, 3H, H-29), 1.40 (s, 3H, H-27), 1.44–1.45 (m, 1H, H-22), 1.46–1.48 (m, 1H, H-7’), 1.49–1.53 (m, 1H, H-6’), 1.60–1.62 (m, 1H, H-6), 1.63 (dd, J = 13.2, 4.0 Hz, 1H, H-19’), 1.67–1.69 (m, 1H, H-2’), 1.72–1.74 (m, 1H, H-7), 1.75–1.78 (m, 1H, H-2), 1.86 (ddd, J = 14.4, 14.4, 5.2 Hz, 1H, H-16), 2.04–2.06 (m, 1H, H-21), 2.12–2.14 (m, 1H, H-15), 2.24 (dd, J = 13.2, 4.0 Hz, 1H, H-18), 2.35 (s, 1H, H-9), 2.43–2.45 (m, 1H, H-19), 2.78 (ddd, J = 13.2, 3.2, 3.2 Hz, 1H, H-1), 3.23 (dd, J = 10.8, 5.6 Hz, 1H, H-3), 4.27 (q, J = 7.2 Hz, 2H, CH2CH3), 5.68 (s, 1H, H-12), 6.40 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.08 (d, J = 8.8 Hz, 2H, Bn-H-2 and 6), 7.55 (d, J = 8.8 Hz, 2H, Bn-H-3 and 5), 7.67 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (CDCl3): δ 200.11, 174.78, 168.70, 166.82, 162.12, 145.40, 132.12, 129.14, 128.61, 121.97, 118.33, 78.64, 61.76, 60.61, 54.86, 48.43, 45.33, 44.31, 43.16, 41.00, 39.07, 39.03, 37.67, 37.01, 32.69, 31.88, 31.06, 28.54, 28.04, 28.02, 27.21, 26.39, 26.34, 23.38, 18.61, 17.42, 16.30, 15.54, 14.27; ESI-MS: m/z = 645.31 [M + H]+. Anal. Calcd. for C41H56O6 (644.41): C, 76.36; H, 8.75%. Found: C, 76.31; H, 8.79%.
Isoferulic acid methyl ester 3β-hydroxy-11-oxo-olean-12-en-30-oate (21). Compound 21 was obtained from GA and isoferulic acid methyl ester as a white solid (575 mg, 87%); m.p. 215–217 °C; 1H-NMR (CDCl3): δ 0.70–0.72 (m, 1H, H-5), 0.81 (s, 3H, H-28), 0.89 (s, 3H, H-24), 0.94–0.98 (m, 1H, H-1’), 1.01 (s, 3H, H-23), 1.06–1.09 (m, 1H, H-15’), 1.14 (s, 3H, H-26), 1.15 (s, 3H, H-25), 1.19–1.21 (m, 1H, H-16’), 1.24–1.26 (m, 2H, H-22’ and 21’), 1.36 (s, 3H, H-29), 1.40 (s, 3H, H-27), 1.44 (m, 1H, H-22), 1.47 (m, 1H, H-7’), 1.49 (m, 1H, H-6’), 1.62 (m, 1H, H-6), 1.64 (dd, 1H, J = 13.2, 4.0 Hz, H-19’), 1.67 (m, 1H, H-2’), 1.70 (m, 1H, H-7), 1.75 (m, 1H, H-2), 1.88 (ddd, 1H, J = 14.4, 14.4, 5.2 Hz, H-16), 2.06 (m, 1H, H-21), 2.10 (m, 1H, H-15), 2.13 (dd, 1H, J = 13.2, 4.0 Hz, H-18), 2.35 (s, 1H, H-9), 2.42 (m, 1H, H-19), 2.78 (ddd, 1H, J = 13.2, 3.2, 3.2 Hz, H-1), 3.23 (dd, 1H, J = 10.8, 5.6 Hz, H-3), 3.79 (s, 3H, COOCH3), 3.86 (s, 3H, OCH3), 5.70 (s, 1H, H-12), 6.30 (d, 1H, Jtrans = 16.0 Hz, H-β), 6.96 (d, 1H, J = 8.8 Hz, Bn-H-3), 7.17 (d, 1H, J = 2.0 Hz, Bn-H-6), 7.36 (dd, 1H, J = 8.8, 2.0 Hz, Bn-H-4), 7.61 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (CDCl3): δ 200.23, 174.38, 169.22, 167.43, 152.86, 143.66, 139.91, 128.42, 127.56, 127.40, 121.68, 116.24, 112.25, 78.63, 61.76, 55.81, 54.85, 51.62, 47.95, 45.32, 44.34, 43.12, 41.16, 39.07, 39.06, 37.42, 37.00, 32.69, 31.81, 31.15, 28.53, 28.29, 28.04, 27.20, 26.45, 26.36, 23.34, 18.63, 17.42, 16.31, 15.55; ESI-MS: m/z = 661.28 [M + H]+. Anal. Calcd. for C41H56O7 (660.40): C, 74.51; H, 8.54%. Found: C, 74.46; H, 8.56%.
Isoferulic acid ethyl ester 3β-hydroxy-11-oxo-olean-12-en-30-oate (22). Compound 22 was obtained from GA and isoferulic acid ethyl ester as a white solid (567 mg, 84%); m.p. 210–213 °C; 1H-NMR (CDCl3): δ 0.69–0.72 (m, 1H, H-5), 0.81 (s, 3H, H-28 ), 0.88 (s, 3H, H-24), 0.94–0.98 (m, 1H, H-1’), 1.01 (s, 3H, H-23), 1.05–1.09 (m, 1H, H-15’), 1.14 (s, 3H, H-26), 1.15 (s, 3H, H-25), 1.19–1.21 (m, 1H, H-16’), 1.24–1.26 (m, 2H, H-22’ and 21’), 1.33 (t, 3H, J = 7.2 Hz, CH3), 1.36 (s, 3H, H-29), 1.40 (s, 3H, H-27), 1.44 (m, 1H, H-22), 1.47 (m, 1H, H-7’), 1.50 (m, 1H, H-6’), 1.62 (m, 1H, H-6), 1.64 (dd, 1H, J = 13.2, 4.0 Hz, H-19’), 1.67 (m, 1H, H-2’), 1.70 (m, 1H, H-7), 1.75 (m, 1H, H-2), 1.87 (ddd, 1H, J = 14.0, 14.0, 4.8 Hz, H-16), 2.06 (m, 1H, H-21), 2.10 (m, 1H, H-15), 2.13 (dd, 1H, J = 12.8, 3.6 Hz, H-18), 2.35 (s, 1H, H-9), 2.42 (m, 1H, H-19), 2.78 (ddd, 1H, J = 13.6, 3.6, 3.6 Hz, H-1), 3.23 (dd, 1H, J = 11.2, 5.2 Hz, H-3), 3.86 (s, 3H, OCH3), 4.25 (q, 2H, J = 7.2 Hz, CH2CH3), 5.70 (s, 1H, H-12), 6.30 (d, 1H, Jtrans = 16.0 Hz, H-β), 6.96 (d, 1H, J = 8.8 Hz, Bn-H-3), 7.18 (d, 1H, J = 2.0 Hz, Bn-H-6), 7.36 (dd, 1H, J = 8.8, 2.0 Hz, Bn-H-4), 7.60 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (CDCl3): δ 200.23, 174.38, 169.22, 167.43, 152.86, 143.66, 139.91, 128.42, 127.56, 127.40, 121.68, 116.24, 112.25, 78.63, 61.76, 55.81, 54.85, 51.62, 47.95, 45.32, 44.34, 43.12, 41.16, 39.07, 39.06, 37.42, 37.00, 32.69, 31.81, 31.15, 28.53, 28.29, 28.04, 27.20, 26.45, 26.36, 23.34, 18.63, 17.42, 16.31, 15.55; ESI-MS: m/z = 675.26 [M + H]+. Anal. Calcd. for C42H58O7 (674.42): C, 74.74; H, 8.66%. Found: C, 74.70; H, 8.69%.
trans-3-Hydroxycinnamic acid methyl ester 3β-hydroxy-11-oxo-olean-12-en-30-oate (23). Compound 23 was obtained from GA and trans-3-hydroxycinnamic acid methyl ester as a white solid (511 mg, 81%); m.p. 188–190 °C; 1H-NMR (CDCl3): δ 0.72–0.75 (m, 1H, H-5), 0.83 (s, 3H, H-28), 0.90 (s, 3H, H-24), 0.97–1.00 (m, 1H, H-1’), 1.03 (s, 3H, H-23), 1.08–1.12 (m, 1H, H-15’), 1.16 (s, 3H, H-26), 1.17 (s, 3H, H-25), 1.20–1.23 (m, 1H, H-16’), 1.27–1.28 (m, 2H, H-22’ and 21’), 1.38 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.44 (m, 1H, H-22), 1.47 (m, 1H, H-7’), 1.50 (m, 1H, H-6’), 1.62 (m, 1H, H-6), 1.66 (dd, 1H, J = 13.2, 4.0 Hz, H-19’), 1.69 (m, 1H, H-2’), 1.74 (m, 1H, H-7), 1.77 (m, 1H, H-2), 1.89 (ddd, 1H, J = 14.8, 14.8, 5.6 Hz, H-16), 2.08 (m, 1H, H-21), 2.16 (m, 1H, H-15), 2.28 (dd, 1H, J = 13.6, 4.4 Hz, H-18), 2.37 (s, 1H, H-9), 2.43 (m, 1H, H-19), 2.81 (ddd, 1H, J = 12.8, 2.8, 2.8 Hz, H-1), 3.25 (dd, 1H, J = 10.4, 6.0 Hz, H-3), 3.84 (s, 3H, COOCH3), 5.70 (s, 1H, H-12), 6.46 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.09 (dt, 1H, J = 6.8, 2.4 Hz, Bn-H-6), 7.21 (s, 1H, Bn-H-2), 7.42 (s, 1H, Bn-H-4), 7.44 (t, 1H, J = 7.6 Hz, Bn-H-5), 7.70 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (CDCl3): δ 200.07, 174.89, 168.68, 167.04, 151.12, 145.65, 135.96, 129.89, 128.61, 125.61, 123.24, 120.69, 118.92, 78.65, 61.77, 54.86, 51.74, 48.36, 45.33, 44.28, 43.15, 40.97, 39.07, 39.04, 37.74, 37.02, 32.69, 31.88, 31.08, 28.58, 28.07, 28.05, 27.22, 26.39, 26.34, 23.39, 18.62, 17.42, 16.30, 15.54; ESI-MS: m/z = 631.23 [M + H]+. Anal. Calcd. for C40H54O6 (630.39): C, 76.16; H, 8.63%. Found: C, 76.11; H, 8.67%.
trans-3-Hydroxycinnamic acid ethyl ester 3β-hydroxy-11-oxo-olean-12-en-30-oate (24). Compound 24 was obtained from GA and trans-3-hydroxycinnamic acid ethyl ester as a white solid (561 mg, 87%); m.p. 173–175 °C; 1H-NMR (CDCl3): δ 0.72–0.75 (m, 1H, H-5), 0.83 (s, 3H, H-28), 0.90 (s, 3H, H-24), 0.97–1.00 (m, 1H, H-1’), 1.03 (s, 3H, H-23), 1.08–1.12 (m, 1H, H-15’), 1.16 (s, 3H, H-26), 1.17 (s, 3H, H-25), 1.20–1.23 (m, 1H, H-16’), 1.27–1.28 (m, 2H, H-22’ and 21’), 1.36 (t, 3H, J = 7.2 Hz, CH3), 1.38 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.44 (m, 1H, H-22), 1.47 (m, 1H, H-7’), 1.50 (m, 1H, H-6’), 1.62 (m, 1H, H-6), 1.66 (dd, 1H, J = 13.2, 4.0 Hz, H-19’), 1.69 (m, 1H, H-2’), 1.74 (m, 1H, H-7), 1.77 (m, 1H, H-2), 1.89 (ddd, 1H, J = 14.4, 14.4, 5.2 Hz, H-16), 2.08 (m, 1H, H-21), 2.16 (m, 1H, H-15), 2.28 (dd, 1H, J = 13.2, 4.0 Hz, H-18), 2.37 (s, 1H, H-9), 2.43 (m, 1H, H-19), 2.81 (ddd, 1H, J = 13.2, 3.2, 3.2 Hz, H-1), 3.25 (dd, 1H, J = 10.8, 5.6 Hz, H-3), 4.29 (q, 2H, J = 7.2 Hz, CH2CH3), 5.70 (s, 1H, H-12), 6.46 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.08 (dt, 1H, J = 6.8, 2.4 Hz, Bn-H-6), 7.22 (s, 1H, Bn-H-2), 7.42 (s, 1H, Bn-H-4), 7.44 (t, 1H, J = 7.6 Hz, Bn-H-5), 7.68 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (CDCl3): δ 200.15, 174.76, 168.73, 166.96, 151.00, 145.48, 135.85, 129.73, 128.50, 125.49, 123.02, 120.57, 118.80, 78.56, 61.69, 60.32, 54.72, 48.21, 45.23, 44.20, 43.02, 40.86, 38.94, 38.90, 37.62, 36.95, 32.56, 31.73, 30.98, 28.40, 27.97, 27.93, 27.12, 26.25, 26.21, 23.23, 18.52, 17.30, 16.23, 15.46, 14.30; ESI-MS: m/z = 667.29 [M + Na]+. Anal. Calcd. for C41H56O6 (644.41): C, 76.36; H, 8.75%. Found: C, 76.29; H, 8.78%.
3.5. General Method for Synthesizing Compouds 25–40
The protected amino acid (N-Boc-l-methionine or N-Boc-l-selenomethionine, 0.5 mmol) was dissolved in dry DCM and stirred at 0 °C for 5 min. Then EDCI (115 mg, 0.6 mmol) and DMAP (12 mg, 0.1 mmol) were added to the solution, and the mixture was stirred at 0 °C for 1 h. The appropriate GA derivative 17–24 (0.5 mmol) was then added to the mixture. After stirring at 0 °C for another 1 h, the reaction mixture was then stirred at room temperature for 12 h. The organic layer was washed with 1 M HCl solution and concentrated in vacuo. The residue was purified by column chromatography on silica gel with ethyl acetate/petroleum to yield pure compound 25–40.
