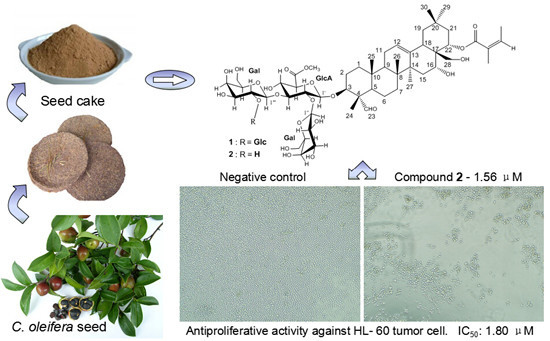
Two New Oleanane-Type Saponins with Anti-Proliferative Activity from Camellia oleifera Abel. Seed Cake
Abstract
:1. Introduction
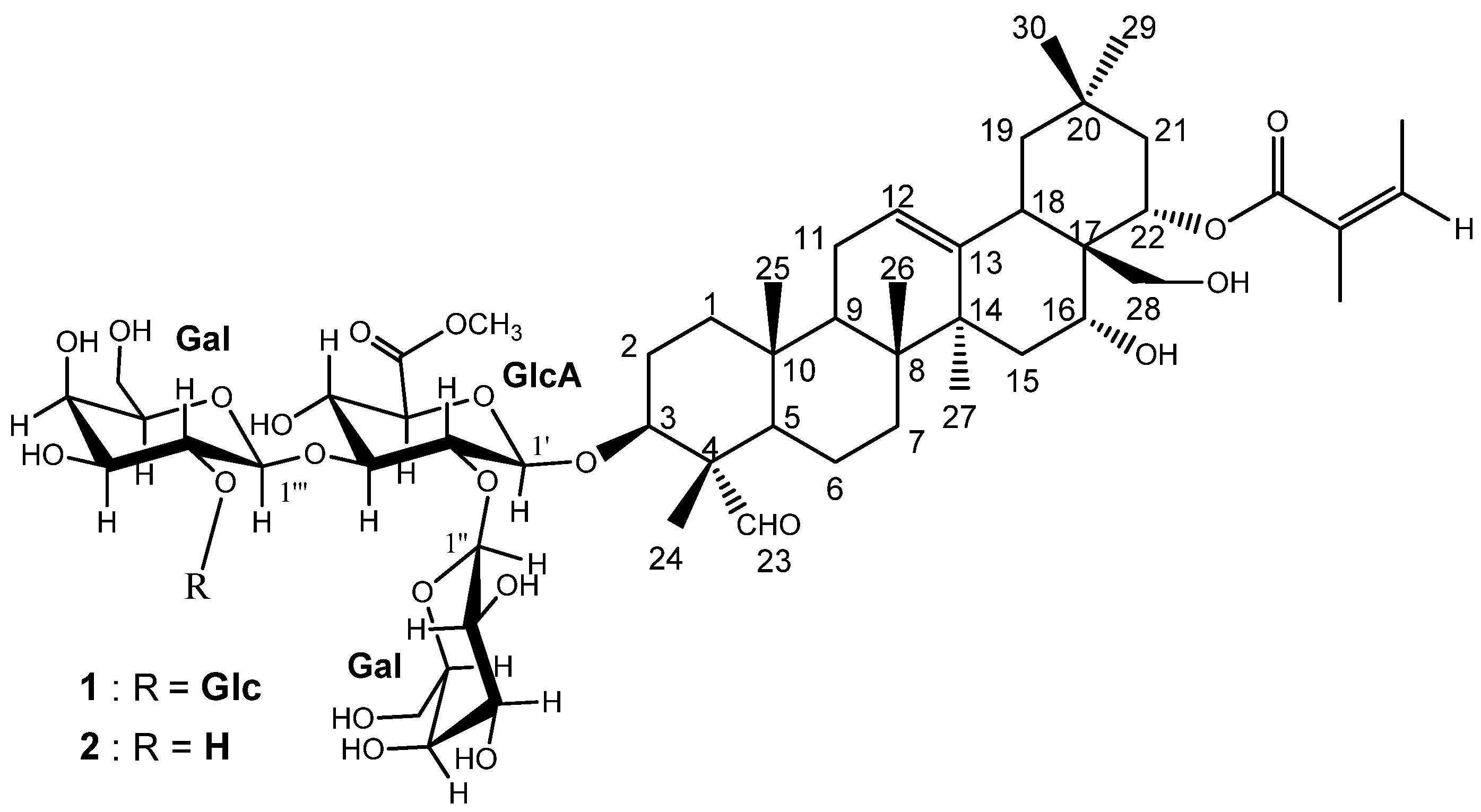
2. Results and Discussion
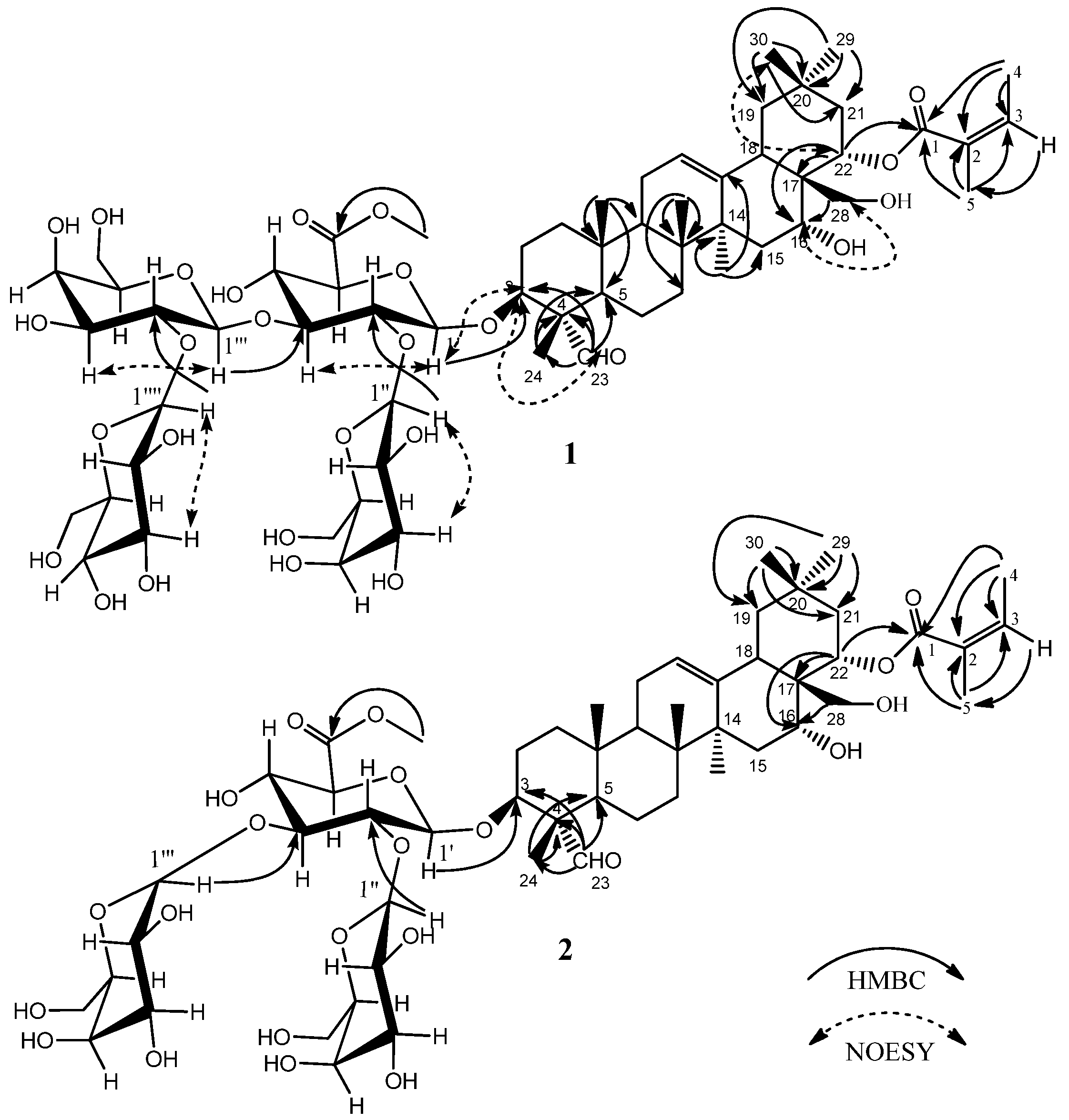
2.1. Isolation and Characterization of the Triterpenoid Saponins
| Position | Oleiferasaponin C4 (1) | Oleiferasaponin C5 (2) | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| 1 | 38.0 | 0.87 m, 1.37 m | 38.0 | 0.90 m, 1.41 m |
| 2 | 25.1 | 1.78 m, 2.05 m | 25.1 | 1.83 m, 2.05 m |
| 3 | 84.1 | 4.02 m | 84.5 | 4.04 m |
| 4 | 55.0 | 55.0 | ||
| 5 | 48.3 | 1.37 m | 48.5 | 1.37 m |
| 6 | 20.3 | 0.90 m, 1.35 m | 20.3 | 0.96 m, 1.35 m |
| 7 | 32.2 | 1.11 m, 1.51 m | 32.3 | 1.13 m, 1.54 m |
| 8 | 40.2 | 40.2 | ||
| 9 | 46.7 | 1.75 m | 46.7 | 1.78 m |
| 10 | 35.9 | 36.0 | ||
| 11 | 23.7 | 1.76 m, 1.84 m | 23.7 | 1.77 m, 1.86 m |
| 12 | 122.9 | 5.35 br s | 122.9 | 5.36 br s |
| 13 | 143.7 | 143.7 | ||
| 14 | 41.6 | 41.7 | ||
| 15 | 35.0 | 1.49 m, 1.83 m | 35.0 | 1.50 m, 1.85 m |
| 16 | 69.9 | 4.60 br s | 70.0 | 4.61 br s |
| 17 | 44.7 | 44.8 | ||
| 18 | 40.8 | 3.05 m | 40.8 | 3.04 m |
| 19 | 47.3 | 1.31 m, 2.88 m | 47.4 | 1.32 m, 2.89 m |
| 20 | 32.0 | 32.0 | ||
| 21 | 41.5 | 2.05 m, 2.83 m | 41.6 | 2.05 m, 2.83 m |
| 22 | 72.8 | 6.22 dd (6.0, 12.0) | 72.9 | 6.23 dd (6.0, 12.0) |
| 23 | 209.9 | 9.91 s | 210.0 | 9.93 s |
| 24 | 11.0 | 1.42 s | 11.0 | 1.46 s |
| 25 | 15.7 | 0.78 s | 15.7 | 0.80 s |
| 26 | 16.7 | 0.81 s | 17.0 | 0.82 s |
| 27 | 27.5 | 1.80 s | 27.5 | 1.81 s |
| 28 | 63.4 | 3.54 m, 3.66 m | 63.5 | 3.56 m, 3.66 m |
| 29 | 33.4 | 1.05 s | 33.4 | 1.05 s |
