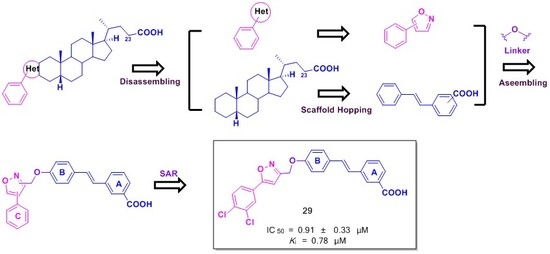
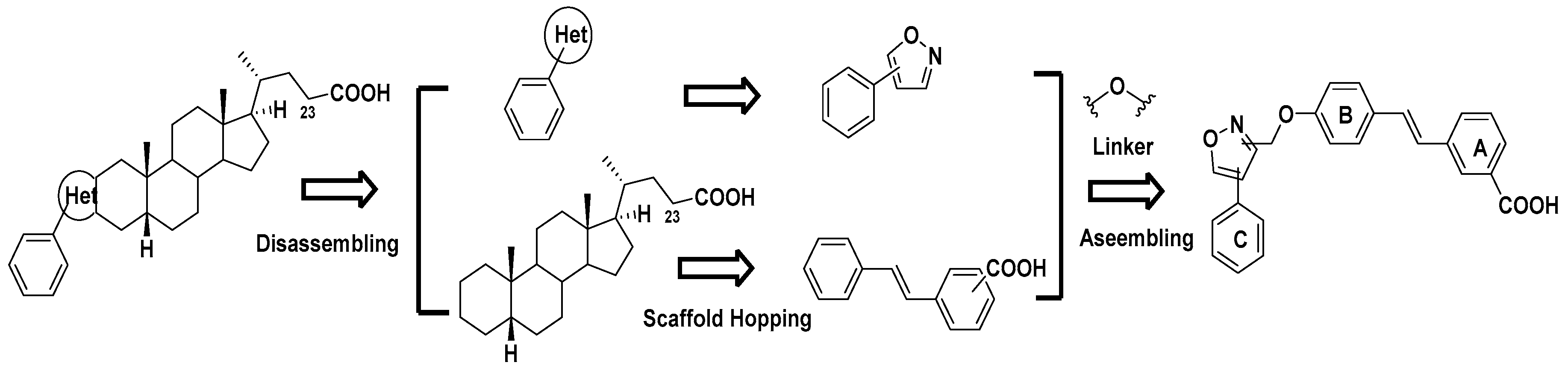
Design, Synthesis and Biological Evaluation of Stilbene Derivatives as Novel Inhibitors of Protein Tyrosine Phosphatase 1B
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. In Vitro Biological Evaluation
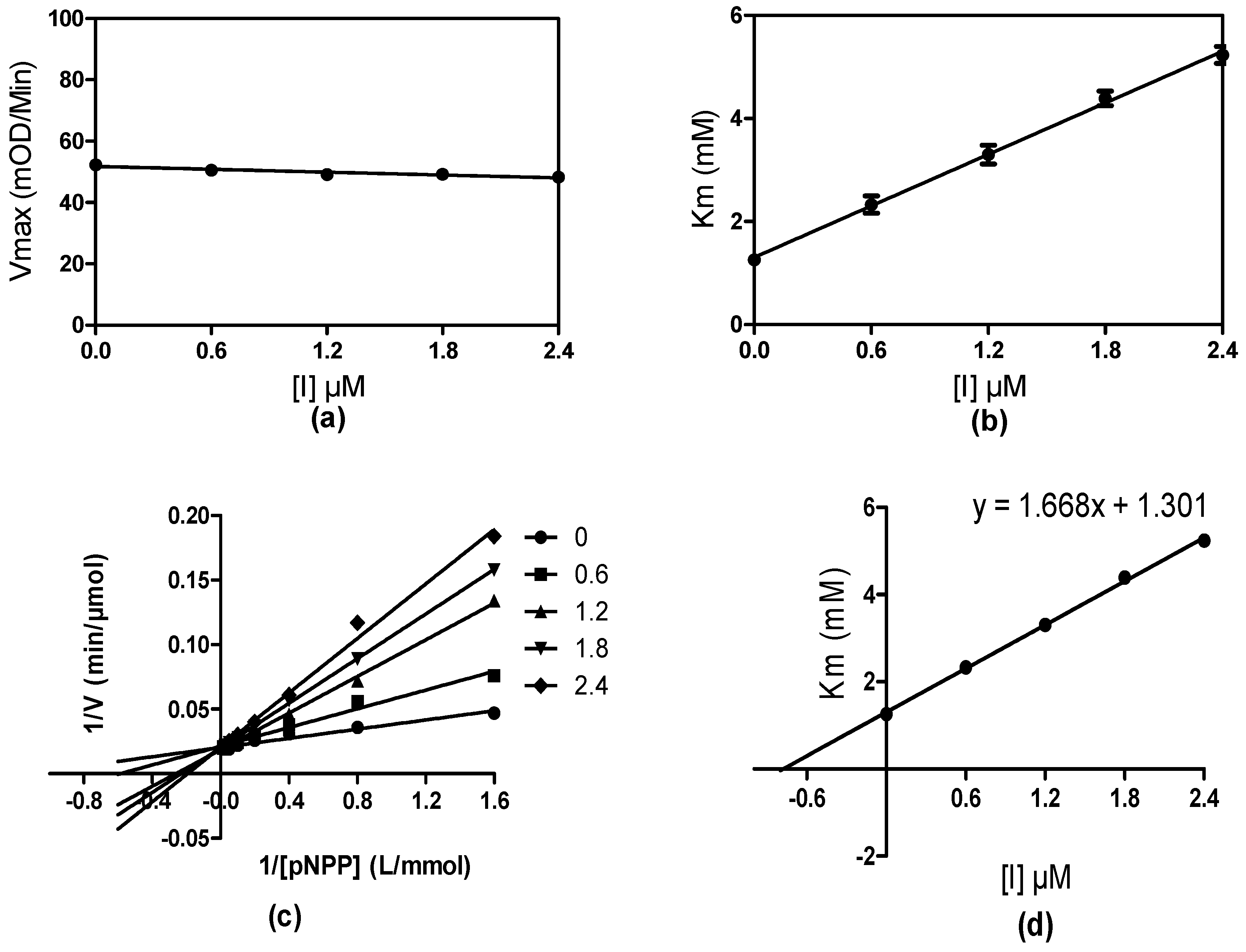
2.3. Enzyme Kinetic Study
3. Experimental Section
3.1. Chemsitry
3.1.1. General Information
3.1.2. Synthesis of Methyl 3-((Diethoxyphosphoryl)methyl)benzoate (11)
3.1.3. Synthesis of (E)-3-(4-Methoxystyryl)benzoic Acid (12)
3.1.4. Synthesis of (E)-3-(4-Hydroxystyryl)benzoic Acid (13)
3.1.5. Synthesis of Methyl (E)-3-(4-Hydroxystyryl)benzoate (14)
3.1.6. Synthesis of (3-Phenylisoxazol-4-yl)methanol (15b)
3.1.7. Synthesis of 4-(Chloromethyl)-3-phenylisoxazole (15c)
3.1.8. Synthesis of (E)-3-(4-((3-Phenylisoxazol-4-yl)methoxy)styryl)benzoic Acid (15)
3.2. Biological Activity against PTP1B and TCPTP
3.3. Characterization of Compound 29
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alonso, A.; Sasin, J.; Bottini, N.; Friedberg, I.; Osterman, A.; Godzik, A.; Hunter, T.; Dixon, J.; Mustelin, T. Protein tyrosine phosphatases in the human genome. Cell 2004, 117, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. Signaling-2000 and beyond. Cell 2000, 100, 113–127. [Google Scholar] [CrossRef]
- Neel, B.G.; Tonks, N.K. Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell Biol. 1997, 9, 193–204. [Google Scholar] [CrossRef]
- Tonks, N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006, 7, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Elchebly, M.; Payette, P.; Michaliszyn, E.; Cromlish, W.; Collins, S.; Loy, A.L.; Normandin, D.; Cheng, A.; Himms-Hagen, J.; Chan, C.C.; et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 1999, 283, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Klaman, L.D.; Boss, O.; Peroni, O.D.; Kim, J.K.; Martino, J.L.; Zabolotny, J.M.; Moghal, N.; Lubkin, M.; Kim, Y.B.; Sharpe, A.H.; et al. Increased energy expenditure, decreased adiposity, and tissuc insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 2000, 20, 5479–5489. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Uetani, N.; Simoncic, P.D.; Chaubey, V.P.; Lee-Loy, A.; McGlade, C.J.; Kennedy, B.P.; Tremblay, M.L. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell 2002, 2, 497–503. [Google Scholar] [CrossRef]