Ferulic acid methyl ester 3β-(Boc-l-methionine)-11-oxo-olean-12-en-30-oate (25). Obtained from 17 and N-Boc-l-methionine as a colourless powder (357 mg, 80%); m.p. 120–123 °C; 1H-NMR (CDCl3): δ 0.82–0.84 (m, 1H, H-5), 0.89 (s, 3H, H-28), 0.91 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.07–1.11 (m, 1H, H-15’), 1.14–1.16 (m, 1H, H-1’), 1.17 (s, 3H, H-26), 1.19 (s, 3H, H-25), 1.22–1.25 (m, 1H, H-16’), 1.27–1.31 (m, 2H, H-22’ and 21’), 1.38 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.47 (s, 9H, Boc-CH3), 1.49–1.51 (m, 1H, H-22), 1.53–1.55 (m, 1H, H-7’), 1.57–1.59 (m, 1H, H-6’), 1.62–1.64 (m, 1H, H-6), 1.67 (dd, J = 13.6, 4.4 Hz, 1H, H-19’), 1.70–1.72 (m, 1H, H-2’), 1.73–1.74 (m, 1H, H-7), 1.77–1.80 (m, 1H, H-2), 1.89 (ddd, J = 14.8, 14.8, 5.6 Hz, 1H, H-16), 2.03–2.05 (m, 1H, H-21), 2.09–2.11 (m, 1H, H-15), 2.12 (s, 3H, SCH3), 2.15 (dd, J = 13.6, 4.4 Hz, 1H, H-18), 2.38 (s, 1H, H-9), 2.43–2.45 (m, 1H, H-19), 2.57–2.60 (m, 2H, SCH2), 2.83 (ddd, J = 13.6, 2.8, 2.8 Hz, 1H, H-1), 3.83 (s, 3H, COOCH3), 3.88 (s, 3H, OCH3), 4.80 (dd, J = 11.2, 5.2 Hz, 1H, H-3), 5.14 (t, J = 8.4 Hz, 1H, COOCH3), 5.73 (s, 1H, H-12), 6.42 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.02 (d, J = 8.0 Hz, 1H, Bn-H-5), 7.13 (s, 1H, Bn-H-3), 7.15 (d, J = 8.0 Hz, 1H, Bn-H-6), 7.68 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (CDCl3): δ 199.82, 174.40, 171.90, 169.23, 166.85, 154.87, 151.38, 149.94, 145.89, 128.40, 123.02, 121.15, 118.31, 113.49, 109.10, 81.93, 79.69, 61.54, 55.69, 54.94, 52.73, 51.67, 48.00, 45.33, 44.47, 43.11, 41.18, 38.06, 38.02, 37.40, 36.86, 32.64, 32.10, 31.81, 31.15, 29.63, 28.47, 28.26, 28.09, 28.04, 26.40, 26.34, 23.48, 23.28, 18.59, 17.32, 16.74, 16.30, 15.36; ESI-MS: m/z = 892.25 [M + H]+. Anal. Calcd. for C51H73NO10S (891.50): C, 68.66; H, 8.25; N, 1.57; S, 3.59%. Found: C, 68.60; H, 8.28; N, 1.54; S, 3.60%.
Ferulic acid methyl ester 3β-(Boc-l-selenomethionine)-11-oxo-olean-12-en-30-oate (26). Obtained from 17 and N-Boc-l-selenomethionine as a colourless powder (389 mg, 83%); m.p. 125–127 °C; 1H-NMR (CDCl3): δ 0.82–0.85 (m, 1H, H-5), 0.90 (s, 3H, H-28), 0.91 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.07–1.11 (m, 1H, H-15’), 1.14–1.16 (m, 1H, H-1’), 1.18 (s, 3H, H-26), 1.19 (s, 3H, H-25), 1.23–1.25 (m, 1H, H-16’), 1.27–1.30 (m, 2H, H-22’ and 21’), 1.38 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.46 (s, 9H, Boc-CH3), 1.49–1.52 (m, 1H, H-22), 1.54–1.56 (m, 1H, H-7’), 1.58–1.60 (m, 1H, H-6’), 1.62–1.65 (m, 1H, H-6), 1.67 (dd, J = 13.6, 4.4 Hz, 1H, H-19’), 1.70–1.72 (m, 1H, H-2’), 1.73–1.76 (m, 1H, H-7), 1.77–1.79 (m, 1H, H-2), 1.88 (ddd, J = 14.8, 14.8, 5.6 Hz, 1H, H-16), 1.89-1.92 (m, 1H, H-21), 1.98–2.00 (m, 1H, H-15), 2.02 (s, 3H, SeCH3), 2.12 (dd, J = 13.6, 4.4 Hz, 1H, H-18), 2.39 (s, 1H, H-9), 2.44–2.47 (m, 1H, H-19), 2.58–2.61 (m, 2H, SeCH2), 2.83 (ddd, J = 13.6, 2.8, 2.8 Hz, 1H, H-1), 3.84 (s, 3H, COOCH3), 3.89 (s, 3H, OCH3), 4.59 (dd, J = 11.2, 5.2 Hz, 1H, H-3), 5.12 (t, J = 8.4 Hz, 1H, CHCOO), 5.73 (s, 1H, H-12), 6.42 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.02 (d, J = 8.0 Hz, 1H, Bn-H-5), 7.13 (s, 1H, Bn-H-3), 7.15 (d, J = 8.0 Hz, 1H, Bn-H-6), 7.68 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (CDCl3): δ 199.82, 174.40, 171.82, 169.17, 166.85, 154.90, 151.40, 149.94, 145.89, 128.40, 123.02, 121.15, 118.32, 113.49, 109.10, 82.12, 79.72, 61.54, 55.70, 54.97, 52.71, 51.67, 48.02, 45.34, 44.47, 43.15, 41.20, 38.10, 38.06, 37.43, 36.86, 32.65, 32.07, 31.84, 31.15, 29.64, 28.50, 28.27, 28.12, 28.02, 26.41, 26.35, 23.45, 23.30, 18.60, 17.32, 16.75, 16.30, 15.37; ESI-MS: m/z = 962.18 [M + Na]+. Anal. Calcd. for C51H73NO10Se (939.44): C, 65.23; H, 7.84; N, 1.49%. Found: C, 65.18; H, 7.89; N, 1.46%.
Ferulic acid ethyl ester 3β-(Boc-l-methionine)-11-oxo-olean-12-en-30-oate (27). Obtained from 18 and N-Boc-l-methionine as a colourless powder (357.8 mg, 79%); m.p. 111–113 °C; 1H-NMR (CDCl3): δ 0.80–0.83 (m, 1H, H-5), 0.88 (s, 3H, H-28), 0.89 (s, 3H, H-24), 0.90 (s, 3H, H-23), 1.05–1.08 (m, 1H, H-15’), 1.11–1.13 (m, 1H, H-1’), 1.15 (s, 3H, H-26), 1.17 (s, 3H, H-25), 1.20–1.23 (m, 1H, H-16’), 1.26–1.28 (m, 2H, H-22’ and 21’), 1.34 (t, J = 7.2 Hz, 3H, CH3), 1.35 (s, 3H, H-29), 1.39 (s, 3H, H-27), 1.45 (s, 9H, Boc-CH3), 1.48–1.50 (m, 1H, H-22), 1.51–1.54 (m, 1H, H-7’), 1.58–1.60 (m, 1H, H-6’), 1.61–1.63 (m, 1H, H-6), 1.68 (dd, J = 13.6, 4.4 Hz, 1H, H-19’), 1.71–1.73 (m, 1H, H-2’), 1.75–1.77 (m, 1H, H-7), 1.78–1.81 (m, 1H, H-2), 1.89 (ddd, J = 14.4, 14.4, 5.2 Hz, 1H, H-16), 2.04–2.06 (m, 1H, H-21), 2.08–2.09 (m, 1H, H-15), 2.10 (s, 3H, SCH3), 2.15 (dd, J = 13.2, 4.0 Hz, 1H, H-18), 2.37–2.39 (m, 1H, H-9), 2.41–2.44 (m, 1H, H-19), 2.55–2.57 (m, 2H, SCH2), 2.81 (ddd, J = 14.0, 3.2, 3.2 Hz, 1H, H-1), 3.86 (s, 3H, OCH3), 4.27 (q, J = 7.2 Hz, 2H, CH2CH3), 4.57 (dd, J = 11.6, 4.8 Hz, 1H, H-3), 5.12 (t, J = 8.4 Hz, 1H, CHCOO), 5.71 (s, 1H, H-12), 6.39 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.00 (d, J = 7.6 Hz, 1H, Bn-H-5), 7.11 (s, 1H, Bn-H-3), 7.12 (d, J = 7.6 Hz, 1H, Bn-H-6), 7.65 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (CDCl3): δ 199.92, 174.28, 171.90, 169.32, 166.77, 155.10, 151.33, 149.92, 145.86, 128.38, 123.00, 121.10, 118.30, 113.49, 109.10, 82.03, 79.80, 61.60, 60.50, 55.67, 54.94, 52.71, 48.00, 45.32, 44.36, 43.13, 41.16, 38.05, 38.04, 37.36, 36.83, 32.61, 32.12, 31.81, 31.16, 29.63, 28.47, 28.26, 28.12, 28.04, 26.45, 26.34, 23.50, 23.28, 18.62, 17.30, 16.74, 16.32, 15.37, 14.26; ESI-MS: m/z = 928.27 [M + Na]+. Anal. Calcd. for C52H75NO10S (905.51): C, 68.92; H, 8.34; N, 1.55; S, 3.54%. Found: C, 68.86; H, 8.37; N, 1.51; S, 3.57%.
Ferulic acid ethyl ester 3β-(Boc-l-selenomethionine)-11-oxo-olean-12-en-30-oate (28). Obtained from 18 and N-Boc-l-selenomethionine as a colourless powder (390 mg, 82%); m.p. 118–120 °C; 1H-NMR (CDCl3): δ 0.82–0.85 (m, 1H, H-5), 0.90 (s, 3H, H-28), 0.91 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.07–1.10 (m, 1H, H-15’), 1.11–1.13 (m, 1H, H-1’), 1.17 (s, 3H, H-26), 1.19 (s, 3H, H-25), 1.22–1.26 (m, 1H, H-16’), 1.27–1.30 (m, 2H, H-22’ and 21’), 1.37 (t, J = 7.2 Hz, 3H, CH3), 1.38 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.47 (s, 9H, Boc-CH3), 1.49–1.51 (m, 1H, H-22), 1.53–1.56 (m, 1H, H-7’), 1.58–1.60 (m, 1H, H-6’), 1.63–1.65 (m, 1H, H-6), 1.67 (dd, J = 13.6, 4.4 Hz, 1H, H-19’), 1.73–1.75 (m, 1H, H-2’), 1.76–1.78 (m, 1H, H-7), 1.80–1.83 (m, 1H, H-2), 1.89 (ddd, J = 14.4, 14.4, 5.2 Hz, 1H, H-16), 1.97–1.99 (m, 1H, H-21), 2.00–2.01 (m, 1H, H-15), 2.02 (s, 3H, SeCH3), 2.12 (dd, J = 13.2, 4.0 Hz, 1H, H-18), 2.39 (s, 1H, H-9), 2.43–2.46 (m, 1H, H-19), 2.59–2.61 (m, 2H, SeCH2), 2.83 (ddd, J = 14.0, 3.2, 3.2 Hz, 1H, H-1), 3.88 (s, 3H, OCH3), 4.29 (q, J = 7.2 Hz, 2H, CH2CH3), 4.59 (dd, J = 11.6, 4.8 Hz, 1H, H-3), 5.13 (t, J = 8.4 Hz, 1H, CHCOO), 5.73 (s, 1H, H-12), 6.41 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.02 (d, J = 8.0 Hz, 1H, Bn-H-5), 7.13 (s, 1H, Bn-H-3), 7.14 (d, J = 8.0 Hz, 1H, Bn-H-6), 7.67 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (CDCl3): δ 199.92, 174.28, 171.83, 169.24, 166.77, 155.12, 151.36, 149.95, 145.86, 128.38, 123.03, 121.10, 118.30, 113.49, 109.10, 82.09, 79.83, 61.60, 60.50, 55.68, 54.95, 52.68, 48.02, 45.32, 44.36, 43.14, 41.18, 38.08, 38.04, 37.37, 36.83, 32.62, 32.10, 31.81, 31.16, 29.63, 28.49, 28.27, 28.15, 28.03, 26.46, 26.36, 23.47, 23.29, 18.64, 17.30, 16.76, 16.32, 15.37, 14.26; ESI-MS: m/z = 978.12 [M + Na]+. Anal. Calcd. for C52H75NO10Se (953.11): C, 65.53; H, 7.93; N, 1.47%. Found: C, 65.46; H, 7.98; N, 1.44%.