| 30 | 25.2 | 1.29 s | 25.2 | 1.29 s |
| 22-O-Ang | ||||
| Ang-1 | 167.9 | 167.9 | ||
| Ang-2 | 129.4 | 129.4 | ||
| Ang-3 | 136.6 | 5.92 (dq-like) | 136.5 | 5.92 (dq-like) |
| Ang-4 | 15.8 | 2.09 d (7.2) | 15.8 | 2.09 d (6.6) |
| Ang-5 | 20.9 | 1.95 s | 20.9 | 1.96 s |
| 3-O- | ||||
| GlcA-1′ | 103.9 | 4.80d (7.2) | 103.7 | 4.83 d (7.2) |
| GlcA-2′ | 78.4 | 4.61 m | 78.1 | 4.25 m |
| GlcA-3′ | 83.9 | 4.36 m | 87.4 | 4.17 m |
| GlcA-4′ | 70.4 | 4.37 m | 71.3 | 4.30 m |
| GlcA-5′ | 76.9 | 4.11 m | 76.2 | 4.36 m |
| GlcA-6′ | 170.0 | 169.8 | ||
| COOMe | 52.1 | 3.69s | 52.1 | 3.69 s |
| Gal-1′′ | 103.1 | 5.79 d (7.8) | 104.3 | 5.50 d (7.8) |
| Gal-2′′ | 73.6 | 4.50 m | 73.6 | 4.46 m |
| Gal-3′′ | 74.8 | 4.33 m | 75.3 | 4.11 m |
| Gal-4′′ | 70.3 | 4.47 m | 70.0 | 4.44 m |
| Gal-5′′ | 76.3 | 4.23 m | 76.7 | 3.96 m |
| Gal-6′′ | 61.7 | 4.00 m, 4.35 m | 61.9 | 4.45 m, 4.53 m |
| Gal-1′′′ | 101.6 | 5.75 d (7.2) | 105.1 | 5.19 d (7.2) |
| Gal-2′′′ | 83.5 | 4.55 m | 72.9 | 4.49 m |
| Gal-3′′′ | 75.1 | 4.31 m | 75.5 | 4.12 m |
| Gal-4′′′ | 69.6 | 4.51 m | 70.1 | 4.57 m |
| Gal-5′′′ | 76.5 | 4.46 m | 77.3 | 4.10 m |
| Gal-6′′′ | 62.2 | 4.50 m, 4.55 m | 61.9 | 4.31 m, 4.35 m |
| Glc-1′′′′ | 106.9 | 5.13 d (7.8) | ||
| Glc-2′′′′ | 76.3 | 4.28 m | ||
| Glc-3′′′′ | 78.5 | 3.74 m | ||
| Glc-4′′′′ | 71.2 | 4.36 m | ||
| Glc-5′′′′ | 78.3 | 4.12 m | ||
| Glc-6′′′′ | 62.4 | 4.39 (2H, m) | ||
2.2. Anti-Proliferative Assay on Human Tumor Cells
| Compound | Cell Lines IC50 | ||||
|---|---|---|---|---|---|
| BEL-7402 | BGC-823 | MCF-7 | HL-60 | KB | |
| 1 (μM) | 10.385 | 11.242 | 15.094 | 6.489 | 12.302 |
| 2 (μM) | 4.218 | 5.505 | 3.915 | 1.797 | 3.468 |
