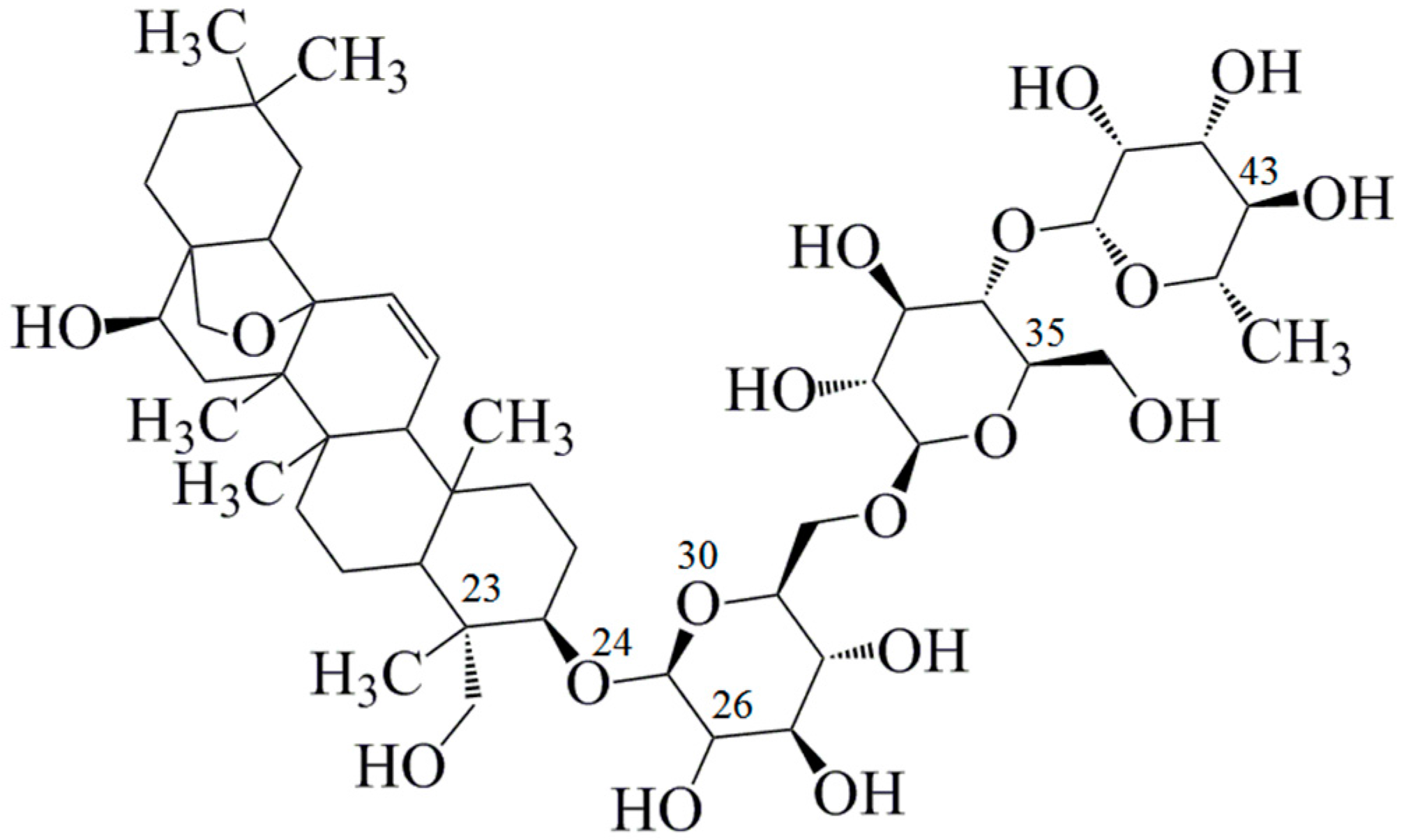
Binding between Saikosaponin C and Human Serum Albumin by Fluorescence Spectroscopy and Molecular Docking
Abstract
:1. Introduction
2. Results and Discussion
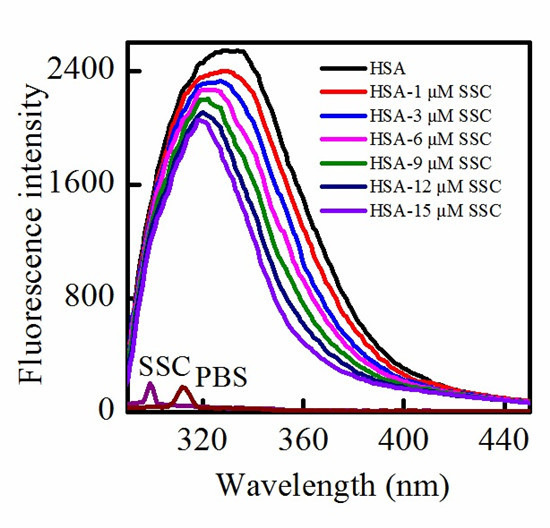
2.1. Fluorescence Quenching of HSA by SSC

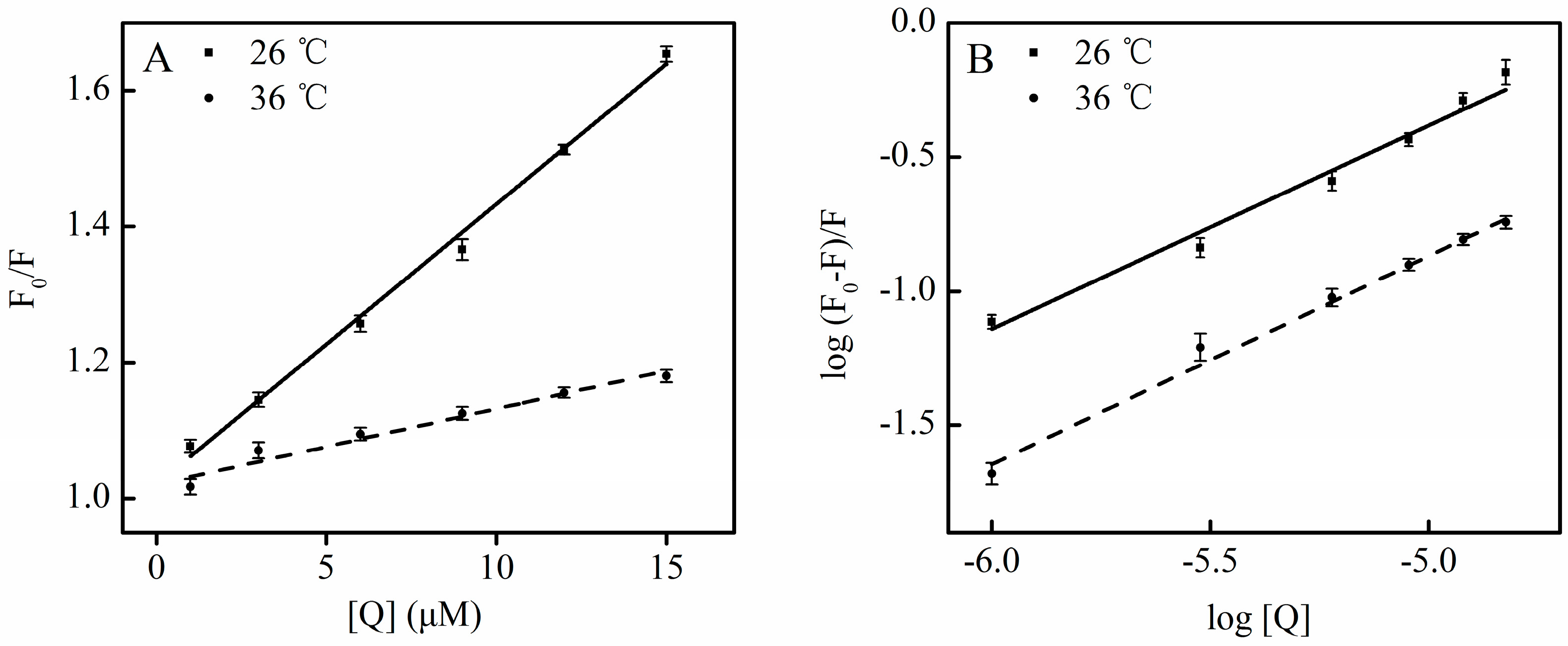
2.2. SSC-Induced Static Quenching
| Fluorescence | T (°C) | Detection | Kq (L·mol−1·S−1) | R |
|---|---|---|---|---|
| Conventional | 26 | λex = 280 nm | 4.19 × 1012 | 0.996 |
| 36 | λex = 280 nm | 1.17 × 1012 * | 0.965 | |
| Synchronous | 26 | ∆λ = 15 nm | 2.97 × 1011 | 0.963 |
| 26 | ∆λ = 60 nm | 2.89 × 1012 | 0.995 |
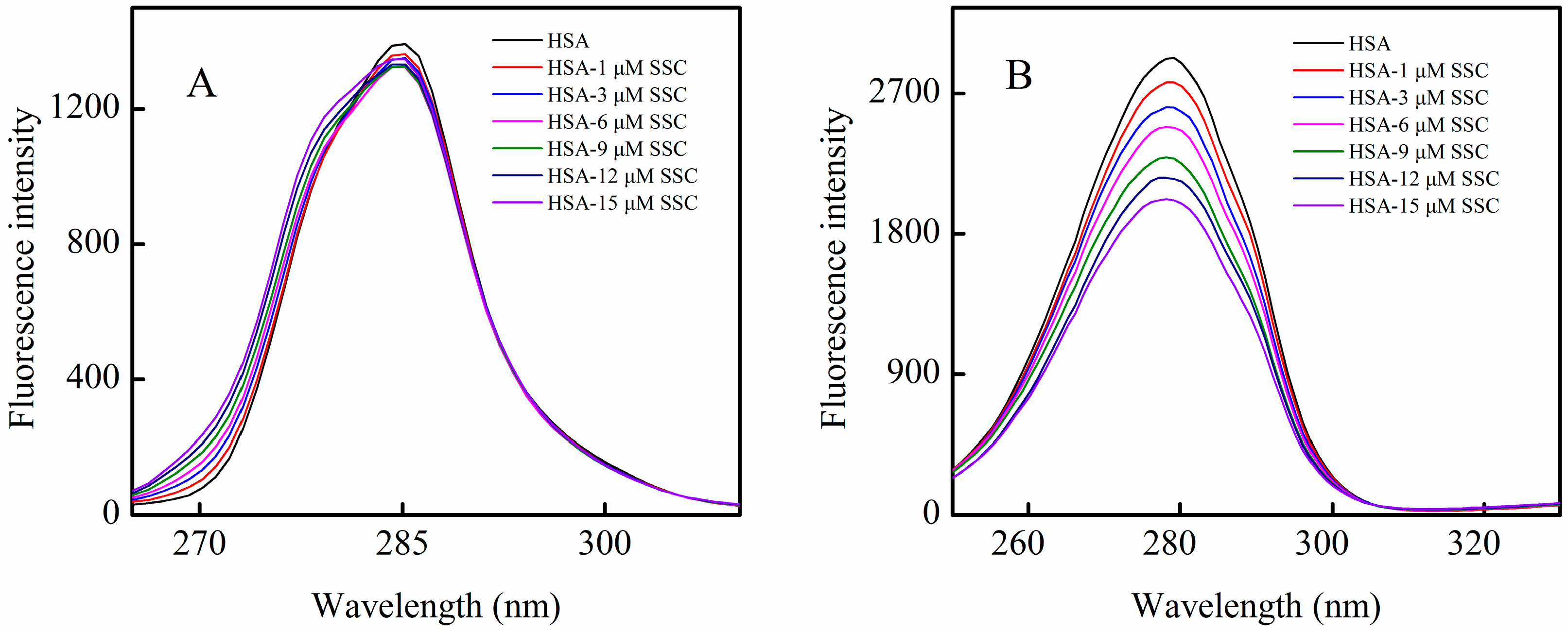
2.3. SSC-Induced Conformational Change in HSA
2.3.1. Investigation of Synchronous Fluorescence
2.3.2. HSA Conformational Change by CD Measurements

2.4. Binding Constants, Sites and Forces between SSC and HSA
2.4.1. Binding Constants and Numbers of Binding Sites
| Fluorescence | T (°C) | Detection | Ka (L·mol−1) | R | n |
|---|---|---|---|---|---|
| Conventional | 26 | λex = 280 nm | 3.72 × 103 | 0.983 | 0.79 |
| 36 | λex = 280 nm | 2.99 × 103 | 0.959 | 0.83 | |
| Synchronous | 26 | ∆λ = 15 nm | 0.90 × 102 | 0.986 | 0.57 |
| 26 | ∆λ = 60 nm | 1.85 × 103 | 0.989 | 0.77 |
2.4.2. Identification of Warfarin and Ibuprofen Binding Sites

| System | Equation | Ka (L·mol−1) ± SD | R |
|---|---|---|---|
| Warfarin | Y = 0.78X + 3.64 | 4.38 × 104 ± 11.65 * | 0.991 |
| Ibuprofen | Y = 0.87X + 3.35 | 2.22 × 103 ± 3.75 * | 0.985 |
| HSA-SSC | Y = 0.79X + 3.57 | 3.72 × 103 ± 4.76 | 0.983 |
2.4.3. Binding Forces between SSC and HSA
| Fluorescence | T (°C) | λex | ∆G (kJ·mol−1) | ∆H (kJ·mol−1) | ∆S (J·mol−1·K−1) |
|---|---|---|---|---|---|
| Conventional | 26 | 280 nm | −20.34 | −47.34 | −90.31 |
