Antioxidant Activity of 3-[N-(Acylhydrazono)ethyl]-4-hydroxy-coumarins
Abstract
:1. Introduction
2. Results and Discussion
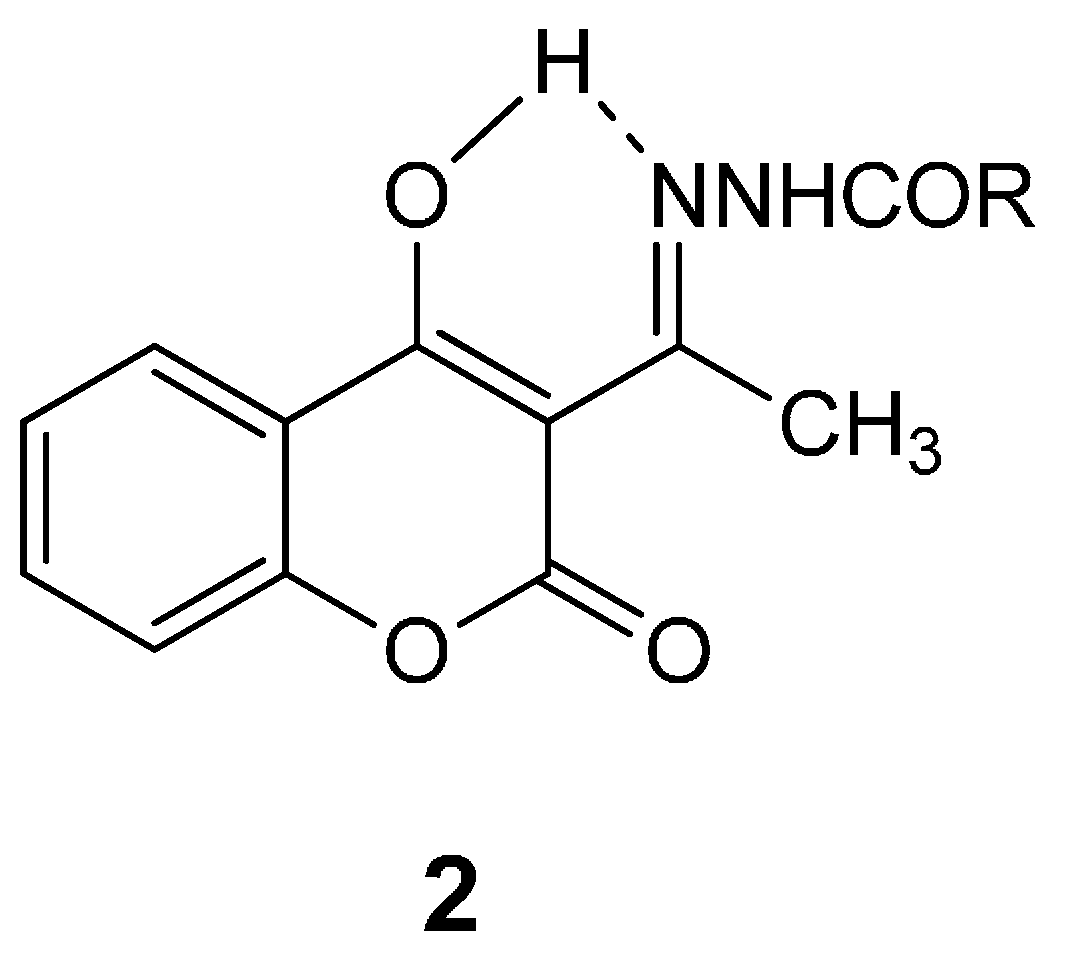
2.1. Pharmacology
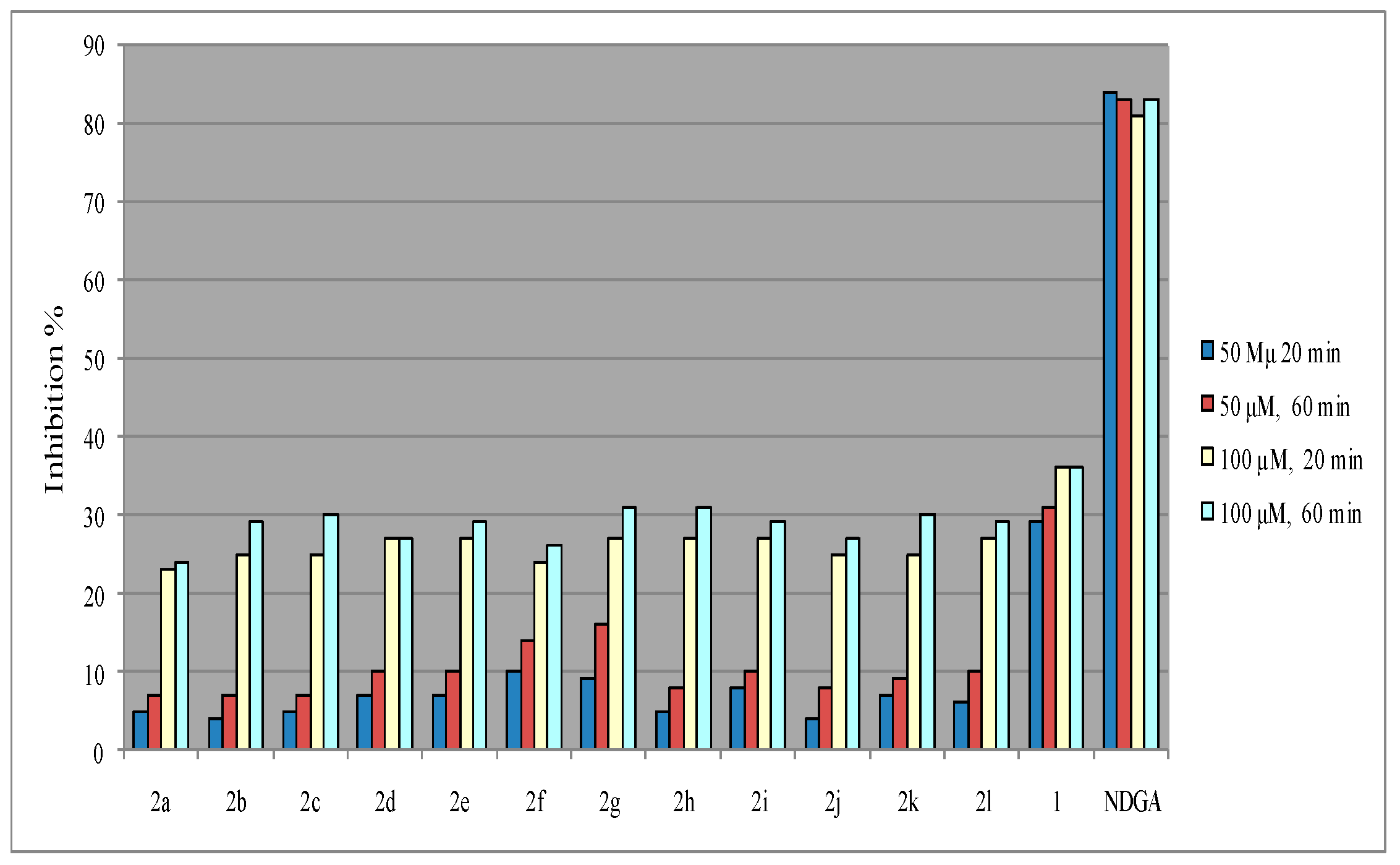
Antioxidant Activity
| Compd. | RA%, 50 μM, 20 min | RA%, 50 μM, 60 min | RA%, 100 µM, 20 min | RA%, 100 μM, 60 min | Clog P | LP a 60 s, 100 μM | LOX b IC50 (μM) |
|---|---|---|---|---|---|---|---|
| 2a | 5 ± 0.2 | 7 ± 0.3 | 23 ± 3.0 | 24 ± 2.0 | 1.85 | 100 ± 9.8 | 62.5 ± 2.3 |
| 2b | 4 ± 0.2 | 7 ± 0.1 | 25 ± 2.0 | 29 ± 1.1 | 3.62 | 100 ± 5.5 | 40 ± 0.5 |
| 2c | 5 ± 0.1 | 7 ± 0.4 | 25 ± 1.2 | 30 ± 2.8 | 3.29 | 98 ± 5.4 | 58 ± 2.7 |
| 2d | 7 ± 0.3 | 10 ± 0.2 | 27 ± 2.2 | 27 ± 1.4 | 3.79 | 100 ± 3.2 | No c |
| 2e | 7 ± 0.2 | 10 ± 0.5 | 27 ± 0.9 | 29 ± 0.8 | 4.20 | 94 ± 4.8 | 55 ± 2.1 |
| 2f | 10 ± 0.5 | 14 ± 1.2 | 24 ± 2.2 | 26 ± 1.2 | 2.96 | 98 ± 2.9 | 70 ± 4.3 |
| 2g | 9 ± 0.3 | 16 ± 0.6 | 27 ± 0.8 | 31 ± 1.4 | 2.38 | 95 ± 7.2 | 46.5 ± 2.3 |
| 2h | 5 ± 0.1 | 8 ± 0.2 | 27 ± 1.5 | 31 ± 1.6 | 2.53 | 99 ± 3.7 | No c |
| 2i | 8 ± 0.5 | 10 ± 0.1 | 27 ± 0.2 | 29 ± 1.7 | 2.96 | 100 ± 8.2 | 49.5 ± 1.2 |
| 2j | 4 ± 0.2 | 8 ± 0.3 | 25 ± 1.8 | 27 ± 0.8 | 2.46 | 95 ± 4.1 | 90 ± 5.1 |
| 2k | 7 ± 0.1 | 9 ± 0.2 | 25 ± 2.1 | 30 ± 2.2 | 3.13 | 98 ± 3.9 | 43.5 ± 3.2 |
| 2l | 6 ± 0.3 | 10 ± 0.2 | 27 ± 2.2 | 29 ± 1.0 | 3.46 | 95 ± 6.2 | 35 ± 0.2 |
| 1 | 29 ± 0.5 | 31± 0.3 | 36± 1.3 | 36 ± 0.8 | 1.91 | 8 ± 0.2 | 44 (± 0.3) d |
| NDGA | 84 ± 2.0 | 83 ± 3.3 | 81 ± 5.2 | 83 ± 4.7 | 5.5 ± 0.1 | ||
| TROLOX | 63 ± 0.2 |

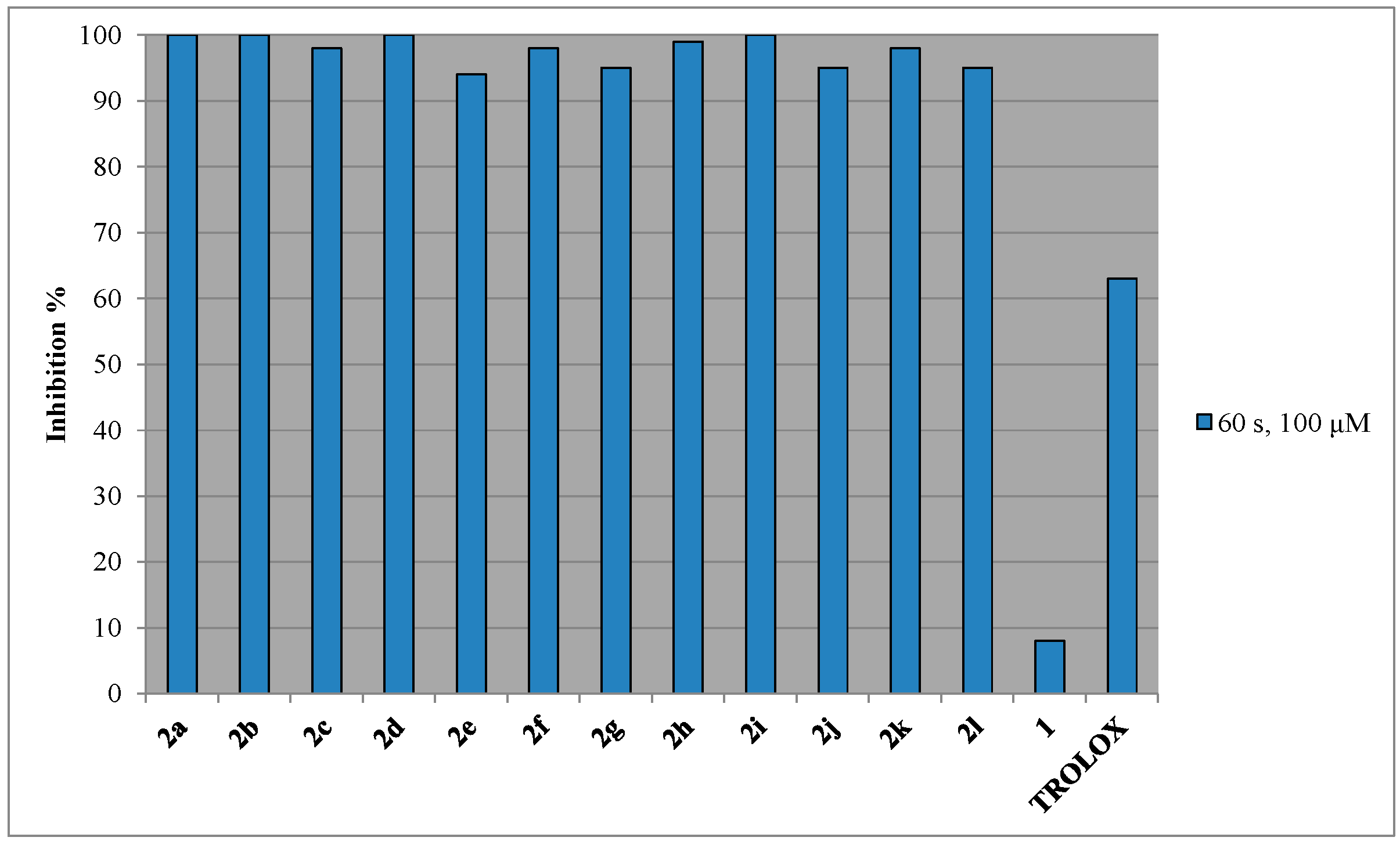
2.2. In Vitro Inhibition of Soybean Lipoxygenase (LOX)

