Detecting β-Casein Variation in Bovine Milk
Abstract
:1. Introduction

2. Results and Discussion
2.1. Comparison between β-CN and β-LG Behavior in the Presence of TCA
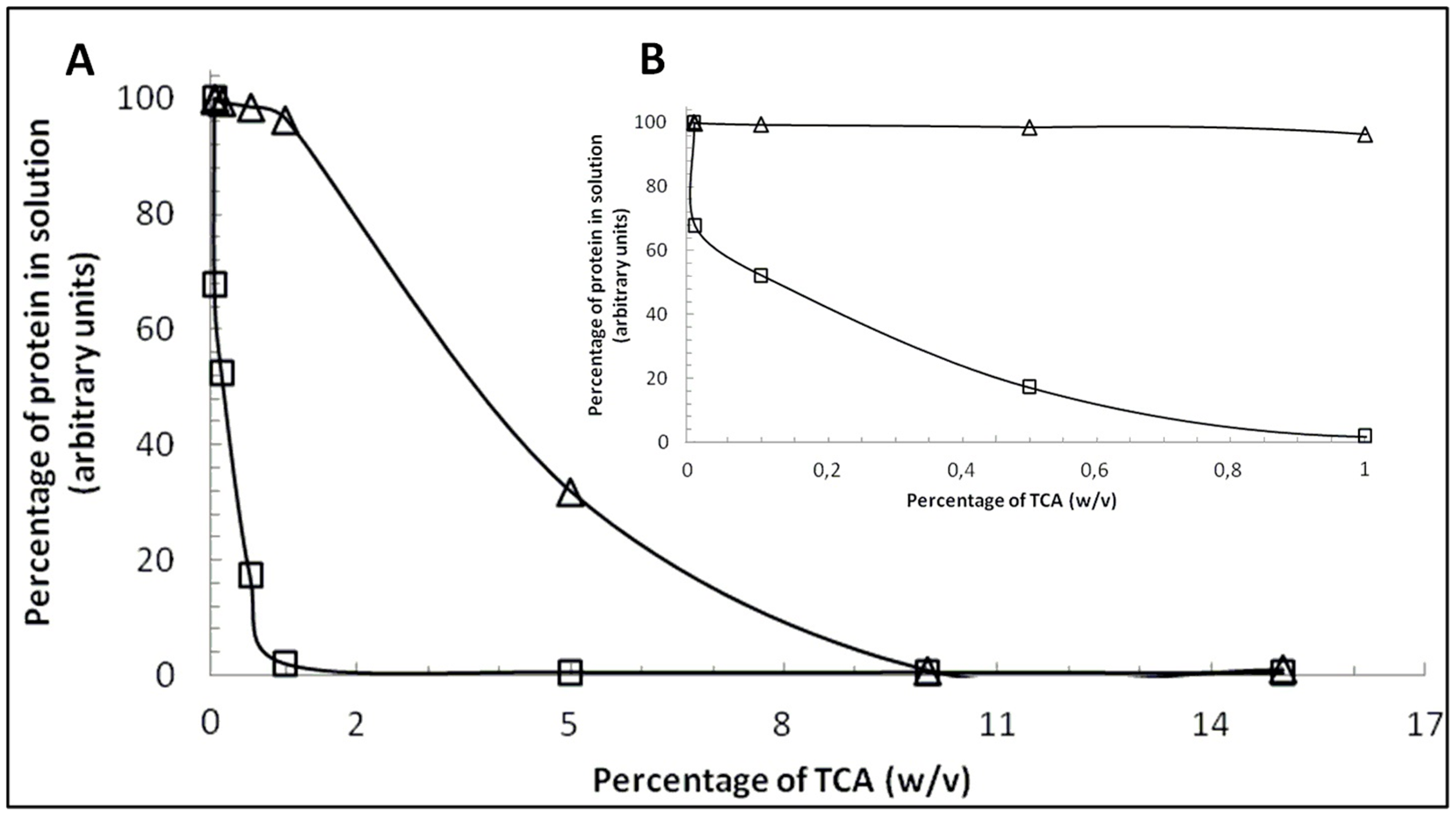
2.2. Content of β-CN in Bulk Milk Samples
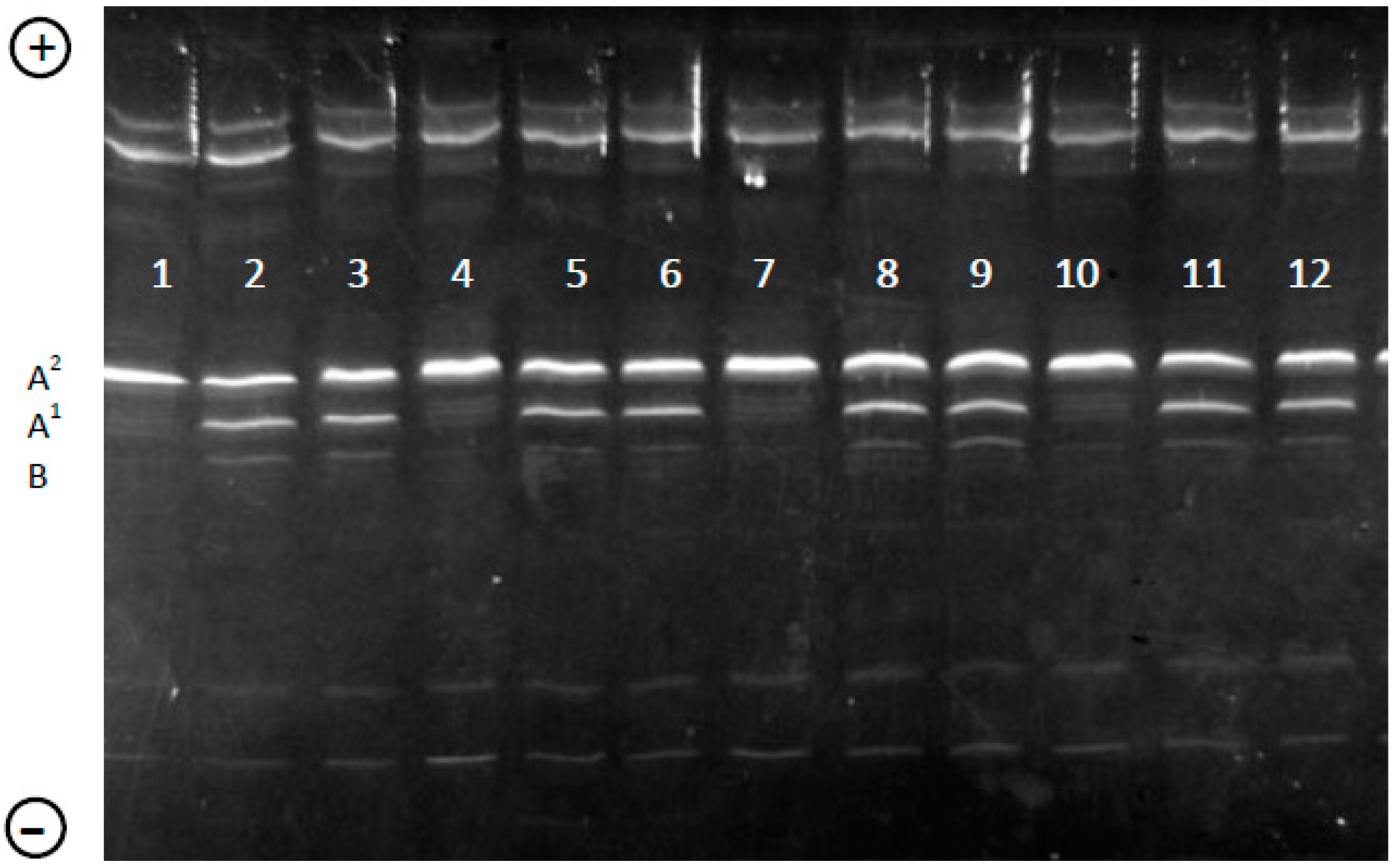
2.3. Genotyping of β-CN in Individual Milk Samples

3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farrell, H.M., Jr.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, F.; Swaisgood, H.E. Nomenclature of the proteins of cows’ milk-sixth edition. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Caroli, A.M.; Chessa, S.; Erhardt, G.J. Milk protein polymorphisms in cattle: Effect on animal breeding and human nutrition. J. Dairy Sci. 2009, 92, 5335–5352. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.J.; Murray, B.A. Bioactive peptides and lactic fermentations. Int. J. Dairy Technol. 2006, 59, 118–125. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Kitts, D.D.; Weiler, K. Bioactive proteins and peptides from food sources: Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H. Overview on milk protein derived peptides. Int. Dairy J. 1998, 8, 363–373. [Google Scholar] [CrossRef]
- Lorenzini, E.; Chessa, S.; Chiatti, F.; Caroli, A.; Pagnacco, G. Peptidi bioattivi di latte e derivati. Sci. Tecn. Latt. Cas. 2007, 58, 113–156. [Google Scholar]
- Hartwig, A.; Teschemacher, H.; Lehmann, W.; Gauly, M.; Erhardt, G. Influence of genetic polymorphisms in bovine milk on the occurence of bioactive peptides. In Proceedings IDF “Milk Protein Polymorphism Seminar II”; International Dairy Federation: Brussels, Belgium, 1997; pp. 459–460. [Google Scholar]
- Jinsmaa, Y.; Yoshikawa, M. Enzymatic release of neocasomorphin and β-casomorphin from bovine β-casein. Peptides 1999, 20, 957–962. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Review of the potential health impact of β-casomorphins and related peptides. EFSA Sci. Rep. 2009, 231, 1–107. [Google Scholar]