| 36 | 280 nm | −19.52 | −90.04 |
2.5. Energy Transfer between HSA and SSC
| Fluorescence | T (°C) | Detection | J (cm3·L·mol−1) | R0 (nm) | E (J) | r (nm) |
|---|---|---|---|---|---|---|
| Conventional | 26 | λex = 280 nm | 6.54 × 10−16 | 2.14 | 0.23 | 2.61 |
| 36 | λex = 280 nm | 3.06 × 10−16 | 1.89 | 0.096 | 2.74 | |
| Synchronous | 26 | ∆λ = 15 nm | 1.14 × 10−15 | 2.34 | 0.037 | 4.03 |
| 26 | ∆λ = 60 nm | 2.65 × 10−15 | 2.69 | 0.19 | 3.45 |
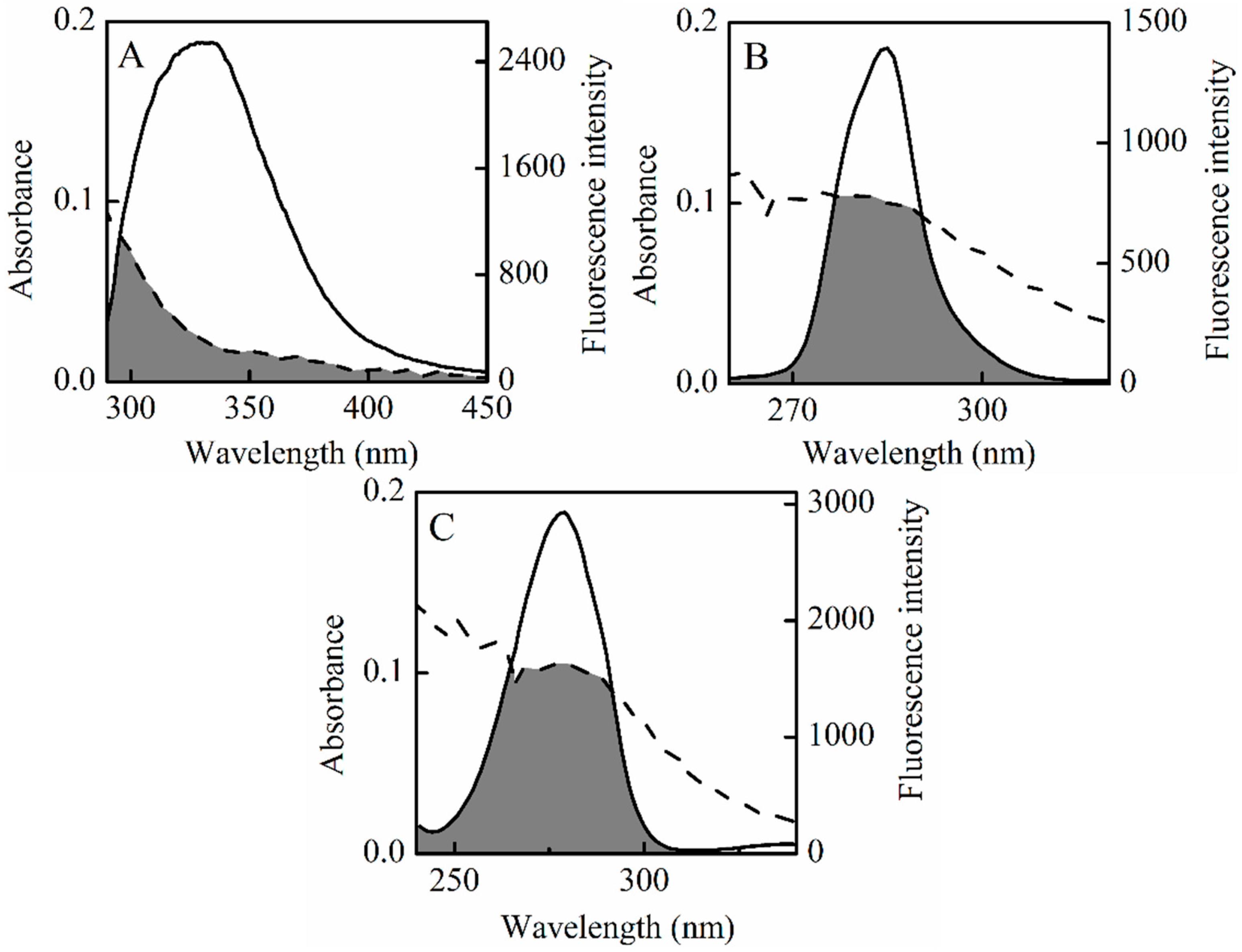
2.6. Docking Results

| Subdomain | Amino Acid Residues/HSA | Atoms/SSC * | Length |
|---|---|---|---|
| Sudlow I | Ser192 | CH2OH(19) | 2.9 Å |
| Lys199 | O(23) | 3.1 Å | |
| Lys199 | O(25) | 2.5 Å | |
| Sudlow II | Arg-410 | O(23) | 2.4 Å |
| Ser489 | CH2OH(19) | 3.1 Å | |
| Ser489 | O(25) | 3.2 Å | |
| Cys438 | OH(45) | 2.5 Å | |
| Val433 | OH(45) | 3.0 Å | |
| IIA-IIB | Asp324 | OH(26) | 2.4 Å |
| Glu354 | CH2OH(37) | 2.8 Å | |
| Glu479 | OH(45) | 2.8 Å |
3. Materials and Methods
3.1. Materials
3.2. Absorbance Measurements
3.3. Fluorescence Measurements
3.3.1. Conventional Fluorescence Measurements for SSC-HSA Binding
3.3.2. Synchronous Fluorescence Measurements for SSC-HSA Binding
3.3.3. Synchronous Fluorescence Measurements for SSC-Tyrosine or SSC-Tryptophan Binding
3.3.4. Site Marker Competitive Fluorescence Experiments
3.3.5 Correction of the Internal Filter
3.4. CD Spectroscopy
3.5. Molecular Docking
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Curry, S.; Mandelkow, H.; Brick, P.; Franks, N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat. Struct. Biol. 1998, 5, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.; Shokoohinia, Y.; Ghobadi, S.; Bijari, N.; Gholamzadeh, S.; Moradi, N.; Ashrafi-Kooshk, M.R.; Aghaei, A.; Khodarahmi, R. Studies of the interaction between isoimperatorin and human serum albumin by multispectroscopic method: identification of possible binding site of the compound using esterase activity of the protein. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Mahammed, A.; Gary, H.B.; Weaver, J.J.; Sorasaenee, K.; Gross, Z. Amphiphilic corroles bind tightly to human serum albumin. Bioconjugate Chem. 2004, 15, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.R.; Zunszain, P.A.; Ha, C.E.; Yang, J.S.; Bhagavan, N.V.; Petitpas, I.; Curry, S.; Hamilton, J.A. Locating high-affinity fatty acid-binding sites on albumin by x-ray crystallography and NMR spectroscopy. Proc. Natl. Acad. Sci. USA 2005, 102, 17958–17963. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.X.; Long, M.; Liu, Y.; Qin, C.; Wang, Y.D. Characterization of the interaction between human serum albumin and morin. BBA-Gen. Subj. 2006, 1760, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Sudhamalla, B.; Gokara, M.; Ahalawat, N.; Amooru, D.G.; Subramanyam, R. Molecular dynamics simulation and binding studies of β-sitosterol with human serum albumin and its biological relevance. J. Phys. Chem. B 2010, 114, 9054–9062. [Google Scholar] [CrossRef] [PubMed]
- Eftink, M.R.; Hagaman, K.A. Fluorescence lifetime and anisotropy studies with liver alcohol dehydrogenase and its complexes. Biochemistry 1986, 25, 6631–6637. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, J.; Zhu, Y.; Chen, J. Characterization of interaction between isoliquiritigenin and bovine serum albumin: Spectroscopic and molecular docking methods. J. Lumin. 2014, 145, 643–650. [Google Scholar] [CrossRef]
- Meneghini, C.; Leboffe, L.; Bionducci, M.; Fanali, G.; Meli, M.; Colombo, G.; Fasano, M.; Ascenzi, P.; Mobilio, S. The five-to-six-coordination transition of ferric human serum heme-albumin is allosterically-modulated by ibuprofen and warfarin: A combined XAS and MD study. PLoS ONE 2014, 9, e104231. [Google Scholar]