3. Experimental Section
3.1. General
3.2. Chemistry
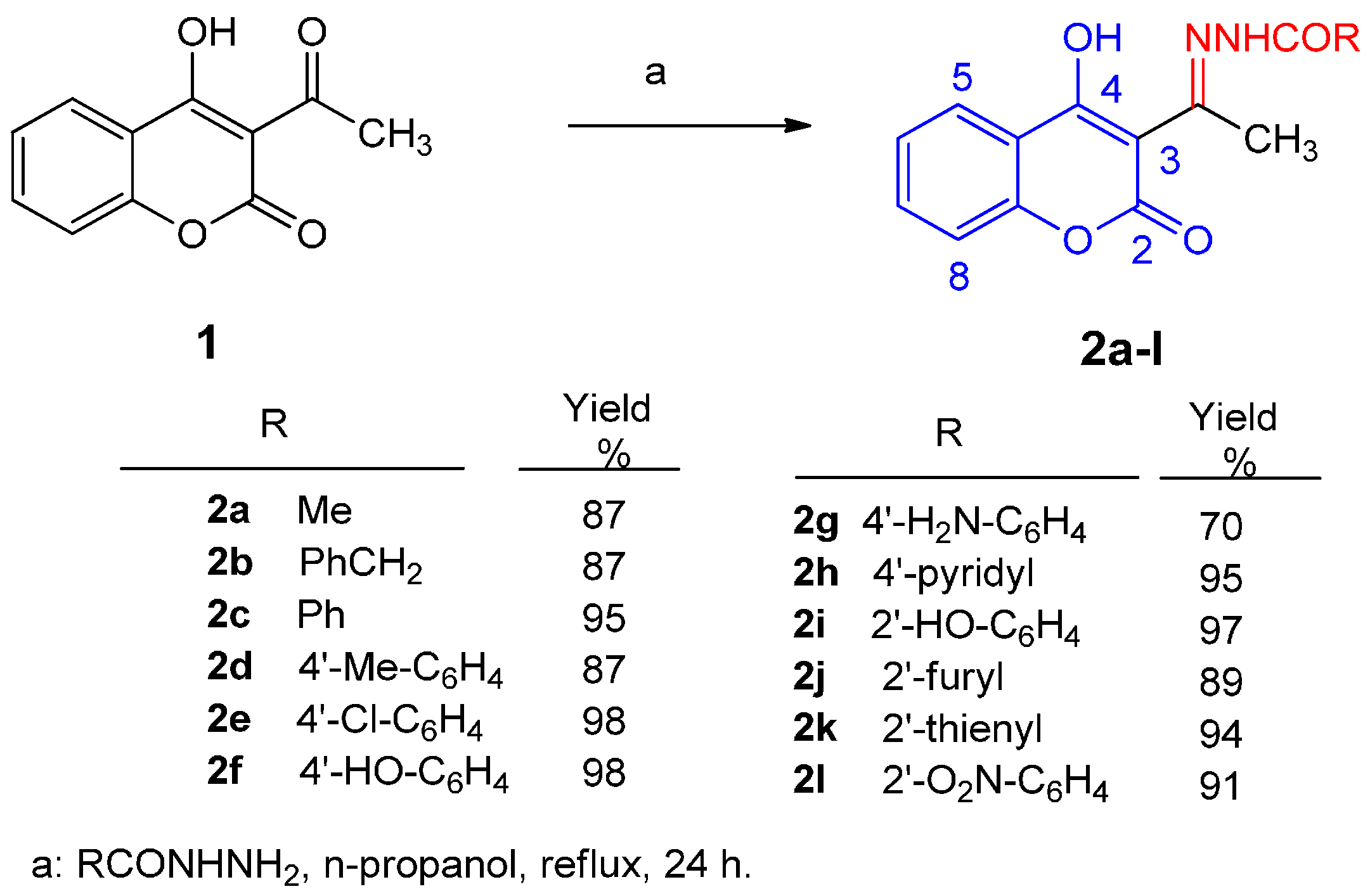
Synthesis of 3-[1-(Acyl-hydrazono)ethyl]-4-hydroxycoumarins (2a–l)
3.3. Pharmacology
3.3.1. Determination of the Reducing Activity of the DPPH (RA%)
3.3.2. Inhibition of Linoleic Acid Lipid Peroxidation
3.3.3. Soybean Lipoxygenase Inhibition Study In Vitro
3.3.4. Physicochemical Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Bedair, A.H.; El-Hady, N.A.; Abd El-Latif, M.S.; Fakery, A.H.; El-Agrody, A.M. 4-Hydroxycoumarin in heterocyclic synthesis Part III: Synthesis of some new pyrano[2,3-d]pyrimidine, 2-substituted[1,2,4]triazolo[1,5-c]pyrimidine and pyrimido[1,6-b][1,24]triazine derivatives. Farmaco 2000, 55, 708–714. [Google Scholar] [CrossRef]
- Cravotto, G.; Tagliapietra, S.; Cappello, R.; Palmisano, G.; Curini, M.; Boccalini, M. Long-chain 3-acyl-4-hydroxycoumarins: Structure and antibacterial activity. Arch. Pharm. Chem. Life Sci. 2006, 339, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anti Cancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Kirkiacharian, S.; Thuy, D.T.; Sicsic, S.; Bakhchinian, R.; Kurkjian, R.; Tonnaire, T. Structure-activity relationships of some 3-substituted-4-hydroxycoumarins as HIV-1 protease inhibitors. Farmaco 2002, 57, 703–708. [Google Scholar] [CrossRef]
- Manolov, I.; Danchev, N.D. Synthesis, toxicological and pharmacological assessment of some 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 1995, 30, 531–535. [Google Scholar] [CrossRef]