trans-4-Hydroxycinnamic acid methyl ester 3β-(Boc-l-methionine)-11-oxo-olean-12-en-30-oate (29). Obtained from 19 and N-Boc-l-methionine as a colourless powder (362 mg, 84%); m.p. 107–109 °C; 1H-NMR (CDCl3): δ 0.82-0.85 (m, 1H, H-5), 0.88 (s, 3H, H-28), 0.91 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.08-1.11 (m, 1H, H-15’), 1.13–1.15 (m, 1H, H-1’), 1.16 (s, 3H, H-26), 1.18 (s, 3H, H-25), 1.23–1.25 (m, 1H, H-16’), 1.27–1.31 (m, 2H, H-22’ and 21’), 1.37 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.47 (s, 9H, Boc-CH3), 1.48–1.50 (m, 1H, H-22), 1.51–1.53 (m, 1H, H-7’), 1.60–1.62 (m, 1H, H-6’), 1.64–1.66 (m, 1H, H-6), 1.67 (dd, J = 13.6, 4.4 Hz, 1H, H-19’), 1.74–1.76 (m, 1H, H-2’), 1.77–1.78 (m, 1H, H-7), 1.80–1.83 (m, 1H, H-2), 1.89 (ddd, J = 15.2, 15.2, 6.0 Hz, 1H, H-16), 2.06–2.08 (m, 1H, H-21), 2.10–2.11 (m, 1H, H-15), 2.12-2.15 (s, 3H, SCH3), 2.26 (dd, J = 14.0, 4.8 Hz, 1H, H-18), 2.39 (s, 1H, H-9), 2.43–2.46 (m, 1H, H-19), 2.57–2.60 (m, 2H, SCH2), 2.83 (ddd, J = 13.2, 2.4, 2.4 Hz, 1H, H-1), 3.83 (s, 3H, COOCH3), 4.59 (dd, J = 10.8, 5.6 Hz, 1H, H-3), 5.14 (t, J = 9.2 Hz, 1H, CHCOO), 5.70 (s, 1H, H-12), 6.43 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.10 (d, J = 8.4 Hz, 2H, Bn-H-2 and 6), 7.57 (d, J = 8.4 Hz, 2H, Bn-H-3 and 5), 7.70 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (CDCl3): δ 199.80, 174.83, 172.02, 168.76, 166.90, 162.24, 155.22, 145.41, 132.17, 129.13, 128.58, 121.92, 118.41, 81.98, 79.85, 61.73, 54.94, 52.88, 51.83, 48.43, 45.36, 44.39, 43.24, 41.10, 38.16, 38.12, 37.78, 37.15, 32.66, 32.23, 31.88, 31.13, 29.87, 28.54, 28.18, 28.16, 28.06, 26.43, 26.40, 23.52, 23.39, 18.65, 17.33, 16.74, 16.42, 15.42; ESI-MS: m/z = 884.22 [M + Na]+. Anal. Calcd. for C50H71NO9S (861.48): C, 69.65; H, 8.30; N, 1.62; S, 3.72%. Found: C, 69.59; H, 8.33; N, 1.58; S, 3.75%.
trans-4-Hydroxycinnamic acid methyl ester 3β-(Boc-l-selenomethionine)-11-oxo-olean-12-en-30-oate (30). Obtained from 19 and N-Boc-l-selenomethionine as a colourless powder (363 mg, 80%); m.p. 115–118 °C; 1H-NMR (400 MHz, CDCl3): δ 0.82–0.85 (m, 1H, H-5), 0.88 (s, 3H, H-28), 0.91 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.08–1.11 (m, 1H, H-15’), 1.13–1.15 (m, 1H, H-1’), 1.17 (s, 3H, H-26), 1.19 (s, 3H, H-25), 1.22–1.26 (m, 1H, H-16’), 1.28–1.31 (m, 2H, H-22’ and 21’), 1.38 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.47 (s, 9H, Boc-CH3), 1.49–1.51 (m, 1H, H-22), 1.53–1.55 (m, 1H, H-7’), 1.60–1.62 (m, 1H, H-6’), 1.64–1.66 (m, 1H, H-6), 1.68 (dd, J = 13.6, 4.4 Hz, 1H, H-19’), 1.74–1.76 (m, 1H, H-2’), 1.77–1.79 (m, 1H, H-7), 1.81–1.84 (m, 1H, H-2), 1.90 (ddd, J = 15.2, 15.2, 6.0 Hz, 1H, H-16), 1.97–1.99 (m, 1H, H-21), 2.00–2.01 (m, 1H, H-15), 2.02 (s, 3H, SeCH3), 2.26 (dd, J = 14.0, 4.8 Hz, 1H, H-18), 2.39 (s, 1H, H-9), 2.43–2.46 (m, 1H, H-19), 2.58–2.60 (m, 2H, SeCH2), 2.84 (ddd, J = 13.2, 2.4, 2.4 Hz, 1H, H-1), 3.83 (s, 3H, COOCH3), 4.59 (dd, J = 10.8, 5.6 Hz, 1H, H-3), 5.13 (t, J = 9.2 Hz, 1H, CHCOO), 5.70 (s, 1H, H-12), 6.43 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.10 (d, J = 8.4 Hz, 2H, Bn-H-2 and 6), 7.57 (d, J = 8.4 Hz, 2H, Bn-H-3 and 5), 7.71 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (CDCl3): δ 199.80, 174.83, 171.90, 168.67, 166.90, 162.25, 155.25, 145.41, 132.16, 129.13, 128.58, 121.92, 118.44, 82.07, 79.88, 61.73, 54.98, 52.85, 51.83, 48.46, 45.36, 44.39, 43.27, 41.13, 38.20, 38.12, 37.80, 37.15, 32.62, 32.20, 31.90, 31.15, 29.87, 28.57, 28.20, 28.18, 28.04, 26.44, 26.42, 23.49, 23.40, 18.67, 17.33, 16.75, 16.42, 15.42; ESI-MS: m/z = 932.16 [M + Na]+. Anal. Calcd. for C50H71NO9Se (909.43): C, 66.06; H, 7.87; N, 1.54%. Found: C, 66.00; H, 7.91; N, 1.51%.
trans-4-Hydroxycinnamic acid ethyl ester 3β-(Boc-l-methionine)-11-oxo-olean-12-en-30-oate (31). Obtained from 20 and N-Boc-l-methionine as a colourless powder (359 mg, 82%); m.p. 102–104 °C; 1H-NMR (CDCl3): δ 0.82–0.85 (m, 1H, H-5), 0.88 (s, 3H, H-28), 0.91 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.08–1.11 (m, 1H, H-15’), 1.13–1.15 (m, 1H, H-1’), 1.16 (s, 3H, H-26), 1.18 (s, 3H, H-25), 1.23–1.26 (m, 1H, H-16’), 1.28–1.30 (m, 2H, H-22’ and 21’), 1.36 (t, J = 7.2 Hz, 3H, CH3), 1.37 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.47 (s, 9H, Boc-CH3), 1.49–1.50 (m, 1H, H-22), 1.51–1.54 (m, 1H, H-7’), 1.61–1.63 (m, 1H, H-6’), 1.64–1.66 (m, 1H, H-6), 1.67 (dd, J = 13.6, 4.4 Hz, 1H, H-19’), 1.73–1.75 (m, 1H, H-2’), 1.77–1.79 (m, 1H, H-7), 1.80–1.83 (m, 1H, H-2), 1.88 (ddd, J = 14.8, 14.8, 5.6 Hz, 1H, H-16), 2.07–2.09 (m, 1H, H-21), 2.10–2.11 (m, 1H, H-15), 2.12 (s, 3H, SCH3), 2.27 (dd, J = 13.6, 4.4 Hz, 1H, H-18), 2.39 (s, 1H, H-9), 2.43–2.46 (m, 1H, H-19), 2.57–2.59 (m, 2H, SCH2), 2.83 (ddd, J = 13.6, 2.8, 2.8 Hz, 1H, H-1), 4.29 (q, J = 7.2 Hz, 2H, CH2CH3), 4.59 (dd, J = 11.2, 5.2 Hz, 1H, H-3), 5.14 (t, J = 9.2 Hz, 1H, CHCOO), 5.70 (s, 1H, H-12), 6.42 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.10 (d, J = 8.4 Hz, 2H, Bn-H-2 and 6), 7.57 (d, J = 8.4 Hz, 2H, Bn-H-3 and 5), 7.69 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (CDCl3): δ 199.83, 174.76, 171.93, 168.81, 166.79, 162.11, 155.21, 145.37, 132.13, 129.14, 128.56, 121.95, 118.38, 82.00, 79.83, 61.62, 60.49, 54.94, 52.85, 48.44, 45.33, 44.31, 43.16, 40.99, 38.07, 38.04, 37.67, 36.84, 32.60, 32.25, 31.88, 31.06, 29.84, 28.53, 28.26, 28.12, 28.01, 26.39, 26.33, 23.47, 23.33, 18.61, 17.28, 16.75, 16.33, 15.41, 14.26; ESI-MS: m/z = 898.25 [M + Na]+. Anal. Calcd. for C51H73NO9S (875.50): C, 69.91; H, 8.40; N, 1.60; S, 3.66%. Found: C, 69.84; H, 8.43; N, 1.55; S, 3.73%.
trans-4-Hydroxycinnamic acid ethyl ester 3β-(Boc-l-selenomethionine)-11-oxo-olean-12-en-30-oate (32). Obtained from 20 and N-Boc-l-selenomethionine as a colourless powder (383 mg, 83%); m.p. 110–112 °C; 1H-NMR (CDCl3): δ 0.82–0.85 (m, 1H, H-5), 0.89 (s, 3H, H-28), 0.91 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.08–1.11 (m, 1H, H-15’), 1.13–1.15 (m, 1H, H-1’), 1.17 (s, 3H, H-26), 1.19 (s, 3H, H-25), 1.23–1.26 (m, 1H, H-16’), 1.28–1.30 (m, 2H, H-22’ and 21’), 1.37 (t, J = 7.2 Hz, 3H, CH3), 1.38 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.47 (s, 9H, Boc-CH3), 1.48–1.50 (m, 1H, H-22), 1.51–1.53 (m, 1H, H-7’), 1.60–1.62 (m, 1H, H-6’), 1.64–1.66 (m, 1H, H-6), 1.67 (dd, J = 13.6, 4.4 Hz, 1H, H-19’), 1.73–1.75 (m, 1H, H-2’), 1.77–1.79 (m, 1H, H-7), 1.81–1.84 (m, 1H, H-2), 1.89 (ddd, J = 14.8, 14.8, 5.6 Hz, 1H, H-16), 1.92–1.94 (m, 1H, H-21), 1.97–1.99 (m, 1H, H-15), 2.02 (s, 3H, SeCH3), 2.26 (dd, J = 13.6, 4.4 Hz, 1H, H-18), 2.39 (s, 1H, H-9), 2.46–2.49 (m, 1H, H-19), 2.57–2.60 (m, 2H, SeCH2), 2.84 (ddd, J = 13.6, 2.8, 2.8 Hz, 1H, H-1), 4.29 (q, J = 7.2 Hz, 2H, CH2CH3), 4.59 (dd, J = 11.2, 5.2 Hz, 1H, H-3), 5.13 (t, J = 9.2 Hz, 1H, CHCOO), 5.70 (s, 1H, H-12), 6.42 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.10 (d, J = 8.4 Hz, 2H, Bn-H-2 and 6), 7.57 (d, J = 8.4 Hz, 2H, Bn-H-3 and 5), 7.70 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (CDCl3): δ 199.83, 174.75, 171.84, 168.78, 166.79, 162.13, 155.25, 145.37, 132.16, 129.14, 128.56, 121.94, 118.38, 82.09, 79.87, 61.62, 60.47, 54.96, 52.80, 48.49, 45.33, 44.36, 43.15, 41.03, 38.05, 38.04, 37.65, 36.78, 32.60, 32.23, 31.88, 31.00, 29.82, 28.59, 28.30, 28.15, 27.96, 26.41, 26.36, 23.42, 23.35, 18.60, 17.28, 16.77, 16.33, 15.40, 14.28; ESI-MS: m/z = 946.23 [M + Na]+. Anal. Calcd. for C51H73NO9Se (923.45): C, 66.36; H, 7.97; N, 1.52%. Found: C, 66.33; H, 8.02; N, 1.48%.
Isoferulic acid methyl ester 3β-(Boc-l-methionine)-11-oxo-olean-12-en-30-oate (33). Obtained from 21 and N-Boc-l-methionine as a colourless powder (361 mg, 81%); m.p. 117–119 °C; 1H-NMR (CDCl3): δ 0.82–0.85 (m, 1H, H-5), 0.89 (s, 3H, H-28), 0.90 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.07–1.11 (m, 1H, H-15’), 1.14–1.16 (m, 1H, H-1’), 1.17 (s, 3H, H-26), 1.18 (s, 3H, H-25), 1.22–1.26 (m, 1H, H-16’), 1.28–1.29 (m, 2H, H-22’ and 21’), 1.38 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.46 (s, 9H, Boc-CH3), 1.49 (m, 1H, H-22), 1.53 (m, 1H, H-7’), 1.58 (m, 1H, H-6’), 1.62 (m, 1H, H-6), 1.67 (dd, 1H, J = 13.6, 4.4 Hz, H-19’), 1.73 (m, 1H, H-2’), 1.77 (m, 1H, H-7), 1.80 (m, 1H, H-2), 1.88 (ddd, 1H, J = 14.8, 14.8, 5.2 Hz, H-16), 2.06 (m, 1H, H-21), 2.10 (m, 1H, H-15), 2.12 (s, 3H, SCH3), 2.15 (dd, 1H, J = 13.2, 4.4 Hz, H-18), 2.39 (s, 1H, H-9), 2.44 (m, 1H, H-19), 2.57 (m, 2H, SCH2), 2.83 (ddd, 1H, J = 13.2, 2.8, 2.8 Hz, H-1), 3.81 (s, 3H, COOCH3), 3.88 (s, 3H, OCH3), 4.59 (dd, 1H, J = 11.2, 5.2 Hz, H-3), 5.14 (t, 1H, J = 8.4 Hz, CHCOO), 5.72 (s, 1H, H-12), 6.32 (d, 1H, Jtrans = 16.0 Hz, H-β), 6.98 (d, 1H, J = 8.4 Hz, Bn-H-3), 7.19 (d, 1H, J = 2.0 Hz, Bn-H-6), 7.38 (dd, 1H, J = 8.4, 2.0 Hz, Bn-H-4), 7.63 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (CDCl3): δ 199.95, 174.34, 171.83, 169.03, 166.96, 154.82, 152.83, 143.64, 138.11, 128.42, 127.43, 127.38, 121.70, 116.21, 112.26, 81.90, 79.66, 61.56, 55.71, 54.87, 52.69, 51.53, 47.93, 45.29, 44.36, 43.03, 41.02, 38.95, 38.85, 37.84, 36.90, 32.63, 32.12, 31.83, 31.12, 29.60, 28.47, 28.33, 28.07, 28.05, 26.35, 26.28, 23.50, 23.13, 18.57, 17.30, 16.73, 16.25, 15.37; ESI-MS: m/z = 914.28 [M + Na]+. Anal. Calcd. for C51H73NO10S (891.50): C, 68.66; H, 8.25; N, 1.57; S, 3.59%. Found: C, 68.60; H, 8.31; N, 1.53; S, 3.63%.