- Samari, F.; Shamsipur, M.; Hemmateenejad, B.; Khayamian, T.; Gharaghani, S. Investigation of the interaction between amodiaquine and human serum albumin by fluorescence spectroscopy and molecular modeling. Eur. J. Med. Chem. 2012, 54, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.B.; Liang, Z.S.; Hu, R.L.; Dong, J.E. Growth and Saikosaponin production of the medicinal herb Bupleurum chinense DC. Under different levels of nitrogen and phosphorus. Ind. Crop. Prod. 2009, 29, 96–101. [Google Scholar] [CrossRef]
- Chiang, L.C.; Ng, L.T.; Liu, L.T.; Shieh, D.E.; Lin, C.C. Cytotoxicity and anti-hepatitis B virus activities of Saikosaponins from Bupleurum species. Planta Med. 2003, 69, 705–709. [Google Scholar] [PubMed]
- Law, B.Y.; Mo, J.F.; Wong, V.K. Autophagic effects of Chaihu (dried roots of Bupleurum chinense DC or Bupleurum scorzoneraefolium WILD). Chin. Med. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Que, R.; Li, Y. Advances in mechanisms of Saikosaponin preventing and treating hepatocellular carcinoma. J. Liaoning Univ. TCM 2014, 16, 128–131. (in Chinese). [Google Scholar]
- Shyu, K.; Tsai, S.; Wang, B.; Liu, Y.; Lee, C. Saikosaponin C induces endothelial cells growth, migration and capillary tube formation. Life Sci. 2004, 76, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Chang, J.; Kim, B.M. Saikosaponin C inhibits lipopolysaccharide-induced apoptosis by suppressing caspase-3 activation and subsequent degradation of focal adhesion kinase in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2014, 445, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Liu, Y.; Xiao, X.H. Investigation of the