- Manolov, I.; Maichle-Moessmer, C.; Danchev, N. Synthesis, structure, toxicological and pharmacological investigations of 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2006, 41, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.G.; Sadowski, J.A.; Matschiner, J.T. Mechanism of action of warfarin. Warfarin and metabolism of vitamin K1. Biochemistry 1972, 11, 1959–1961. [Google Scholar] [CrossRef] [PubMed]
- Au, N.; Rettie, A.E. Pharmacogenomics of 4-hydroxycoumarin anticoagulants. Drug Metab. Rev. 2008, 40, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Stanchev, S.V.; Hadjimitova, T.; Traykov, T.; Boyanov, I.; Manolov, I. Investigation of the antioxidant properties of some new 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2009, 44, 3077–3082. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayed, A.S. Synthesis of new substituted chromen[4,3-c]pyrazol-4-ones and their antioxidant activities. Molecules 2011, 10292–10302. [Google Scholar] [CrossRef] [PubMed]
- Tosum, A. Biotechnological production of coumarins. In Biotechnological Production of Plant Secondary Metabolites; Orhan, I.E., Ed.; Bentham e-Books: Sharjah, The United Arab Emirates, 2012; pp. 36–52. [Google Scholar]
- Harper, S. The active principles of leguminous fish-poison plants. Part VI. Robustic acid. J. Chem. Soc. 1942, 181–182. [Google Scholar] [CrossRef]
- Miski, M.; Jakupovic, J. Cyclic farnesyl-coumarin and farnesyl-chromone derivatives from Ferula communis subsp. Communis. Phytochemistry 1990, 29, 1995–1998. [Google Scholar] [CrossRef]
- Lamnaouer, D.; Fraigui, O.; Martin, M.T.; Bodo, B. Structure of ferulenol derivatives from Ferula communis var. genuine. Phytochemistry 1991, 30, 2383–2386. [Google Scholar] [CrossRef]