- Barnett, M.P.G.; McNabb, W.C.; Roy, N.C.; Woodford, K.B.; Clarke, A.J. Dietary A1 β-casein affects gastrointestinal transit time, dipeptidyl peptidase-4 activity, and inflammatory status relative to A2 β-casein in Wistar rats. Int. J. Food Sci. Nutr. 2014, 65, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.R.U.; Kapila, R.; Sharma, R.; Saliganti, V.; Kapila, S. Comparative evaluation of cow β-casein variants (A1/A2) consumption on Th2-mediated inflammatory response in mouse gut. Eur. J. Nutr. 2014, 53, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.R.U.; Kapila, R. Release of β-casomorphin-7/5 during simulated gastrointestinal digestion of milk β-casein variants from Indian crossbred cattle (Karan Fries). Food Chem. 2015, 168, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Woodford, K.; Kukuljan, S.; Pal, S. Comparative effects of A1 versus A2 β-casein on gastrointestinal measures: a blinded randomised cross-over pilot study. EJCN 2014, 68, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Bonfatti, V.; Grigoletto, L.; Cecchinato, A.; Gallo, L.; Carnier, P. Validation of a new reversed-phase high-performance liquid chromatography method for separation and quantification of bovine milk protein genetic variants. J. Chromatogr. A 2008, 119, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Givens, I.; Aikman, P.; Gibson, T.; Brown, R. Proportions of A1, A2, B and C β-casein protein variants in retail milk in the UK. Food Chem. 2013, 139, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, O.; Raineri, M.; Gigliotti, C.; Caroli, A. Metodo per la quantificazione delle varianti genetiche di β-caseina bovina. Sci. Tecn. Latt. Cas. 2013, 64, 101–107. [Google Scholar]
- Hallén, E.; Wedholm, A.; Andrén, A.; Lundén, A. Effect of β-casein, κ-casein and β-lactoglobulin genotypes on concentration of milk protein variants. J. Anim. Breed. Genet. 2008, 125, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Heck, J.M.L.; Schennink, A.; van Valenberg, H.J.F.; Bovenhuis, H.; Visker, M.H.P.W.; van Arendonk, J.A.M.; van Hooijdonk, A.C.M. Effects of milk protein variants on the protein composition of bovine milk. J. Dairy Sci. 2009, 92, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Chessa, S.; Bulgari, O.; Rossoni, A.; Ceriotti, G.; Caroli, A.M. Bovine β-casein: Detection of two single nucleotide polymorphisms by bidirectional allele specific polymerase chain reaction (BAS-PCR) and monitoring of their variation. OJAS 2013, 3, 36–41. [Google Scholar] [CrossRef]