interaction between berberine and human serum albumin. Biomacromolecules 2009, 10, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.E.; Pralle, A.; Yao, X.; Swaminath, G.; Gandhi, C.S.; Jan, Y.N.; Kobilka, B.K.; Isacoff, E.Y.; Jan, L.Y. A fluorescent probe designed for studying protein conformational change. Proc. Natl. Acad. Sci. USA 2005, 102, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Xie, M.X.; Zheng, D.; Liu, Y.; Li, X.Y.; Chen, X. Spectroscopic studies on the interaction of cinnamic acid and its hydroxyl derivatives with human serum albumin. J. Mol. Struct. 2004, 692, 71–80. [Google Scholar]
- Kang, J.; Liu, Y.; Xie, M.X.; Li, S.; Jiang, M.; Wang, Y.D. Interactions of human serum albumin with chlorogenic acid and ferulic acid. BBA-Gen. Subj. 2004, 1674, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, R.; Zhao, X.; Yang, B.; Gao, C.; Hao, X.; Wu, Y. New strategy for the evaluation of CdTe quantum dot toxicity targeted to bovine serum albumin. Sci. Total Environ. 2009, 407, 5019–5023. [Google Scholar] [CrossRef] [PubMed]
- Mahesha, H.G.; Singh, S.A.; Srinivasan, N.; Rao, A.G.A. A spectroscopic study of the interaction of isoflavones with human serum albumin. FEBS J. 2006, 273, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Protein Chem. 1994, 45, 153–203. [Google Scholar]
- Ji, Z.; Yuan, H.; Liu, M.; Hu, J. 1H-NMR study of the effect of acetonitrile on the interaction of ibuprofen with human serum albumin. J. Pharm. Biomed. Anal. 2002, 30, 151–159. [Google Scholar] [CrossRef]