- Saidkhodzhaev, A.I.; Kushmuradov, A.Y.; Maikov, V.M. Fepaldine-Terpenoid Coumarin from Ferrula-Pallida. Khim. Prip. Soedin. 1980, 6, 716–718. [Google Scholar]
- O’Kennedy, R.; Thornes, R.D. Coumarins: Biology, Applications and Mode of Action; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Galm, U.; Dessoy, M.A.; Schmidt, J.; Wessjohann, L.A.; Heide, I. In vitro and in vivo production of new aminocoumarins by a combined biochemical, genetic, and synthetic approach. Chem. Biol. 2004, 11, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Hu, S.; Pacholec, M.; Walsh, C.T. Synthesis of proposed oxidation-cyclization-methylation intermediates of the coumarin antibiotic biosynthetic pathway. Org. Lett. 2003, 5, 3233–3236. [Google Scholar] [CrossRef] [PubMed]
- Marcu, M.G.; Schulte, T.W.; Neckers, L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J. Natl. Cancer Inst. 2000, 92, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kotali, A. Transformation of phenolic hydroxyl into acyl group: A new tool in organic synthesis. Arkivoc 2009, 1, 81–96. [Google Scholar]
- Kotali, A.; Kotali, E.; Lafazanis, I.S.; Harris, P.A. Reactions of nitrogen derivatives of carbonyl compounds with phenyliodoso diacetate in organic synthesis. Curr. Org. Synth. 2010, 7, 62–77. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Kotali, A.; Lafazanis, I.S.; Papageorgiou, A.; Xrysogelou, E.; Lialiaris, T.; Sinakos, Z. Synthesis, characterization and antileucemic activity of 7-hydroxy-8-acetylcoumarin benzoylhydrazone. Molbank 2008, 2, M574. [Google Scholar] [CrossRef]