| Total Saponins (μg/mL) | 11.023 | 5.981 | 10.611 | 12.546 | 19.761 |
3. Materials and Methods
3.1. General Information
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Spectroscopic Data
3.5. Acid hydrolysis and GC-MS Analysis of the Sugar Moieties in 1 and 2
3.6. Anti-Proliferative Activity Assay in Vitro
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuo, P.C.; Lin, T.C.; Yang, C.W.; Lin, C.L.; Chen, G.F.; Huang, G.W. Bioactive saponin from tea seed pomace with inhibitory effects against Rhizoctonia. solani. J. Agric. Food Chem. 2010, 58, 8618–8622. [Google Scholar] [PubMed]
- Hu, J.L.; Nie, S.P.; Huang, D.F.; Li, C.; Xie, M.Y.; Wan, Y. Antimicrobial activity of saponin-rich fraction from Camellia oleifera cake and its effect on cell viability of mouse macrophage RAW 264.7. J. Sci. Food Agric. 2012, 92, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Yang, S.L.; Han, Y.Y.; Zhao, L.; Lu, G.L.; Xia, T.; Gao, L.P. Qualitative and Quantitative Analysis of Triterpene Saponins from Tea Seed Pomace (Camellia oleifera Abel) and Their Activities against Bacteria and Fungi. Molecules 2014, 19, 7568–7580. [Google Scholar] [PubMed]
- Hu, J.L.; Nie, S.P.; Huang, D.F.; Li, C.; Xie, M.Y. Extraction of saponin from Camellia oleifera cake and evaluation of its antioxidant activity. Int. J. Food Sci. Technol. 2012, 47, 1676–1687. [Google Scholar]
- Sugimoto, S.; Chi, G.; Kato, Y.; Nakamura, S.; Matsuda, H.; Yoshikawa, M. Medicinal Flowers. XXVI. Structures of Acylated Oleanane-Type Triterpene Oligoglycosides, Yuchasaponins A, B, C, and D, from the Flower Buds of Camellia oleifera—Gastroprotective, Aldose Reductase Inhibitory, and Radical Scavenging Effects-. Chem. Pharm. Bull. 