- Jann, O.; Ceriotti, G.; Caroli, A.; Erhardt, G. A new variant in exon VII of bovine β-casein gene (CSN2) and its distribution among European breeds. J. Anim. Breed. Genet. 2002, 119, 65–68. [Google Scholar] [CrossRef]
- Oldfield, D.J.; Singh, H.; Taylor, M.W.; Pearce, K.N. Heat-induced interactions of β-lactoglobulin and α-lactalbumin with the casein micelle in pH-adjusted skim milk. Int. Dairy J. 2000, 10, 509–518. [Google Scholar] [CrossRef]
- Caroli, A.M.; Chessa, S.; Erhardt, G.J. Milk protein variants for animal breeding and human nutrition. In Proceedings of the 9th World Congress on Genetics Applied to Livestock Production, Leipzig, Germany, 1–6 August 2010.
- The UniProt Consortium. UniProt: A hub for protein information. Nucleic Acids Res. 2015, 43, D204–D212. [Google Scholar]
- Sagar, A.J.; Pandit, M.W. Denaturation studies on bovine pancreatic ribonuclease: Effect of trichloroacetic acid. Biochim. Biophys. Acta 1983, 743, 303–309. [Google Scholar] [CrossRef]
- Erhardt, G.; Juszczak, J.; Panicke, L.; Krick-Saleck, H. Genetic polymorphism of milk proteins in Polish Red Cattle: A new genetic variant of β-lactoglobulin. J. Anim. Breed. Genet. 1998, 115, 63–71. [Google Scholar] [CrossRef]
- Sample Availability: Samples are not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caroli, A.M.; Savino, S.; Bulgari, O.; Monti, E. Detecting β-Casein Variation in Bovine Milk. Molecules 2016, 21, 141. https://doi.org/10.3390/molecules21020141
Caroli AM, Savino S, Bulgari O, Monti E. Detecting β-Casein Variation in Bovine Milk. Molecules. 2016; 21(2):141. https://doi.org/10.3390/molecules21020141
Chicago/Turabian StyleCaroli, Anna Maria, Salvatore Savino, Omar Bulgari, and Eugenio Monti. 2016. "Detecting β-Casein Variation in Bovine Milk" Molecules 21, no. 2: 141. https://doi.org/10.3390/molecules21020141