- Ponnurengam, M.S.; Malliappan, S.; Sethn, K.G.; Doble, M. QSAR studies on chalcones and flavonoids as anti-tuberculosis agents using genetic function approximation (GFA) method. Chem. Pharm. Bull. 2007, 55, 44–49. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Vanucci-Bacqué, C.; Carayon, C.; Bernis, C.; Camare, C.; Nègre-Salvayre, A.; Bedos-Belval, F.; Baltas, M. Synthesis, antioxidant and cytoprotective evaluation of potential antiatherogenic phenolic hydrazones. A structure-activity relationship insight. Bioorg. Chem. Med. 2014, 22, 4269–4276. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Kotali, A.; Nasiopoulou, D.A.; Harris, P.A.; Helliwell, M.; Joule, J.A. Transformation of a hydroxyl into an acyl group on α-pyrone ring: A novel route to 3,4-diacylcoumarins. Tetrahedron 2012, 68, 761–766. [Google Scholar] [CrossRef]
- Melagraki, G.; Chatzidakis, H.; Afantitis, A.; Igglessi-Markopoulou, O.; Detsi, A.; Koufaki, M.; Kontogiorgis, C.; Hadjipavlou-Litina, D.J. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. J. Eur. J. Med. Chem. 2009, 44, 3020–3026. [Google Scholar] [CrossRef] [PubMed]
- Kotali, A.; Lafazanis, I.S.; Harris, P.A. A novel and facile synthesis of 7,8-diacylcoumarins. Tetrahedron Lett. 2007, 48, 7181–7183. [Google Scholar] [CrossRef]
- Kotali, A.; Lafazanis, I.S.; Harris, P.A. Synthesis of angular 7,8-pyridazinocoumarins via the transformation of a hydroxy group into a carbonyl group. Synthesis 2009, 5, 836–840. [Google Scholar] [CrossRef]
- Kotali, A.; Lafazanis, I.S.; Harris, P.A. Synthesis of 6,7-diacylcoumarins via the transformation of a hydroxy into a carbonyl group. Synth. Commun. 2008, 38, 3996–4006. [Google Scholar] [CrossRef]