Isoferulic acid methyl ester 3β-(Boc-l-selenomethionine)-11-oxo-olean-12-en-30-oate (34). Obtained from 21 and N-Boc-l-selenomethionine as a colourless powder (375 mg, 80%); m.p. 124–125 °C; 1H-NMR (CDCl3): δ 0.82–0.85 (m, 1H, H-5), 0.89 (s, 3H, H-28), 0.90 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.07–1.11 (m, 1H, H-15’), 1.14–1.16 (m, 1H, H-1’), 1.18 (s, 3H, H-26), 1.19 (s, 3H, H-25), 1.22–1.26 (m, 1H, H-16’), 1.28–1.29 (m, 2H, H-22’ and 21’), 1.38 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.47 (s, 9H, Boc-CH3), 1.49 (m, 1H, H-22), 1.52 (m, 1H, H-7’), 1.60 (m, 1H, H-6’), 1.62 (m, 1H, H-6), 1.67 (dd, 1H, J = 13.6, 4.4 Hz, H-19’), 1.73 (m, 1H, H-2’), 1.77 (m, 1H, H-7), 1.80 (m, 1H, H-2), 1.88 (ddd, 1H, J = 14.8, 14.8, 5.2 Hz, H-16), 1.93 (m, 1H, H-21), 2.00 (m, 1H, H-15), 2.02 (s, 3H, SeCH3), 2.13 (dd, 1H, J = 13.2, 4.4 Hz, H-18), 2.39 (s, 1H, H-9), 2.44 (m, 1H, H-19), 2.58 (m, 2H, SeCH2), 2.83 (ddd, 1H, J = 13.2, 2.8, 2.8 Hz, H-1), 3.81 (s, 3H, COOCH3), 3.88 (s, 3H, OCH3), 4.59 (dd, 1H, J = 11.2, 5.2 Hz, H-3), 5.12 (t, 1H, J = 8.4 Hz, CHCOO), 5.73 (s, 1H, H-12), 6.32 (d, 1H, Jtrans = 16.0 Hz, H-β), 6.98 (d, 1H, J = 8.4 Hz, Bn-H-3), 7.19 (d, 1H, J = 2.0 Hz, Bn-H-6), 7.38 (dd, 1H, J = 8.4, 2.0 Hz, Bn-H-4), 7.63 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (CDCl3): δ 199.95, 174.34, 171.75, 169.00, 166.97, 154.85, 152.85, 143.64, 138.11, 128.42, 127.43, 127.38, 121.70, 116.21, 112.26, 81.90, 79.70, 61.56, 55.73, 54.88, 52.67, 51.53, 47.96, 45.31, 44.36, 43.06, 41.05, 39.02, 38.85, 37.86, 36.90, 32.65, 32.04, 31.85, 31.13, 29.58, 28.52, 28.33, 28.10, 28.03, 26.37, 26.30, 23.47, 23.10, 18.59, 17.30, 16.74, 16.26, 15.39; ESI-MS: m/z = 962.12 [M + Na]+. Anal. Calcd. for C51H73NO10Se (939.44): C, 65.23; H, 7.84; N, 1.49%. Found: C, 65.15; H, 7.89; N, 1.43%.
Isoferulic acid ethyl ester 3β-(Boc-l-methionine)-11-oxo-olean-12-en-30-oate (35). Obtained from 22 and N-Boc-l-methionine as a colourless powder (376 mg, 83%); m.p. 111–114 °C; 1H-NMR (CDCl3): δ 0.80–0.83 (m, 1H, H-5), 0.88 (s, 3H, H-28), 0.89 (s, 3H, H-24), 0.90 (s, 3H, H-23), 1.05–1.09 (m, 1H, H-15’), 1.14–1.16 (m, 1H, H-1’), 1.16 (s, 3H, H-26), 1.17 (s, 3H, H-25), 1.20–1.24 (m, 1H, H-16’), 1.26–1.28 (m, 2H, H-22’ and 21’), 1.33 (t, 3H, J = 7.2 Hz, CH3), 1.36 (s, 3H, H-29), 1.40 (s, 3H, H-27), 1.45 (s, 9H, Boc-CH3), 1.48 (m, 1H, H-22), 1.51 (m, 1H, H-7’), 1.58 (m, 1H, H-6’), 1.61 (m, 1H, H-6), 1.64 (dd, 1H, J = 13.6, 4.4 Hz, H-19’), 1.72 (m, 1H, H-2’), 1.75 (m, 1H, H-7), 1.78 (m, 1H, H-2), 1.88 (ddd, 1H, J = 14.4, 14.4, 5.2 Hz, H-16), 2.05 (m, 1H, H-21), 2.10 (m, 1H, H-15), 2.10 (s, 3H, SCH3), 2.14 (dd, 1H, J = 13.2, 4.0 Hz, H-18), 2.37 (s, 1H, H-9), 2.43 (m, 1H, H-19), 2.55 (m, 2H, SCH2), 2.81 (ddd, 1H, J = 14.0, 3.2, 3.2 Hz, H-1), 3.86 (s, 3H, OCH3), 4.25 (q, 2H, J = 7.2 Hz, CH2CH3), 4.57 (dd, 1H, J = 11.6, 4.8 Hz, H-3), 5.12 (t, 1H, J = 8.4 Hz, CHCOO), 5.71 (s, 1H, H-12), 6.30 (d, 1H, Jtrans = 16.0 Hz, H-β), 6.96 (d, 1H, J = 8.4 Hz, Bn-H-3), 7.18 (d, 1H, J = 2.0 Hz, Bn-H-6), 7.36 (dd, 1H, J = 8.4, 2.0 Hz, Bn-H-4), 7.60 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (CDCl3): δ 200.13, 174.23, 171.83, 169.13, 166.88, 155.04, 152.80, 143.61, 138.08, 128.40, 127.40, 127.38, 121.65, 116.20, 112.26, 81.93, 79.78, 61.65, 60.65, 55.70, 54.87, 52.70, 47.93, 45.27, 44.27, 43.05, 41.05, 38.96, 38.85, 37.80, 36.87, 32.60, 32.15, 31.83, 31.14, 29.61, 28.47, 28.33, 28.10, 28.05, 26.37, 26.30, 23.52, 23.13, 18.59, 17.29, 16.73, 16.28, 15.38, 14.23; ESI-MS: m/z = 928.24 [M + Na]+. Anal. Calcd. for C52H75NO10S (905.51): C, 68.92; H, 8.34; N, 1.55; S, 3.54%. Found: C, 68.87; H, 8.39; N, 1.51; S, 3.56%.
Isoferulic acid methyl ester 3β-(Boc-l-selenomethionine)-11-oxo-olean-12-en-30-oate (36). Obtained from 22 and N-Boc-l-selenomethionine as a colourless powder (405 mg, 85%); m.p. 119–121 °C; 1H-NMR (CDCl3): δ 0.80–0.83 (m, 1H, H-5), 0.88 (s, 3H, H-28), 0.89 (s, 3H, H-24), 0.90 (s, 3H, H-23), 1.05–1.09 (m, 1H, H-15’), 1.12–1.14 (m, 1H, H-1’), 1.16 (s, 3H, H-26), 1.17 (s, 3H, H-25), 1.20–1.24 (m, 1H, H-16’), 1.26–1.28 (m, 2H, H-22’ and 21’), 1.33 (t, 3H, J = 7.2 Hz, CH3), 1.36 (s, 3H, H-29), 1.40 (s, 3H, H-27), 1.45 (s, 9H, Boc-CH3), 1.48 (m, 1H, H-22), 1.51 (m, 1H, H-7’), 1.59 (m, 1H, H-6’), 1.62 (m, 1H, H-6), 1.64 (dd, 1H, J = 13.6, 4.4 Hz, H-19’), 1.71 (m, 1H, H-2’), 1.75 (m, 1H, H-7), 1.78 (m, 1H, H-2), 1.88 (ddd, 1H, J = 14.4, 14.4, 5.2 Hz, H-16), 1.96 (m, 1H, H-21), 1.98(m, 1H, H-15), 2.00 (s, 3H, SeCH3), 2.10 (dd, 1H, J = 13.2, 4.0 Hz, H-18), 2.37 (s, 1H, H-9), 2.43 (m, 1H, H-19), 2.55 (m, 2H, SeCH2), 2.81 (ddd, 1H, J = 14.0, 3.2, 3.2 Hz, H-1), 3.86 (s, 3H, OCH3), 4.25 (q, 2H, J = 7.2 Hz, CH2CH3), 4.57 (dd, 1H, J = 11.6, 4.8 Hz, H-3), 5.10 (t, 1H, J = 8.4 Hz, CHCOO), 5.71 (s, 1H, H-12), 6.30 (d, 1H, Jtrans = 16.0 Hz, H-β), 6.96 (d, 1H, J = 8.8 Hz, Bn-H-3), 7.18 (d, 1H, J = 2.0 Hz, Bn-H-6), 7.36 (dd, 1H, J = 8.8, 2.0 Hz, Bn-H-4), 7.60 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (CDCl3): δ 200.14 174.23, 171.77, 169.05, 166.88, 155.06, 152.83, 143.62, 138.08, 128.40, 127.40, 127.38, 121.65, 116.20, 112.26, 81.93, 79.80, 61.65, 60.65, 55.72, 54.89, 52.66, 47.94, 45.27, 44.27, 43.06, 41.08, 38.96, 38.86, 37.82, 36.87, 32.61, 32.12, 31.83, 31.14, 29.61, 28.48, 28.31, 28.12, 28.04, 26.39, 26.30, 23.49, 23.15, 18.61, 17.29, 16.75, 16.28, 15.38, 14.23; ESI-MS: m/z = 976.15 [M + Na]+. Anal. Calcd. for C52H75NO10Se (953.11): C, 65.53; H, 7.93; N, 1.47%. Found: C, 65.48; H, 8.00; N, 1.43%.
trans-3-Hydroxycinnamic acid methyl ester 3β-(Boc-l-methionine)-11-oxo-olean-12-en-30-oate (37). Obtained from 23 and N-Boc-l-methionine as a colourless powder (349 mg, 81%); m.p. 109–111 °C; 1H-NMR (CDCl3): δ 0.82–0.85 (m, 1H, H-5), 0.90 (s, 3H, H-28), 0.91 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.08–1.12 (m, 1H, H-15’), 1.13–1.15 (m, 1H, H-1’), 1.17(s, 3H, H-26), 1.19 (s, 3H, H-25), 1.23–1.25 (m, 1H, H-16’), 1.26–1.28 (m, 2H, H-22’ and 21’), 1.39 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.47 (s, 9H, Boc-CH3), 1.50 (m, 1H, H-22), 1.53 (m, 1H, H-7’), 1.61 (m, 1H, H-6’), 1.64 (m, 1H, H-6), 1.67 (dd, 1H, J = 13.6, 4.4 Hz, H-19’), 1.74 (m, 1H, H-2’), 1.77 (m, 1H, H-7), 1.80 (m, 1H, H-2), 1.89 (ddd, 1H, J = 15.2, 15.2, 6.0 Hz, H-16), 2.07 (m, 1H, H-21), 2.10 (m, 1H, H-15), 2.13 (s, 3H, SCH3), 2.28 (dd, 1H, J = 14.0, 4.8 Hz, H-18), 2.39 (s, 1H, H-9), 2.46 (m, 1H, H-19), 2.57 (m, 2H, SCH2), 2.84 (ddd, 1H, J = 13.2, 2.4, 2.4 Hz, H-1), 3.83 (s, 3H, COOCH3), 4.59 (dd, 1H, J = 10.8, 5.6 Hz, H-3), 5.14 (t, 1H, J = 9.2 Hz, CHCOO), 5.71 (s, 1H, H-12), 6.46 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.09 (dt, 1H, J = 6.8, 2.4 Hz, Bn-H-6), 7.21 (s, 1H, Bn-H-2), 7.42 (s, 1H, Bn-H-4), 7.44 (t, 1H, J = 7.6 Hz, Bn-H-5), 7.70 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (CDCl3): δ 199.80, 174.87, 171.91, 168.82, 167.02, 155.24, 151.11, 143.61, 135.95, 129.88, 128.57, 125.50, 123.22, 120.67, 118.89, 81.99, 79.82, 61.61, 54.92, 52.85, 51.76, 48.36, 45.32, 44.28, 43.15, 40.94, 38.57, 38.07, 37.72, 36.84, 32.59, 32.23, 31.88, 31.07, 29.84, 28.57, 28.26, 28.12, 28.07, 26.37, 26.32, 23.53, 23.33, 18.61, 17.28, 16.75, 16.31, 15.41; ESI-MS: m/z = 884.27 [M + Na]+. Anal. Calcd. for C50H71NO9S (861.48): C, 69.65; H, 8.30; N, 1.62; S, 3.72%. Found: C, 69.61; H, 8.35; N, 1.57; S, 3.78%.