2009, 57, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.L.; Liao, X.X.; Zhu, L.W.; Zhang, W.M.; Jiang, J.X. Synergism and foaming properties in binary mixtures of a biosurfactant derived from Camellia oleifera Abel and synthetic surfactants. J. Colloid Interface Sci. 2011, 359, 487–492. [Google Scholar] [PubMed]
- Chen, Y.Z.; Ma, L.; Liu, Z.C.; Peng, S.F.; Chen, X.L.; Chen, L.S.; Wang, X.N.; Wang, R. Molluscicidal effect of Camellia oleifera saponin. J. Central South. Univ. For. Technol. 2011, 31, 147–150. [Google Scholar]
- Chen, Y.F.; Yang, C.H.; Chang, M.S.; Ciou, Y.P.; Huang, Y.C. Foam Properties and Detergent Abilities of the Saponins from Camellia oleifera. Int. J. Mol. Sci. 2010, 11, 4417–4425. [Google Scholar] [PubMed]
- Chen, L.Y.; Chen, J.; Xu, H.H. Sasanquasaponin from Camellia oleifera Abel. induces cell cycle arrest and apoptosis in human breast cancer MCF-7 cells. Fitoterapia 2013, 84, 123–129. [Google Scholar] [PubMed]
- Zong, J.F.; Wang, R.L.; Bao, G.H.; Ling, T.J.; Zhang, L.; Zhang, X.F.; Hou, R.Y. Novel Triterpenoid Saponins from Residual Seed Cake of Camellia oleifera Abel. show Anti-proliferative Activity against Tumor Cells. Fitoterapia 2015, 104, 7–13. [Google Scholar] [PubMed]
- Li, X.; Zhao, J.P.; Peng, C.P.; Chen, Z.; Liu, Y.L.; Xu, Q.M.; Khan, I.A.; Yang, S.L. Cytotoxic Triterpenoid Glycosides from the Roots of Camellia oleifera. Planta Med. 2014, 80, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, C.Z.; Ye, J.Z.; Chen, H.X. New triterpene saponins from the seed cake of Camellia oleifera and their cytotoxic activity. Phytochem. Lett. 2014, 8, 46–51. [Google Scholar]