- Somogyi, L.; Sohár, P. Tricarbonylmethane acylhydrazones: Reactions under acylating conditions and formation of fused isoxazole derivatives with concomitant N-N bond cleavage. Liebigs Ann. Chem. 1995, 1995, 1903–1906. [Google Scholar] [CrossRef]
- Eisenhauer, H.R.; Link, K.P. Studies on 4-Hydroxycoumarins. XIII. The Mechanism for the reaction of 4-hydroxycoumarin with aliphatic acid chlorides. J. Am. Chem. Soc. 1953, 75, 2044–2045. [Google Scholar] [CrossRef]
- Traven, V.F.; Negrebetsky, V.V.; Vorobjeva, L.I.; Carberry, E.A. Keto-enol tautomerism, NMR spectra and H-D exchange of 4-hydroxycoumarins. Can. J. Chem. 1997, 75, 377–383. [Google Scholar] [CrossRef]
- Gautam, D.R.; Protopappas, J.; Fylaktakidou, K.C.; Litinas, K.E.; Nicolaides, D.N.; Tsoleridis, C.A. Unexpected one-pot synthesis of new polycyclic coumarin[4,3-c]pyridine derivatives via a tandem hetero-Diels–Alder and 1,3-dipolar cycloaddition reaction. Tetrahedron Lett. 2009, 50, 448–451. [Google Scholar] [CrossRef]
- Kotali, A.; Nasiopoulou, D.A.; Harris, P.A.; Helliwell, M.; Joule, J.A. N′-[1-(2,4-Dioxo-3,4-dihydro-2H-1-benzopyran-3-yl-idene)eth-yl]thiophene-2-carbo-hydrazide. Acta Cryst. Sect. E 2010, E67, o1014. [Google Scholar]
- Lyssenko, K.A.; Antipin, M.Y. Hydrogen bond in 3-acetyl-4-hydroxycoumarin: X-ray diffraction study and quantum-chemical calculations. Russ. Chem. Bull. 2001, 50, 418–431. [Google Scholar] [CrossRef]
- Naceur, H.; Fischmeister, C.; Puerta, M.C.; Valerga, P. A rapid access to new coumarinyl chalcone and substituted chromeno [4, 3-c] pyrazol-4 (1H)-ones and their antibacterial and DPPH radical scavenging activities. Med. Chem. Res. 2011, 20, 522–530. [Google Scholar]