trans-3-Hydroxycinnamic acid methyl ester 3β-(Boc-l-selenomethionine)-11-oxo-olean-12-en-30-oate (38). Obtained from 23 and N-Boc-l-selenomethionine as a colourless powder (377 mg, 83%); m.p. 112–115 °C; 1H-NMR (CDCl3): δ 0.82–0.85 (m, 1H, H-5), 0.90 (s, 3H, H-28), 0.91 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.08–1.11 (m, 1H, H-15’), 1.13–1.15 (m, 1H, H-1’), 1.17(s, 3H, H-26), 1.19 (s, 3H, H-25), 1.23–1.25 (m, 1H, H-16’), 1.26–1.28 (m, 2H, H-22’ and 21’), 1.39 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.47 (s, 9H, Boc-CH3), 1.50 (m, 1H, H-22), 1.53 (m, 1H, H-7’), 1.61 (m, 1H, H-6’), 1.65 (m, 1H, H-6), 1.67 (dd, 1H, J = 13.6, 4.4 Hz, H-19’), 1.74 (m, 1H, H-2’), 1.77 (m, 1H, H-7), 1.80 (m, 1H, H-2), 1.89 (ddd, 1H, J = 15.2, 15.2, 6.0 Hz, H-16), 1.97 (m, 1H, H-21), 2.01 (m, 1H, H-15), 2.02 (s, 3H, SeCH3), 2.16 (dd, 1H, J = 14.0, 4.8 Hz, H-18), 2.39 (s, 1H, H-9), 2.46 (m, 1H, H-19), 2.58 (m, 2H, SeCH2), 2.84 (ddd, 1H, J = 13.2, 2.4, 2.4 Hz, H-1), 3.83 (s, 3H, COOCH3), 4.59 (dd, 1H, J = 10.8, 5.6 Hz, H-3), 5.13 (t, 1H, J = 9.2 Hz, CHCOO), 5.71 (s, 1H, H-12), 6.46 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.09 (dt, 1H, J = 6.8, 2.4 Hz, Bn-H-6), 7.21 (s, 1H, Bn-H-2), 7.42 (s, 1H, Bn-H-4), 7.44 (t, 1H, J = 7.6 Hz, Bn-H-5), 7.70 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (CDCl3): δ 199.80, 174.87, 171.83, 168.78, 167.02, 155.26, 151.12, 143.61, 135.96, 129.88, 128.57, 125.50, 123.22, 120.67, 118.90, 82.06, 79.84, 61.61, 54.93, 52.83, 51.76, 48.37, 45.32, 44.28, 43.16, 40.95, 38.59, 38.07, 37.73, 36.84, 32.60, 32.21, 31.88, 31.07, 29.84, 28.58, 28.27, 28.14, 28.06, 26.38, 26.33, 23.49, 23.34, 18.62, 17.28, 16.76, 16.31, 15.41; ESI-MS: m/z = 932.22 [M + Na]+. Anal. Calcd. for C50H71NO9Se (909.43): C, 66.06; H, 7.87; N, 1.54%. Found: C, 66.01; H, 7.91; N, 1.52%.
trans-3-Hydroxycinnamic acid ethyl ester 3β-(Boc-l-methionine)-11-oxo-olean-12-en-30-oate (39). Obtained from 24 and N-Boc-l-methionine as a colourless powder (341 mg, 78%); m.p. 107–108 °C; 1H-NMR (CDCl3): δ 0.82–0.85 (m, 1H, H-5), 0.90 (s, 3H, H-28), 0.91 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.08–1.12 (m, 1H, H-15’), 1.13–1.15 (m, 1H, H-1’), 1.17(s, 3H, H-26), 1.19 (s, 3H, H-25), 1.23–1.25 (m, 1H, H-16’), 1.26–1.28 (m, 2H, H-22’ and 21’), 1.36 (t, 3H, J = 7.2 Hz, CH3), 1.38 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.47 (s, 9H, Boc-CH3), 1.50 (m, 1H, H-22), 1.53 (m, 1H, H-7’), 1.61 (m, 1H, H-6’), 1.64 (m, 1H, H-6), 1.67 (dd, 1H, J = 13.6, 4.4 Hz, H-19’), 1.74 (m, 1H, H-2’), 1.77 (m, 1H, H-7), 1.80 (m, 1H, H-2), 1.89 (ddd, 1H, J = 14.8, 14.8, 5.6 Hz, H-16), 2.07 (m, 1H, H-21), 2.10 (m, 1H, H-15), 2.13 (s, 3H, SCH3), 2.28 (dd, 1H, J = 13.6, 4.4 Hz, H-18), 2.39 (s, 1H, H-9), 2.46 (m, 1H, H-19), 2.56 (m, 2H, SCH2), 2.84 (ddd, 1H, J = 13.6, 2.8, 2.8 Hz, H-1), 4.29 (q, 2H, J = 7.2 Hz, CH2CH3), 4.59 (dd, 1H, J = 11.2, 5.2 Hz, H-3), 5.15 (t, 1H, J = 9.2 Hz, CHCOO), 5.71 (s, 1H, H-12), 6.46 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.08 (dt, 1H, J = 6.8, 2.4 Hz, Bn-H-6), 7.22 (s, 1H, Bn-H-2), 7.42 (s, 1H, Bn-H-4), 7.44 (t, 1H, J = 7.6 Hz, Bn-H-5), 7.69 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (CDCl3): δ 199.92, 174.72, 171.91, 168.98, 166.93, 155.64, 150.97, 143.48, 135.84, 129.72, 128.45, 125.38, 123.00, 120.54, 118.78, 82.10, 79.97, 61.73, 60.45, 54.92, 52.83, 48.36, 45.32, 44.10, 43.16, 40.85, 38.58, 38.06, 37.69, 36.82, 32.57, 32.26, 31.85, 31.09, 29.85, 28.57, 28.26, 28.14, 28.07, 26.45, 26.32, 23.54, 23.33, 18.63, 17.27, 16.75, 16.32, 15.41, 14.30; ESI-MS: m/z = 898.30 [M + Na]+. Anal. Calcd. for C51H73NO9S (875.50): C, 69.91; H, 8.40; N, 1.60; S, 3.66%. Found: C, 69.87; H, 8.46; N, 1.54; S, 3.70%.
trans-3-Hydroxycinnamic acid ethyl ester 3β-(Boc-l-selenomethionine)-11-oxo-olean-12-en-30-oate (40). Obtained from 24 and N-Boc-l-selenomethionine as a colourless powder (364 mg, 79%); m.p. 109–112 °C; 1H-NMR (CDCl3): δ 0.82–0.85 (m, 1H, H-5), 0.90 (s, 3H, H-28), 0.91 (s, 3H, H-24), 0.92 (s, 3H, H-23), 1.08–1.11 (m, 1H, H-15’), 1.13–1.15 (m, 1H, H-1’), 1.17(s, 3H, H-26), 1.19 (s, 3H, H-25), 1.23–1.25 (m, 1H, H-16’), 1.26–1.28 (m, 2H, H-22’ and 21’), 1.36 (t, 3H, J = 7.2 Hz, CH3), 1.38 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.47 (s, 9H, Boc-CH3), 1.50 (m, 1H, H-22), 1.53 (m, 1H, H-7’), 1.61 (m, 1H, H-6’), 1.64 (m, 1H, H-6), 1.70 (dd, 1H, J = 13.6, 4.4 Hz, H-19’), 1.74 (m, 1H, H-2’), 1.77 (m, 1H, H-7), 1.80 (m, 1H, H-2), 1.90 (ddd, 1H, J = 14.8, 14.8, 5.6 Hz, H-16), 1.98 (m, 1H, H-21), 2.01 (m, 1H, H-15), 2.02 (s, 3H, SeCH3), 2.29 (dd, 1H, J = 13.6, 4.4 Hz, H-18), 2.39 (s, 1H, H-9), 2.46 (m, 1H, H-19), 2.58 (m, 2H, SeCH2), 2.84 (ddd, 1H, J = 13.6, 2.8, 2.8 Hz, H-1), 4.29 (q, 2H, J = 7.2 Hz, CH2CH3), 4.59 (dd, 1H, J = 11.2, 5.2 Hz, H-3), 5.13 (t, 1H, J = 9.2 Hz, CHCOO), 5.71 (s, 1H, H-12), 6.46 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.08 (dt, 1H, J = 6.8, 2.4 Hz, Bn-H-6), 7.22 (s, 1H, Bn-H-2), 7.42 (s, 1H, Bn-H-4), 7.44 (t, 1H, J = 7.6 Hz, Bn-H-5), 7.68 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (CDCl3): δ 199.92, 174.72, 171.82, 168.93, 166.93, 155.67, 150.98, 143.48, 135.86, 129.72, 128.45, 125.36, 123.03, 120.51, 118.78, 82.17, 80.00, 61.73, 60.44, 54.95, 52.82, 48.38, 45.32, 44.13, 43.19, 40.87, 38.57, 38.09, 37.68, 36.79, 32.58, 32.26, 31.84, 31.03, 29.85, 28.60, 28.29, 28.15, 28.03, 26.46, 26.36, 23.51, 23.30, 18.62, 17.27, 16.78, 16.32, 15.41, 14.31; ESI-MS: m/z = 946.26 [M + Na]+. Anal. Calcd. for C51H73NO9Se (923.45): C, 66.36; H, 7.97; N, 1.52%. Found: C, 66.33; H, 8.01; N, 1.47%.
3.6. General Method for Synthesizing Compouds 41–56
The Boc-protected compounds 25–40 (0.25 mmol) were dissolved in dry DCM. After saturation with dry hydrogen chloride gas for 10 min, stirring at room temperature was then continued for 24 h. After completion of the reaction (as monitored by TLC), the resulting precipitate was filtered and washed with ethyl acetate until no parent substance can be detected; analytical samples were obtained by re-crystallization to obtain pure compounds 41–56.
Ferulic acid methyl ester 3β-(l-methionine)-11-oxo-olean-12-en-30-oate hydrochloride (41). Obtained from 25 as a white solid (155 mg, 81%); m.p. 203–205 °C; 1H-NMR (DMSO-d6): δ 0.71–0.74 (m, 1H, H-5), 0.83 (s, 3H, H-28), 0.84 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.90–0.93 (m, 1H, H-15’), 1.00–1.03 (m, 1H, H-1’), 1.07 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.12–1.14 (m, 1H, H-16’), 1.20–1.24 (m, 2H, H-22’ and 21’), 1.32 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.43–1.45 (m, 1H, H-22), 1.48–1.50 (m, 1H, H-7’), 1.51–1.52 (m, 1H, H-6’), 1.53–1.55 (m, 1H, H-6), 1.62 (dd, J = 13.2, 4.0 Hz, 1H, H-19’), 1.67–1.69 (m, 1H, H-2’), 1.71–1.73 (m, 1H, H-7), 1.75–1.78 (m, 1H, H-2), 1.86 (ddd, J = 14.8, 14.8, 6.0 Hz, 1H, H-16), 1.91–1.93 (m, 1H, H-21), 1.95–1.97 (m, 1H, H-15), 2.04 (s, 3H, SCH3), 2.28–2.31 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.54–2.56 (m, 1H, H-19), 2.57–2.60 (m, 2H, SCH2), 2.64 (ddd, J = 13.2, 3.2, 3.2 Hz, 1H, H-1), 3.74 (s, 3H, COOCH3), 3.83 (s, 3H, OCH3), 4.47 (dd, J = 11.2, 5.2 Hz, 1H, H-3), 5.46 (s, 1H, H-12), 6.72 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.11 (d, J = 8.0 Hz, 1H, Bn-H-5), 7.33 (dd, J = 8.0, 1.6 Hz, 1H, Bn-H-3), 7.55 (d, J = 1.6 Hz, 1H, Bn-H-6), 7.67 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (DMSO-d6): δ 199.91, 174.52, 173.90, 169.23, 166.87, 151.38, 149.96, 145.89, 128.40, 123.02, 121.15, 118.31, 113.51, 109.11, 81.45, 61.59, 55.69, 54.98, 52.84, 51.69, 48.03, 45.37, 44.50, 43.11, 41.28, 38.10, 38.04, 37.40, 36.86, 32.65, 32.12, 31.83, 31.25, 29.69, 28.52, 28.29, 28.10, 26.40, 26.38, 23.58, 23.30, 18.69, 17.42, 16.74, 16.33, 15.40; ESI-MS: m/z = 792.22 [M + H]+. Anal. Calcd. for C46H66ClNO8S (791.44): C, 66.68; H, 8.03; N, 1.69; S, 3.87%. Found: C, 66.63; H, 8.07; N, 1.66; S, 3.90%.
Ferulic acid methyl ester 3β-(l-selenomethionine)-11-oxo-olean-12-en-30-oate hydrochloride (42). Obtained from 26 as a white solid (156 mg, 77%); m.p. 210–213 °C; 1H-NMR (DMSO-d6): δ 0.71–0.74 (m, 1H, H-5), 0.83 (s, 3H, H-28), 0.84 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.91–0.94 (m, 1H, H-15’), 1.00–1.04 (m, 1H, H-1’), 1.07 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.12–1.15 (m, 1H, H-16’), 1.21–1.24 (m, 2H, H-22’ and 21’), 1.32 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.43–1.45 (m, 1H, H-22), 1.49–1.51 (m, 1H, H-7’), 1.52–1.53 (m, 1H, H-6’), 1.54–1.57 (m, 1H, H-6), 1.63 (dd, J = 13.2, 4.0 Hz, 1H, H-19’), 1.67–1.69 (m, 1H, H-2’), 1.71–1.73 (m, 1H, H-7), 1.76–1.78 (m, 1H, H-2), 1.86 (ddd, J = 14.8, 14.8, 6.0 Hz, 1H, H-16), 1.88–1.90 (m, 1H, H-21), 1.92–1.93 (m, 1H, H-15), 1.94 (s, 3H, SeCH3), 2.28–2.31 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.54–2.56 (m, 1H, H-19), 2.57–2.59 (m, 2H, SeCH2), 2.64 (ddd, J = 13.2, 3.2, 3.2 Hz, 1H, H-1), 3.74 (s, 3H, COOCH3), 3.84 (s, 3H, OCH3), 4.48 (dd, J = 11.2, 5.2 Hz, 1H, H-3), 5.46 (s, 1H, H-12), 6.72 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.11 (d, J = 8.0 Hz, 1H, Bn-H-5), 7.33 (dd, J = 8.0, 1.6 Hz, 1H, Bn-H-3), 7.55 (d, J = 1.6 Hz, 1H, Bn-H-6), 7.67 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (DMSO-d6): δ 199.92, 174.47, 173.82, 169.27, 166.85, 151.40, 149.94, 145.90, 128.42, 123.02, 121.16, 118.32, 113.49, 109.10, 82.42, 61.54, 55.72, 55.00, 52.81, 51.67, 48.02, 45.34, 44.54, 43.15, 41.30, 38.06, 38.00, 37.43, 36.88, 32.65, 32.17, 31.84, 31.28, 29.74, 28.50, 28.27, 28.12, 26.43, 26.36, 23.55, 23.32, 18.71, 17.43, 16.75, 16.34, 15.38; ESI-MS: m/z = 840.26 [M + H]+. Anal. Calcd. for C46H66ClNO8Se (839.39): C, 63.11; H, 7.60; N, 1.60%. Found: C, 63.03; H, 7.65; N, 1.54%.