- Chen, J.H.; Wu, H.Y.; Liau, B.C.; Chang, C.M.J.; Jong, T.T.; Wu, L.C. Identification and evaluation of antioxidants defatted Camellia oleifera seeds by isopropanol salting-out pretreatment. Food Chem. 2010, 121, 1246–1254. [Google Scholar] [CrossRef]
- Zhang, X.F.; Han, Y.Y.; Bao, G.H.; Ling, T.J.; Zhang, L.; Gao, L.P.; Xia, T. A new saponin from tea seed pomace (Camellia oleifera Abel) and its protective effect on PC12 Cells. Molecules 2012, 17, 11721–11728. [Google Scholar] [PubMed]
- Mu, L.H.; Huang, C.L.; Zhou, W.B.; Guo, D.H.; Liu, P. Methanolysis of triterpenoid saponin from Ardisia. gigantifolia stapf. and structure-activity relationship study against cancer cells. Bioorg. Med. Chem. Lett. 2013, 23, 6073–6078. [Google Scholar] [PubMed]
- Lee, S.Y.; Jung, M.Y.; Yoon, S.H. Optimization of the Refining Process of Camellia Seed Oil for Edible Purposes. Food Sci. Biotechnol. 2014, 23, 65–73. [Google Scholar]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, J.-F.; Peng, Y.-R.; Bao, G.-H.; Hou, R.-Y.; Wan, X.-C. Two New Oleanane-Type Saponins with Anti-Proliferative Activity from Camellia oleifera Abel. Seed Cake. Molecules 2016, 21, 188. https://doi.org/10.3390/molecules21020188
Zong J-F, Peng Y-R, Bao G-H, Hou R-Y, Wan X-C. Two New Oleanane-Type Saponins with Anti-Proliferative Activity from Camellia oleifera Abel. Seed Cake. Molecules. 2016; 21(2):188. https://doi.org/10.3390/molecules21020188
Chicago/Turabian StyleZong, Jian-Fa, Yun-Ru Peng, Guan-Hu Bao, Ru-Yan Hou, and Xiao-Chun Wan. 2016. "Two New Oleanane-Type Saponins with Anti-Proliferative Activity from Camellia oleifera Abel. Seed Cake" Molecules 21, no. 2: 188. https://doi.org/10.3390/molecules21020188