- Abdou, M.M. 3-Acetyl-4-hydroxycoumarin: Synthesis, reactions and applications. Arab. J. Chem. 2014. [Google Scholar] [CrossRef]
- Feng, J.Y.; Liu, Z.Q. Phenolic and enolic hydroxyl groups in curcumin: Which plays the major role in scavenging radicals? J. Agric. Food Chem. 2009, 57, 11041–11046. [Google Scholar] [CrossRef] [PubMed]
- Liégeois, C.; Lermusieau, G.; Collin, S. Measuring antioxidant efficiency of wort, malt, and hops against the 2,2′-azobis(2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. Agric. Food Chem. 2000, 48, 1129–1134. [Google Scholar] [CrossRef]
- Müller, K. 5-Lipoxygenase and 12-lipoxygenase: Attractive targets for the development of novel antipsoriatic drugs. Arch. Pharm. 1994, 327, 3–19. [Google Scholar] [CrossRef]
- Kemal, C.; Louis-Flamberg, P.; Krupinski-Olsen, R.; Shorter, A.L. Reductive inactivation of soybean lipoxygenase 1 by catechols: A possible mechanism for regulation of lipoxygenase activity. Biochemistry 1987, 26, 7064–7072. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, J.; Eling, T.E.; Mason, R.P. Formation of free radical metabolites in the reaction between soybean lipoxygenase and its inhibitors. An ESR study. Biochemistry 1989, 28, 8363–8367. [Google Scholar] [CrossRef] [PubMed]
- Simulated with SpinWorks Simulation Program, Version 2.5.5. Available online: http://davinci.chem.umanitoba.ca/pub/marat/SpinWorks/ (accessed on 29 November 2006).
- Software used for determination of clog P: Biobyte Corp., C-QSAR Database 201 West 4th Street, Suite 204, Claremont, CA 91711, USA, 2002.