Ferulic acid ethyl ester 3β-(l-methionine)-11-oxo-olean-12-en-30-oate hydrochloride (43). Obtained from 27 as a white solid (142 mg, 73%); m.p. 191–194 °C; 1H-NMR (DMSO-d6): δ 0.74–0.78 (m, 1H, H-5), 0.83 (s, 3H, H-28), 0.84 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.90–0.93 (m, 1H, H-15’), 1.00–1.02 (m, 1H, H-1’), 1.07 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.12–1.14 (m, 1H, H-16), 1.17–1.23 (m, 2H, H-22’ and 21’), 1.27 (t, J = 7.2 Hz, 3H, CH3), 1.31 (s, 3H, H-29), 1.40 (s, 3H, H-27), 1.45–1.47 (m, 1H, H-22), 1.48–1.50 (m, 1H, H-7’), 1.51–1.52 (m, 1H, H-6’), 1.54–1.57 (m, 1H, H-6), 1.63 (dd, J = 13.2, 4.0 Hz, 1H, H-19’), 1.67–1.69 (m, 1H, H-2’), 1.70–1.73 (m, 1H, H-7), 1.79–1.81 (m, 1H, H-2), 1.86 (ddd, J = 14.4, 14.4, 5.6 Hz, 1H, H-16), 1.92-1.93 (m, 1H, H-21), 1.94–1.96 (m, 1H, H-15), 2.03 (s, 3H, SCH3), 2.28–2.31 (m, 1H, H-18), 2.42 (s, 1H, H-9), 2.54–2.55 (m, 1H, H-19), 2.56–2.59 (m, 2H, SCH2), 2.63 (ddd, J = 13.6, 3.6, 3.6 Hz, 1H, H-1), 3.83 (s, 3H, OCH3), 4.20 (q, J = 7.2 Hz, 2H, CH2CH3), 4.47 (dd, J = 11.6, 4.8 Hz, 1H, H-3), 5.46 (s, 1H, H-12), 6.72 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.10 (d, J = 8.0 Hz, 1H, Bn-H-5), 7.31 (dd, J = 8.0, 1.6 Hz, 1H, Bn-H-3), 7.55 (d, J = 1.6 Hz, 1H, Bn-H-6), 7.64 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (DMSO-d6): δ 200.02, 174.37, 174.03, 169.45, 166.78, 151.33, 149.94, 145.86, 128.38, 123.03, 121.10, 118.30, 113.47, 109.12, 81.43, 61.60, 60.50, 55.67, 54.94, 53.52, 48.02, 45.33, 44.36, 43.15, 41.26, 38.07, 38.05, 37.36, 36.83, 32.63, 32.14, 31.79, 31.21, 29.72, 28.47, 28.37, 28.15, 26.45, 26.34, 23.63, 23.28, 18.75, 17.47, 16.72, 16.39, 15.46, 14.26; ESI-MS: m/z = 828.30 [M + Na]+. Anal. Calcd. for C47H68ClNO8S (805.46): C, 67.00; H, 8.13; N, 1.66; S, 3.81%. Found: C, 66.95; H, 8.20; N, 1.61; S, 3.85%.
Ferulic acid ethyl ester 3β-(l-selenomethionine)-11-oxo-olean-12-en-30-oate hydrochloride (44). Obtained from 28 as a white solid (160 mg, 78%); m.p. 205–207 °C; 1H-NMR (DMSO-d6): δ 0.70–0.74 (m, 1H, H-5), 0.83 (s, 3H, H-28), 0.84 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.91–0.93 (m, 1H, H-15’), 1.00–1.03 (m, 1H, H-1’), 1.07 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.12–1.14 (m, 1H, H-16’), 1.20–1.24 (m, 2H, H-22’ and 21’), 1.27 (t, J = 7.2 Hz, 3H, CH3), 1.32 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.46–1.48 (m, 1H, H-22), 1.49–1.50 (m, 1H, H-7’), 1.51–1.52 (m, 1H, H-6’), 1.54–1.56 (m, 1H, H-6), 1.65 (dd, J = 13.2, 4.0 Hz, 1H, H-19’), 1.68-1.69 (m, 1H, H-2’), 1.70–1.73 (m, 1H, H-7), 1.79–1.82 (m, 1H, H-2), 1.86 (ddd, J = 14.4, 14.4, 5.6 Hz, 1H, H-16), 1.89–1.90 (m, 1H, H-21), 1.91–1.92 (m, 1H, H-15), 1.93 (s, 3H, SeCH3), 2.28–2.31 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.54–2.56 (m, 1H, H-19), 2.57–2.60 (m, 2H, SeCH2), 2.63 (ddd, J = 13.6, 3.6, 3.6 Hz, 1H, H-1), 3.83 (s, 3H, OCH3), 4.20 (q, J = 7.2 Hz, 2H, CH2CH3), 4.47 (dd, J = 11.6, 4.8 Hz, 1H, H-3), 5.46 (s, 1H, H-12), 6.72 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.11 (d, J = 8.0 Hz, 1H, Bn-H-5), 7.32 (dd, J = 8.0, 1.6 Hz, 1H, Bn-H-3), 7.56 (d, J = 1.6 Hz, 1H, Bn-H-6), 7.65 (d, Jtrans = 16.0 Hz, 1H, H-α); 13C-NMR (DMSO-d6): δ 200.06, 174.38, 173.98, 169.39, 166.87, 151.36, 149.95, 145.88, 128.42, 123.05, 121.13, 118.30, 113.51, 109.11, 81.49, 61.60, 60.50, 55.68, 54.97, 53.49, 48.04, 45.32, 44.37, 43.14, 41.28, 38.08, 38.04, 37.39, 36.84, 32.62, 32.13, 31.80, 31.26, 29.73, 28.49, 28.39, 28.13, 26.46, 26.37, 23.59, 23.29, 18.76, 17.50, 16.76, 16.42, 15.47, 14.27; ESI-MS: m/z = 854.18 [M + H]+. Anal. Calcd. for C47H68ClNO8Se (853.40): C, 63.47; H, 7.71; N, 1.57%. Found: C, 63.44; H, 7.75; N, 1.53%.
trans-4-Hydroxycinnamic acid methyl ester 3β-(l-methionine)-11-oxo-olean-12-en-30-oate hydrochloride (45). Obtained from 29 as a white solid (143 mg, 76%); m.p. 205–207 °C; 1H-NMR (DMSO-d6): δ 0.71–0.74 (m, 1H, H-5), 0.83 (s, 3H, H-28), 0.84 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.91–0.93 (m, 1H, H-15’), 1.00–1.04 (m, 1H, H-1’), 1.07 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.17–1.20 (m, 1H, H-16’), 1.24–1.27 (m, 2H, H-22’ and 21’), 1.33 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.45–1.48 (m, 1H, H-22), 1.49–1.50 (m, 1H, H-7’), 1.52-1.53 (m, 1H, H-6’), 1.54–1.57 (m, 1H, H-6), 1.64 (dd, J = 13.2, 4.0 Hz, 1H, H-19’), 1.68–1.70 (m, 1H, H-2’), 1.71–1.73 (m, 1H, H-7), 1.79–1.82 (m, 1H, H-2), 1.87 (ddd, J = 15.2, 15.2, 6.4 Hz, 1H, H-16), 1.93–1.95 (m, 1H, H-21), 1.97–2.00 (m, 1H, H-15), 2.04 (s, 3H, SCH3), 2.15–2.17 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.53–2.55 (m, 1H, H-19), 2.56–2.59 (m, 2H, SCH2), 2.64 (ddd, J = 12.8, 2.8, 2.8 Hz, 1H, H-1), 3.74 (s, 3H, COOCH3), 4.47 (dd, J = 10.8, 5.6 Hz, 1H, H-3), 5.46 (s, 1H, H-12), 6.65 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.17 (d, J = 8.8 Hz. 2H, Bn-H-2 and 6), 7.69 (d, J = 8.8 Hz, 2H, Bn-H-3 and 5), 7.81 (d, Jtrans = 16.0 Hz, 2H, H-α); 13C-NMR (DMSO-d6): δ 199.80, 174.83, 174.01, 168.76, 166.98, 162.27, 145.45, 132.17, 129.13, 128.58, 121.98, 118.45, 81.48, 61.73, 54.94, 53.68, 51.83, 48.43, 45.36, 44.39, 43.24, 41.10, 38.16, 38.12, 37.78, 37.15, 32.66, 32.43, 31.88, 31.13, 29.98, 28.54, 28.18, 28.14, 26.57, 26.40, 23.63, 23.39, 18.65, 17.33, 16.74, 16.42, 15.54; ESI-MS: m/z = 784.23 [M + Na]+. Anal. Calcd. for C45H64ClNO7S (761.43): C, 67.69; H, 8.08; N, 1.75; S, 4.02%. Found: C, 67.65; H, 8.10; N, 1.69; S, 4.10%.
trans-4-Hydroxycinnamic acid methyl ester 3β-(l-selenomethionine)-11-oxo-olean-12-en-30-oate hydrochloride (46). Obtained from 30 as a white solid (156 mg, 80%); m.p. 218–221 °C; 1H-NMR (DMSO-d6): δ 0.71–0.74 (m, 1H, H-5), 0.83 (s, 3H, H-28), 0.84 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.90–0.93 (m, 1H, H-15’), 1.00–1.03 (m, 1H, H-1’), 1.06 (s, 3H, H-26), 1.07 (s, 3H, H-25), 1.16–1.20 (m, 1H, H-16’), 1.23–1.27 (m, 2H, H-22’ and 21’), 1.33 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.44–1.46 (m, 1H, H-22), 1.48–1.50 (m, 1H, H-7’), 1.52–1.55 (m, 1H, H-6’), 1.56–1.59 (m, 1H, H-6), 1.65 (dd, J = 13.2, 4.0 Hz, 1H, H-19’), 1.68–1.69 (m, 1H, H-2’), 1.71–1.74 (m, 1H, H-7), 1.78–1.81 (m, 1H, H-2), 1.86 (ddd, J = 15.2, 15.2, 6.4 Hz, 1H, H-16), 1.88–1.90 (m, 1H, H-21), 1.91–1.92 (m, 1H, H-15), 1.93 (s, 3H, SeCH3), 2.15–2.18 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.53–2.55 (m, 1H, H-19), 2.58–2.61 (m, 2H, SeCH2), 2.64 (ddd, J = 12.8, 2.8, 2.8 Hz, 1H, H-1), 3.73 (s, 3H, COOCH3), 4.47 (dd, J = 10.8, 5.6 Hz, 1H, H-3), 5.45 (s, 1H, H-12), 6.64 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.17 (d, J = 8.8 Hz, 2H, Bn-H-2 and 6), 7.69 (d, J = 8.8 Hz, 2H, Bn-H-3 and 5), 7.81 (d, Jtrans = 16.0 Hz, 2H, H-α); 13C-NMR (DMSO-d6): δ 199.83, 174.83, 173.95, 168.67, 167.03, 162.25, 145.44, 132.20, 129.13, 128.58, 121.97, 118.48, 81.52, 61.73, 54.96, 53.65, 51.83, 48.44, 45.36, 44.35, 43.27, 41.13, 38.18, 38.14, 37.80, 37.15, 32.62, 32.40, 31.93, 31.15, 30.00, 28.59, 28.20, 28.18, 26.59, 26.42, 23.65, 23.40, 18.70, 17.29, 16.72, 16.48, 15.53; ESI-MS: m/z = 810.14 [M + H]+. Anal. Calcd. for C45H64ClNO7Se (809.38): C, 63.93; H, 7.63; N, 1.66%. Found: C, 63.86; H, 7.68; N, 1.61%.