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal structure of human serum albumin at 2.5 A resolution. Protein Eng. 1999, 12, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Goncharova, I.; Orlov, S.; Urbanova, M. The location of the high- and low- affinity bilirubin-binding sites on serum albumin: Ligand-competition analysis investigated by circular dichroism. Biophys. Chem. 2013, 180–181, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, W.; Liu, J.; Sheng, F.; Hu, Z.; Chen, X. Binding of the bioactive component Jatrorrhizine to human serum albumin. BBA-Gen. Subj. 2005, 1722, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Maiti, T.K.; Ghosh, K.S.; Debnath, J.; Dasgupta, S. Binding of all-trans retinoic acid to human serum albumin: Fluorescence, FT-IR and circular dichroism studies. Int. J. Biol. Macromol. 2006, 38, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.G.; Li, Q.L.; Zhou, Z.G.; Wang, Y.L.; Bryant, S.H. Structure-Based Virtual Screening for Drug Discovery: A Problem-Centric Review. AAPS J. 2012, 14, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Soltes, L.; Mach, M. Estimation of drug-protein binding parameters on assuming the validity of thermodynamic equilibrium. J. Chromatogr. B 2002, 768, 113–119. [Google Scholar] [CrossRef]
- Daly, A.K. Optimal dosing of warfarin and other coumarin anticoagulants: The role of genetic polymorphisms. Arch. Toxicol. 2013, 87, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Lesko, S.M.; Mitchell, A.A. An assessment of the safety of pediatric ibuprofen. A practitioner-based randomized clinical trial. J. Pediatr. 1995, 273, 836. [Google Scholar] [CrossRef]
- Zhang, Z. Exploration of Chaihu couplet medicines in Shang Han Lun. Hubei J. TCM 2014, 36, 47–48. (in Chinese). [Google Scholar]