- Payá, M.; Halliwell, B.; Hoult, J.R. Interactions of a series of coumarins with reactive oxygen species. Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem. Pharm. 1992, 44, 205–214. [Google Scholar] [CrossRef]
- Vukovic, N.; Sukdolak, S.; Solujic, S.; Niciforovic, N. An efficient synthesis and antioxidant properties of novel imino and amino derivatives of 4-hydroxy coumarins. Arch. Pharm. Res. 2010, 33, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Curini, M.; Epifano, F.; Genovese, S.; Menghini, L.; Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Bellacchio, E. Lipoxygenase inhibitory activity of boropinic acid, active principle of boronia pinnata. Nat. Prod. Commun. 2006, 1, 1141–1145. [Google Scholar]
- Sample Availability: Samples of the compounds 2d, 2i and 2l are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotali, A.; Nasiopoulou, D.A.; Tsoleridis, C.A.; Harris, P.A.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.J. Antioxidant Activity of 3-[N-(Acylhydrazono)ethyl]-4-hydroxy-coumarins. Molecules 2016, 21, 138. https://doi.org/10.3390/molecules21020138
Kotali A, Nasiopoulou DA, Tsoleridis CA, Harris PA, Kontogiorgis CA, Hadjipavlou-Litina DJ. Antioxidant Activity of 3-[N-(Acylhydrazono)ethyl]-4-hydroxy-coumarins. Molecules. 2016; 21(2):138. https://doi.org/10.3390/molecules21020138
Chicago/Turabian StyleKotali, Antigoni, Despina A. Nasiopoulou, Constantinos A. Tsoleridis, Philip A. Harris, Christos A. Kontogiorgis, and Dimitra J. Hadjipavlou-Litina. 2016. "Antioxidant Activity of 3-[N-(Acylhydrazono)ethyl]-4-hydroxy-coumarins" Molecules 21, no. 2: 138. https://doi.org/10.3390/molecules21020138