trans-4-Hydroxycinnamic acid ethyl ester 3β-(l-methionine)-11-oxo-olean-12-en-30-oatehydrochloride (47). Obtained from 31 as a white solid (138 mg, 74%); m.p. 202–204 °C; 1H-NMR (DMSO-d6): δ 0.71–0.74 (m, 1H, H-5), 0.83 (s, 3H, H-28), 0.84 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.91–0.94 (m, 1H, H-15’), 1.00–1.04 (m, 1H, H-1’), 1.07 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.13–1.14 (m, 1H, H-16’), 1.18–1.22 (m, 2H, H-22’ and 21’), 1.27 (t, J = 7.2 Hz, 3H, CH3), 1.33 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.45–1.47 (m, 1H, H-22), 1.49–1.51 (m, 1H, H-7’), 1.52–1.55 (m, 1H, H-6’), 1.57–1.60 (m, 1H, H-6), 1.64 (dd, J = 13.2, 4.0 Hz, 1H, H-19’), 1.67–1.68 (m, 1H, H-2’), 1.71–1.74 (m, 1H, H-7), 1.79–1.82 (m, 1H, H-2), 1.87 (ddd, J = 14.8, 14.8, 6.0 Hz, 1H, H-16), 1.93–1.95 (m, 1H, H-21), 1.97–1.99 (m, 1H, H-15), 2.04 (s, 3H, SCH3), 2.15–2.17 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.52–2.54 (m, 1H, H-19), 2.56–2.58 (m, 2H, SCH2), 2.64 (ddd, J = 13.2, 3.2, 3.2 Hz, 1H, H-1), 4.20 (q, J = 7.2 Hz, 2H, CH2CH3), 4.47 (dd, J = 11.2, 5.2 Hz, 1H, H-3), 5.46 (s, 1H, H-12), 6.63 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.17 (d, J = 8.8 Hz, 2H, Bn-H-2 and 6), 7.67 (d, J = 8.8 Hz, 2H, Bn-H-3 and 5), 7.81 (d, Jtrans = 16.0 Hz, 2H, H-α); 13C-NMR (DMSO-d6): δ 199.94, 174.85, 174.05, 168.92, 166.91, 162.23, 145.47, 132.17, 129.36, 128.58, 122.02, 118.40, 81.40, 61.62, 60.63, 54.94, 53.68, 48.44, 45.47, 44.40, 43.21, 41.06, 38.05, 38.01, 37.63, 36.90, 32.60, 32.36, 31.88, 31.06, 29.96, 28.52, 28.30, 28.25, 26.46, 26.43, 23.60, 23.36, 18.74, 17.40, 16.75, 16.43, 15.54, 14.46; ESI-MS: m/z = 776.28 [M + H]+. Anal. Calcd. for C46H66ClNO7S (775.45): C, 68.00; H, 8.19; N, 1.72; S, 3.95%. Found: C, 67.96; H, 8.21; N, 1.70; S, 3.97%.
trans-4-Hydroxycinnamic acid ethyl ester 3β-(l-selenomethionine)-11-oxo-olean-12-en-30-oate hydrochloride (48). Obtained from 32 as a white solid (145 mg, 73%); m.p. 208–211 °C; 1H-NMR (DMSO-d6): δ 0.71–0.74 (m, 1H, H-5), 0.83 (s, 3H, H-28), 0.84 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.91–0.94 (m, 1H, H-15’), 1.00–1.04 (m, 1H, H-1’), 1.07 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.13–1.14 (m, 1H, H-16’), 1.18–1.22 (m, 2H, H-22’ and 21’), 1.27 (t, J = 7.2 Hz, 3H, CH3), 1.33 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.45–1.47 (m, 1H, H-22), 1.49–1.51 (m, 1H, H-7’), 1.52–1.55 (m, 1H, H-6’), 1.57–1.59 (m, 1H, H-6), 1.65 (dd, J = 13.2, 4.0 Hz, 1H, H-19’), 1.68–1.70 (m, 1H, H-2’), 1.71–1.73 (m, 1H, H-7), 1.78–1.80 (m, 1H, H-2), 1.87 (ddd, J = 14.8, 14.8, 6.0 Hz, 1H, H-16), 1.89–1.91 (m, 1H, H-21), 1.92–1.93 (m, 1H, H-15), 1.94 (s, 3H, SeCH3), 2.15–2.18 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.52–2.54 (m, 1H, H-19), 2.56–2.59 (m, 2H, SeCH2), 2.64 (ddd, J = 13.2, 3.2, 3.2 Hz, 1H, H-1), 4.20 (q, J = 7.2 Hz, 2H, CH2CH3), 4.47 (dd, J = 11.2, 5.2 Hz, 1H, H-3), 5.46 (s, 1H, H-12), 6.63 (d, Jtrans = 16.0 Hz, 1H, H-β), 7.17 (d, J = 8.8 Hz, 2H, Bn-H-2 and 6), 7.67 (d, J = 8.8 Hz, 2H, Bn-H-3 and 5), 7.81 (d, Jtrans = 16.0 Hz, 2H, H-α); 13C-NMR (DMSO-d6): δ 199.92, 174.88, 173.98, 168.98, 166.88, 162.25, 145.49, 132.19, 129.28, 128.56, 122.06, 118.45, 81.49, 61.62, 60.59, 54.96, 53.64, 48.49, 45.43, 44.36, 43.26, 41.03, 38.05, 38.04, 37.65, 36.89, 32.60, 32.33, 31.88, 31.10, 29.97, 28.59, 28.32, 28.26, 26.44, 26.37, 23.59, 23.35, 18.75, 17.38, 16.77, 16.46, 15.54, 14.48; ESI-MS: m/z = 846.32 [M + Na]+. Anal. Calcd. for C46H66ClNO7Se (823.39): C, 64.29; H, 7.74; N, 1.63%. Found: C, 64.25; H, 7.77; N, 1.59%.
Isoferulic acid methyl ester 3β-(l-methionine)-11-oxo-olean-12-en-30-oate hydrochloride (49). Obtained from 33 as a white solid (143 mg, 75%); m.p. 199–201 °C; 1H-NMR (DMSO-d6): δ 0.71–0.74 (m, 1H, H-5), 0.84 (s, 3H, H-28), 0.85 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.91–0.94 (m, 1H, H-15’), 1.00–1.03 (m, 1H, H-1’), 1.08 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.12–1.14 (m, 1H, H-16’), 1.20–1.24 (m, 2H, H-22’ and 21’), 1.33 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.46 (m, 1H, H-22), 1.50 (m, 1H, H-7’), 1.52 (m, 1H, H-6’), 1.55 (m, 1H, H-6), 1.64 (dd, 1H, J = 13.2, 4.0 Hz, H-19’), 1.68 (m, 1H, H-2’), 1.71 (m, 1H, H-7), 1.80 (m, 1H, H-2), 1.87 (ddd, 1H, J = 14.8, 14.8, 5.6 Hz, H-16), 1.92 (m, 1H, H-21), 1.95 (m, 1H, H-15), 2.04 (s, 3H, SCH3), 2.28 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.54 (m, 1H, H-19), 2.57 (m, 2H, SCH2), 2.64 (ddd, 1H, J = 12.8, 3.2, 3.2 Hz, H-1), 3.72 (s, 3H, COOCH3), 3.82 (s, 3H, OCH3), 4.47 (dd, 1H, J = 11.2, 5.2 Hz, H-3), 5.48 (s, 1H, H-12), 6.60 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.18 (d, 1H, J = 8.4 Hz, Bn-H-3), 7.57 (d, 1H, J = 2.0 Hz, Bn-H-6), 7.62 (dd, 1H, J = 8.4, 2.0 Hz, Bn-H-4), 7.63 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (DMSO-d6): δ 200.03, 174.45, 172.81, 169.03, 166.97, 152.83, 143.64, 138.11, 128.42, 127.43, 127.38, 121.70, 116.21, 112.29, 81.49, 61.60, 55.71, 54.89, 52.80, 51.55, 47.91, 45.32, 44.39, 43.03, 41.13, 38.95, 38.89, 37.84, 36.90, 32.63, 32.15, 31.84, 31.21, 29.63, 28.51, 28.35, 28.12, 26.35, 26.29, 23.59, 23.15, 18.67, 17.40, 16.73, 16.27, 15.41; ESI-MS: m/z = 814.21 [M + Na]+. Anal. Calcd. for C46H66ClNO8S (791.44): C, 66.68; H, 8.03; N, 1.69; S, 3.87%. Found: C, 66.62; H, 8.08; N, 1.66; S, 3.91%.
Isoferulic acid methyl ester 3β-(l-selenomethionine)-11-oxo-olean-12-en-30-oate hydrochloride (50). Obtained from 34 as a white solid (158 mg, 78%); m.p. 201–203 °C; 1H-NMR (DMSO-d6): δ 0.71–0.74 (m, 1H, H-5), 0.84 (s, 3H, H-28), 0.85 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.91–0.94 (m, 1H, H-15’), 1.00–1.03 (m, 1H, H-1’), 1.08 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.13–1.15 (m, 1H, H-16’), 1.20–1.24 (m, 2H, H-22’ and 21’), 1.33 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.46 (m, 1H, H-22), 1.50 (m, 1H, H-7’), 1.52 (m, 1H, H-6’), 1.55 (m, 1H, H-6), 1.64 (dd, 1H, J = 13.2, 4.0 Hz, H-19’), 1.68 (m, 1H, H-2’), 1.71 (m, 1H, H-7), 1.80 (m, 1H, H-2), 1.86 (ddd, 1H, J = 14.8, 14.8, 5.6 Hz, H-16), 1.89 (m, 1H, H-21), 1.92 (m, 1H, H-15), 1.93 (s, 3H, SeCH3), 2.28 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.55 (m, 1H, H-19), 2.58 (m, 2H, SeCH2), 2.64 (ddd, 1H, J = 12.8, 3.2, 3.2 Hz, H-1), 3.72 (s, 3H, COOCH3), 3.82 (s, 3H, OCH3), 4.47 (dd, 1H, J = 11.2, 5.2 Hz, H-3), 5.48 (s, 1H, H-12), 6.60 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.18 (d, 1H, J = 8.4 Hz, Bn-H-3), 7.57 (d, 1H, J = 2.0 Hz, Bn-H-6), 7.62 (dd, 1H, J = 8.4, 2.0 Hz, Bn-H-4), 7.63 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (DMSO-d6): δ 200.05, 174.43, 172.75, 169.11, 166.97, 152.85, 143.64, 138.13, 128.43, 127.43, 127.38, 121.70, 116.21, 112.26, 81.42, 61.56, 55.76, 54.91, 52.76, 51.53, 47.96, 45.31, 44.45, 43.06, 41.14, 39.00, 38.85, 37.86, 36.91, 32.65, 32.15, 31.85, 31.26, 29.66, 28.52, 28.33, 28.13, 26.38, 26.30, 23.54, 23.14, 18.70, 17.33, 16.74, 16.30, 15.40; ESI-MS: m/z = 840.28 [M + H]+. Anal. Calcd. for C46H66ClNO8Se (839.39): C, 63.11; H, 7.60; N, 1.60%. Found: C, 63.05; H, 7.64; N, 1.53%.
Isoferulic acid ethyl ester 3β-(l-methionine)-11-oxo-olean-12-en-30-oate hydrochloride (51). Obtained from 35 as a white solid (144 mg, 74%); m.p. 192–195 °C; 1H-NMR (DMSO-d6): δ 0.71–0.74 (m, 1H, H-5), 0.84 (s, 3H, H-28), 0.85 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.91–0.94 (m, 1H, H-15’), 1.00–1.04 (m, 1H, H-1’), 1.08 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.13–1.15 (m, 1H, H-16’), 1.18–1.21 (m, 2H, H-22’ and 21’), 1.26 (t, 3H, J = 7.2 Hz, CH3), 1.33 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.46 (m, 1H, H-22), 1.50 (m, 1H, H-7’), 1.52 (m, 1H, H-6’), 1.54 (m, 1H, H-6), 1.64 (dd, 1H, J = 13.2, 4.0 Hz, H-19’), 1.68 (m, 1H, H-2’), 1.71 (m, 1H, H-7), 1.80 (m, 1H, H-2), 1.86 (ddd, 1H, J = 14.4, 14.4, 5.6 Hz, H-16), 1.89 (m, 1H, H-21), 1.92 (m, 1H, H-15), 2.04 (s, 3H, SCH3), 2.29 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.53 (m, 1H, H-19), 2.56 (m, 2H, SCH2), 2.64 (ddd, 1H, J = 13.6, 3.6, 3.6 Hz, H-1), 3.82 (s, 3H, OCH3), 4.18 (q, 2H, J = 7.2 Hz, CH2CH3), 4.47 (dd, 1H, J = 11.6, 4.8 Hz, H-3), 5.48 (s, 1H, H-12), 6.59 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.18 (d, 1H, J = 8.4 Hz, Bn-H-3), 7.59 (d, 1H, J = 2.0 Hz, Bn-H-6), 7.61 (dd, 1H, J = 8.4, 2.0 Hz, Bn-H-4), 7.62 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (DMSO-d6): δ 200.21, 174.46, 172.85, 169.25, 166.90, 152.80, 143.61, 138.08, 128.40, 127.41, 127.38, 121.65, 116.17, 112.26, 81.35, 61.65, 60.65, 55.70, 54.87, 53.47, 47.96, 45.27, 44.27, 43.08, 41.17, 38.99, 38.85, 37.80, 36.87, 32.61, 32.18, 31.80, 31.20, 29.70, 28.47, 28.42, 28.17, 26.37, 26.30, 23.64, 23.13, 18.72, 17.38, 16.70, 16.34, 15.43, 14.23; ESI-MS: m/z = 828.33 [M + Na]+. Anal. Calcd. for C47H68ClNO8S (805.46): C, 67.00; H, 8.13; N, 1.66; S, 3.81%. Found: C, 66.96; H, 8.15; N, 1.61; S, 3.84%.