- Tian, J.N.; Liu, J.Q.; He, W.Y.; Hu, Z.D.; Yao, Z.D.; Chen, X.G. Probing the binding of scutellarin to human serum albumin by circular dichroism, fluorescence spectroscopy, FTIR, and molecular modeling method. Biomacromolecules 2004, 5, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Klotz, I.M.; Urquhart, J.M. Effect of temperatrue on binding of organicions by proteins. J. Am. Chem. Soc. 1949, 71, 847–865. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.C.; Liu, R.T.; Chi, Z.X.; Teng, Y.; Qin, P.F. New Insights into the Behavior of Bovine Serum Albumin Adsorbed onto Carbon Nanotubes: Comprehensive Spectroscopic Studies. J. Phys. Chem. B. 2010, 114, 5625–5631. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Pal, A.; Dey, S.; Chatterjee, B.K.; Chakrabarti, P. Interaction of virstatin with human serum albumin: Spectroscopic analysis and molecular modeling. PLoS ONE 2012, 7, e37468. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, X.; Zhang, Y.; Qian, Y.; Gao, H. Interactions of acidic pharmaceuticals with human serum albumin: Insights into the molecular toxicity of emerging pollutants. Amino Acids 2012, 43, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Y.; Li, W.X.; Cao, H. Study of the interaction between trans-resveratrol and BSA by the multispectroscopic method. J. Solut. Chem. 2008, 37, 1609–1623. [Google Scholar] [CrossRef]
- Xiao, J.B.; Chen, X.Q.; Jiang, X.Y.; Hilczer, M.; Tachiya, M. Probing the interaction of trans-resveratrol with bovine serum albumin: A fluorescence quenching study with tachiya model. J. Fluoresc. 2008, 18, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Maghsudi, M.; Rouhani, S. Study on the interaction of food colourant quinoline yellow with bovine serum albumin by spectroscopic techniques. Food Chem. 2012, 135, 1836–1841. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Stryer, L. Proximity relationships in rhodopsin. Proc. Natl. Acad. Sci. USA. 1972, 69, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Shi, J.; Cao, H.; Wu, S.D.; Ren, F.L.; Xu, M. Analysis of binding interaction between puerarin and bovine serum albumin by multi-spectroscopic method. J. Pharmaceut. Biomed. 2007, 45, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of fluorescence spectroscopy. Springer 2006, 15, 518–520. [Google Scholar]