Isoferulic acid ethyl ester 3β-(l-selenomethionine)-11-oxo-olean-12-en-30-oate hydrochloride (52). Obtained from 36 as a white solid (154 mg, 75%); m.p. 196–198 °C; 1H-NMR (DMSO-d6): δ 0.71–0.75 (m, 1H, H-5), 0.84 (s, 3H, H-28), 0.85 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.91–0.93 (m, 1H, H-15’), 1.00–1.04 (m, 1H, H-1’), 1.08 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.13–1.15 (m, 1H, H-16’), 1.18–1.21 (m, 2H, H-22’ and 21’), 1.26 (t, 3H, J = 7.2 Hz, CH3), 1.33 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.46 (m, 1H, H-22), 1.50 (m, 1H, H-7’), 1.52 (m, 1H, H-6’), 1.54 (m, 1H, H-6), 1.64 (dd, 1H, J = 13.2, 4.0 Hz, H-19’), 1.68 (m, 1H, H-2’), 1.70 (m, 1H, H-7), 1.80 (m, 1H, H-2), 1.86 (ddd, 1H, J = 14.4, 14.4, 5.6 Hz, H-16), 1.89 (m, 1H, H-21), 1.92 (m, 1H, H-15), 1.94 (s, 3H, SeCH3), 2.30 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.55 (m, 1H, H-19), 2.58 (m, 2H, SeCH2), 2.64 (ddd, 1H, J = 13.6, 3.6, 3.6 Hz, H-1), 3.82 (s, 3H, OCH3), 4.18 (q, 2H, J = 7.2 Hz, CH2CH3), 4.47 (dd, 1H, J = 11.6, 4.8 Hz, H-3), 5.48 (s, 1H, H-12), 6.59 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.18 (d, 1H, J = 8.4 Hz, Bn-H-3), 7.59 (d, 1H, J = 2.0 Hz, Bn-H-6), 7.61 (dd, 1H, J = 8.4, 2.0 Hz, Bn-H-4), 7.62 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (DMSO-d6): δ 200.24, 174.41, 172.87, 169.20, 166.96, 152.83, 143.62, 138.08, 128.43, 127.41, 127.36, 121.65, 116.20, 112.25, 81.33, 61.65, 60.65, 55.72, 54.91, 53.45, 47.94, 45.27, 44.27, 43.06, 41.18, 38.96, 38.86, 37.82, 36.88, 32.61, 32.15, 31.83, 31.23, 29.68, 28.48, 28.45, 28.15, 26.39, 26.30, 23.60, 23.15, 18.74, 17.42, 16.75, 16.37, 15.47, 14.25; ESI-MS: m/z = 854.24 [M + H]+. Anal. Calcd. for C47H68ClNO8Se (853.40): C, 63.47; H, 7.71; N, 1.57%. Found: C, 63.44; H, 7.74; N, 1.52%.
trans-3-Hydroxycinnamic acid methyl ester 3β-(l-methionine)-11-oxo-olean-12-en-30-oate hydrochloride (53). Obtained from 37 as a white solid (145 mg, 79%); m.p. 190–192 °C; 1H-NMR (DMSO-d6): δ 0.72–0.74 (m, 1H, H-5), 0.84 (s, 3H, H-28), 0.85 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.91–0.93 (m, 1H, H-15’), 1.00–1.04 (m, 1H, H-1’), 1.06 (s, 3H, H-26), 1.07 (s, 3H, H-25), 1.12–1.14 (m, 1H, H-16’), 1.17–1.20 (m, 2H, H-22’ and 21’), 1.34 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.45 (m, 1H, H-22), 1.49 (m, 1H, H-7’), 1.52 (m, 1H, H-6’), 1.54 (m, 1H, H-6), 1.65 (dd, 1H, J = 13.2, 4.0 Hz, H-19’), 1.68 (m, 1H, H-2’), 1.70 (m, 1H, H-7), 1.79 (m, 1H, H-2), 1.86 (ddd, 1H, J = 15.2, 15.2, 6.4 Hz, H-16), 1.94 (m, 1H, H-21), 1.97 (m, 1H, H-15), 2.03 (s, 3H, SCH3), 2.18 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.52 (m, 1H, H-19), 2.54 (m, 2H, SCH2), 2.63 (ddd, 1H, J = 12.8, 2.8, 2.8 Hz, H-1), 3.74 (s, 3H, COOCH3), 4.47 (dd, 1H, J = 10.8, 5.6 Hz, H-3), 5.48 (s, 1H, H-12), 6.74 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.15 (dd, 1H, J = 8.0, 2.0 Hz, Bn-H-6), 7.48 (t, 1H, J = 8.0 Hz, Bn-H-5), 7.54 (s, 1H, Bn-H-2), 7.65 (d, 1H, J = 7.6 Hz, Bn-H-4), 7.71 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (DMSO-d6): δ 199.80, 174.87, 173.94, 168.82, 167.07, 151.16, 143.69, 135.91, 129.83, 128.57, 125.59, 123.26, 120.65, 118.94, 81.50, 61.61, 54.92, 53.63, 51.76, 48.36, 45.32, 44.28, 43.15, 40.94, 38.57, 38.07, 37.72, 36.84, 32.59, 32.41, 31.88, 31.07, 29.95, 28.57, 28.26, 28.16, 26.49, 26.32, 23.61, 23.33, 18.61, 17.28, 16.75, 16.31, 15.54; ESI-MS: m/z = 762.27 [M + H]+. Anal. Calcd. for C45H64ClNO7S (761.43): C, 67.69; H, 8.08; N, 1.75; S, 4.02%. Found: C, 67.64; H, 8.13; N, 1.72; S, 4.10%.
trans-3-Hydroxycinnamic acid methyl ester 3β-(l-selenomethionine)-11-oxo-olean-12-en-30-oate hydro-chloride (54). Obtained from 38 as a white solid (148 mg, 76%); m.p. 194–196 °C; 1H-NMR (DMSO-d6): δ 0.72–0.74 (m, 1H, H-5), 0.84 (s, 3H, H-28), 0.85 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.90–0.93 (m, 1H, H-15’), 1.00–1.03 (m, 1H, H-1’), 1.06 (s, 3H, H-26), 1.07 (s, 3H, H-25), 1.12–1.14 (m, 1H, H-16’), 1.17–1.20 (m, 2H, H-22’ and 21’), 1.34 (s, 3H, H-29), 1.41 (s, 3H, H-27), 1.44 (m, 1H, H-22), 1.49 (m, 1H, H-7’), 1.52 (m, 1H, H-6’), 1.54 (m, 1H, H-6), 1.65 (dd, 1H, J = 13.2, 4.0 Hz, H-19’), 1.68 (m, 1H, H-2’), 1.70 (m, 1H, H-7), 1.75 (m, 1H, H-2), 1.86 (ddd, 1H, J = 15.2, 15.2, 6.4 Hz, H-16), 1.89 (m, 1H, H-21), 1.91 (m, 1H, H-15), 1.93 (s, 3H, SeCH3), 2.18 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.55 (m, 1H, H-19), 2.60 (m, 2H, SeCH2), 2.63 (ddd, 1H, J = 12.8, 2.8, 2.8 Hz, H-1), 3.74 (s, 3H, COOCH3), 4.47 (dd, 1H, J = 10.8, 5.6 Hz, H-3), 5.49 (s, 1H, H-12), 6.74 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.15 (dd, 1H, J = 8.0, 2.0 Hz, Bn-H-6), 7.48 (t, 1H, J = 8.0 Hz, Bn-H-5), 7.54 (s, 1H, Bn-H-2), 7.65 (d, 1H, J = 7.6 Hz, Bn-H-4), 7.71 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (DMSO-d6): δ 199.82, 174.87, 173.89, 168.78, 167.09, 151.12, 143.63, 136.03, 129.88, 128.55, 125.53, 123.25, 120.67, 118.97, 81.56, 61.61, 54.93, 53.60, 51.76, 48.37, 45.32, 44.26, 43.16, 40.95, 38.59, 38.07, 37.76, 36.81, 32.63, 32.43, 31.88, 31.13, 29.94, 28.58, 28.27, 28.18, 26.45, 26.33, 23.66, 23.34, 18.68, 17.25, 16.79, 16.34, 15.57; ESI-MS: m/z = 832.18 [M + Na]+. Anal. Calcd. for C45H64ClNO7Se (809.38): C, 63.93; H, 7.63; N, 1.66%. Found: C, 63.90; H, 7.66; N, 1.63%.
trans-3-Hydroxycinnamic acid ethyl ester 3β-(l-methionine)-11-oxo-olean-12-en-30-oate hydrochloride (55). Obtained from 39 as a white solid (136 mg, 73%); m.p. 183–185 °C; 1H-NMR (DMSO-d6): δ 0.72–0.74 (m, 1H, H-5), 0.84 (s, 3H, H-28), 0.85 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.91–0.93 (m, 1H, H-15’), 1.00–1.04 (m, 1H, H-1’), 1.07 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.12–1.14 (m, 1H, H-16’), 1.17–1.20 (m, 2H, H-22’ and 21’), 1.27 (t, 3H, J = 7.2 Hz, CH3), 1.34 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.45 (m, 1H, H-22), 1.49 (m, 1H, H-7’), 1.52 (m, 1H, H-6’), 1.55 (m, 1H, H-6), 1.65 (dd, 1H, J = 13.2, 4.0 Hz, H-19’), 1.68 (m, 1H, H-2’), 1.70 (m, 1H, H-7), 1.79 (m, 1H, H-2), 1.86 (ddd, 1H, J = 14.8, 14.8, 6.0 Hz, H-16), 1.95 (m, 1H, H-21), 1.98 (m, 1H, H-15), 2.04 (s, 3H, SCH3), 2.19 (m, 1H, H-18), 2.43 (s, 1H, H-9), 2.52 (m, 1H, H-19), 2.56 (m, 2H, SCH2), 2.64 (ddd, 1H, J = 13.2, 3.2, 3.2 Hz, H-1), 4.20 (q, 2H, J = 7.2 Hz, CH2CH3), 4.48 (dd, 1H, J = 11.2, 5.2 Hz, H-3), 5.49 (s, 1H, H-12), 6.74 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.15 (dd, 1H, J = 8.0, 2.0 Hz, Bn-H-6), 7.48 (t, 1H, J = 8.0 Hz, Bn-H-5), 7.56 (s, 1H, Bn-H-2), 7.64 (d, 1H, J = 7.6 Hz, Bn-H-4), 7.70 (d, 1H, Jrans = 16.0 Hz, H-α); 13C-NMR (DMSO-d6): δ 200.03, 174.81, 172.05, 169.08, 167.02, 151.03, 143.58, 135.87, 129.79, 128.49, 125.58, 123.17, 120.60, 118.79, 81.48, 61.73, 60.61, 54.92, 53.65, 48.36, 45.43, 44.21, 43.16, 40.97, 38.58, 38.12, 37.66, 36.87, 32.55, 32.36, 31.85, 31.17, 29.98, 28.57, 28.35, 28.19, 26.41, 26.42, 23.79, 23.33, 18.76, 17.37, 16.75, 16.48, 15.58, 14.51; ESI-MS: m/z = 776.34 [M + H]+. Anal. Calcd. for C46H66ClNO7S (775.45): C, 68.00; H, 8.19; N, 1.72; S, 3.95%. Found: C, 67.97; H, 8.22; N, 1.68; S, 4.01%.
trans-3-Hydroxycinnamic acid ethyl ester 3β-(l-selenomethionine)-11-oxo-olean-12-en-30-oate hydrochloride (56). Obtained from 40 as a white solid (149 mg, 75%); m.p. 191–194 °C; 1H-NMR (DMSO-d6): δ 0.72–0.75 (m, 1H, H-5), 0.84 (s, 3H, H-28), 0.85 (s, 3H, H-24), 0.85 (s, 3H, H-23), 0.91–0.93 (m, 1H, H-15’), 1.01–1.04 (m, 1H, H-1’), 1.07 (s, 3H, H-26), 1.08 (s, 3H, H-25), 1.13–1.15 (m, 1H, H-16’), 1.17–1.21 (m, 2H, H-22’ and 21’), 1.27 (t, 3H, J = 7.2 Hz, CH3), 1.35 (s, 3H, H-29), 1.42 (s, 3H, H-27), 1.45 (m, 1H, H-22), 1.48 (m, 1H, H-7’), 1.52 (m, 1H, H-6’), 1.57 (m, 1H, H-6), 1.65 (dd, 1H, J = 13.2, 4.0 Hz, H-19’), 1.68 (m, 1H, H-2’), 1.71 (m, 1H, H-7), 1.76 (m, 1H, H-2), 1.86 (ddd, 1H, J = 14.8, 14.8, 6.0 Hz, H-16), 1.89 (m, 1H, H-21), 1.91 (m, 1H, H-15), 1.94 (s, 3H, SeCH3), 2.19 (m, 1H, H-18), 2.44 (s, 1H, H-9), 2.54 (m, 1H, H-19), 2.59 (m, 2H, SeCH2), 2.64 (ddd, 1H, J = 13.2, 3.2, 3.2 Hz, H-1), 4.20 (q, 2H, J = 7.2 Hz, CH2CH3), 4.48 (dd, 1H, J = 11.2, 5.2 Hz, H-3), 5.49 (s, 1H, H-12), 6.74 (d, 1H, Jtrans = 16.0 Hz, H-β), 7.15 (dd, 1H, J = 8.0, 2.0 Hz, Bn-H-6), 7.48 (t, 1H, J = 8.0 Hz, Bn-H-5), 7.56 (s, 1H, Bn-H-2), 7.65 (d, 1H, J = 8.0 Hz), 7.69 (d, 1H, Jtrans = 16.0 Hz, H-α); 13C-NMR (DMSO-d6): δ 200.02, 174.82, 171.96, 169.03, 167.05, 151.08, 143.60, 135.91, 129.72, 128.54, 125.49, 123.13, 120.62, 118.78, 81.44, 61.75, 60.58, 54.95, 53.62, 48.38, 45.38, 44.23, 43.19, 40.98, 38.57, 38.09, 37.65, 36.89, 32.58, 32.33, 31.84, 31.13, 29.96, 28.63, 28.40, 28.13, 26.46, 26.47, 23.65, 23.30, 18.72, 17.39, 16.78, 16.45, 15.60, 14.53; ESI-MS: m/z = 824.24 [M + H]+. Anal. Calcd. for C46H66ClNO7Se (823.39): C, 64.29; H, 7.74; N, 1.63%. Found: C, 64.25; H, 7.77; N, 1.58%.
3.7. Anticancer Assays
The cytotoxicities of the compounds were evaluated by the MTT assay, which is based on the conversion of MTT to formazan crystals by mitochondrial dehydrogenases. The three lines of cells (MCF-7, MDA-MB-231 and hTERT-RPE1) were cultured in 96 well plates for 24 h with a density of 0.8 × 104 cells/well, afterwards treated them with varying concentrations of GA, doxorubicin or the derivatives for 24 h at 37 °C. The medium was incubated with 20 μL of 5 mg/mL MTT solution for 3 h in a humidified incubator containing 5% CO2. The purple coloured formazan crystals formed in the wells were dissolved in DMSO and their absorbances were measured at 570 nm with a microplate reader. Cell viability was expressed as a percentage of the value in control cultures.