- Weiss, S. Fluorescence spectroscopy of single biomolecules. Science 1999, 283, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Denicola, A. Protein tryptophan accessibility studied by fluorescence quenching. Biochem. Mol. Biol. Educ. 2002, 30, 175–178. [Google Scholar] [CrossRef]
- RCSB Protein Data Bank—Structure Summary. Available online: http://www.rcsb.org/pdb/explore.do?structureId=2XVU (accessed on 30 October 2015).
- Sample Availability: Samples of saikosaponin C from Bupleurum chinense DC are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Wang, H.-M.; Niu, Q.-X.; Ye, D.-Y.; Liang, G.-W. Binding between Saikosaponin C and Human Serum Albumin by Fluorescence Spectroscopy and Molecular Docking. Molecules 2016, 21, 153. https://doi.org/10.3390/molecules21020153
Chen Y-C, Wang H-M, Niu Q-X, Ye D-Y, Liang G-W. Binding between Saikosaponin C and Human Serum Albumin by Fluorescence Spectroscopy and Molecular Docking. Molecules. 2016; 21(2):153. https://doi.org/10.3390/molecules21020153
Chicago/Turabian StyleChen, Yi-Cun, Hong-Mei Wang, Qing-Xia Niu, Dan-Yan Ye, and Guo-Wu Liang. 2016. "Binding between Saikosaponin C and Human Serum Albumin by Fluorescence Spectroscopy and Molecular Docking" Molecules 21, no. 2: 153. https://doi.org/10.3390/molecules21020153
APA StyleChen, Y.-C., Wang, H.-M., Niu, Q.-X., Ye, D.-Y., & Liang, G.-W. (2016). Binding between Saikosaponin C and Human Serum Albumin by Fluorescence Spectroscopy and Molecular Docking. Molecules, 21(2), 153. https://doi.org/10.3390/molecules21020153