- Zabolotny, J.M.; Bence-Hanulec, K.K.; Stricker-Krongrad, A.; Haj, F.; Wang, Y.; Minokoshi, Y.; Kim, Y.B.; Elmquist, J.K.; Tartaglia, L.A.; Kahn, B.B.; et al. PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2002, 2, 489–495. [Google Scholar] [CrossRef]
- Iversen, L.F.; Moller, K.B.; Pedersen, A.K.; Peters, G.H.; Petersen, A.S.; Andersen, H.S. Structure Determination of T Cell Protein-tyrosine Phosphatase. J. Biol. Chem. 2002, 277, 19982–19990. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Yang, D.L.; Gao, L.X.; Tang, C.L.; Ma, W.P.; Ye, H.H.; Zhang, S.Q.; Zhao, Y.N.; Xu, H.J.; Hu, Z.; et al. 1H-2,3-Dihydroperimidine Derivatives: A New Class of Potent Protein Tyrosine Phosphatase 1B Inhibitors. Molecules 2014, 19, 102–121. [Google Scholar] [CrossRef] [PubMed]
- He, H.B.; Gao, L.X.; Deng, Q.F.; Ma, W.P.; Tang, C.L.; Qiu, W.W.; Tang, J.; Li, J.Y.; Li, J.; Yang, F. Synthesis and biological evaluation of 4,4-dimethyl lithocholic acid derivatives as novel inhibitors of protein tyrosine phosphatase 1B. Bioorg. Med. Chem. Lett. 2012, 22, 7237–7242. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.H.; Pandey, N.R.; Zhou, X. Functional properties of Claramine: A novel PTP1B inhibitor and insulin-mimetic compound. Biochem. Biophys. Res. Commun. 2015, 458, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, F.R. MSI-1436 reduces acute food intake without affecting dopamine transporter activity. Pharmacol. Biochem. Behav. 2010, 97, 138–143. [Google Scholar]
- Dodds, E.C.; Goldberg, L.; Lawson, W.; Robinson, R. Oestrogenic activity of certain synthetic compounds. Nature 1938, 141, 247–248. [Google Scholar] [CrossRef]
- Sun, H.M.; Tawa, G.; Wallqvist, A. Classification of scaffold-hopping approaches. Drug Discov. Today 2012, 17, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Inamori, Y.; Kato, Y.; Kubo, M.; Yasuda, M.; Baba, K.; Kozawa, M. Physiological activities of 3,3′,4,5′-tetrahydroxystilbene isolated from the heartwood of Cassia garrettiana CRAIB. Chem. Pharm. Bull. 1984, 32, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Belofsky, G.; Percivili, D.; Lewis, K.G.; Tegos, P.; Ekart, J. Phenolic Metabolites of Dalea versicolor that Enhance Antibiotic Activity against Model Pathogenic Bacteria. J. Nat. Prod. 2004, 67, 481–484. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Complexation of Pinosylvin, an Analogue of Resveratrol with High Antifungal and Antimicrobial Activity, by Different Types of Cyclodextrins. J. Agric. Food Chem. 2009, 57, 10175–10180. [Google Scholar] [CrossRef] [PubMed]
- Bagula, P.K.; Dindab, A.K.; Banerjeea, S.K. Effect of resveratrol on sirtuins expression and cardiac complications in diabetes. Biochem. Biophys. Res. Commun. 2015, 468, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Xu, Y.; Hu, C.Q.; Zhang, J.; Lin, X.; Li, J.Y.; Yang, B.; He, Q.J.; Hu, Y.Z. Design, synthesis and AChE inhibitory activity of indanone and aurone derivatives. Eur. J. Med. Chem. 2009, 44, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Anthony, N.G.; Breen, D.; Clarke, J.; Donoghue, G.; Drummond, A.J.; Ellis, E.M.; Gemmell, C.G.; Helesbeux, J.J.; Hunter, I.S.; Khalaf, A.I.; et al. Antimicrobial Lexitropsins Containing Amide, Amidine, and Alkene Linking Groups. J. Med. Chem. 2007, 50, 6116–6125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallejos, J.C.; Schouteeten, A.; Wilhelm, D. Process for the Synthesis of Polyhydroxystilbene Compounds. US Patent 8,399,714, 23 December 2010. [Google Scholar]
- Yamamoto, T.; Fujita, K.; Asari, S.; Chiba, A.; Kataba, Y.; Ohsumi, K.; Ohmuta, N.; Iida, Y.; Ijichi, C.; Iwayama, S.; et al. Synthesis and evaluation of isoxazole derivatives as lysophosphatidic acid (LPA) antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 3736–3740. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.T.; Tellitu, I.; Domı́nguez, E.; Hernández, S.; Moreno, I.; SanMartı́n, R. A general and efficient PIFA mediated synthesis of heterocycle-fused quinolinone derivatives. Tetrahedron 2002, 58, 8581–8589. [Google Scholar] [CrossRef]
- Ramana, P.V.; Reddy, A.R. Synthesis of 1,2,3-triazole substituted isoxazoles via copper(I) catalyzed cycloaddition. J. Heterocycl. Chem. 2012, 49, 621–627. [Google Scholar] [CrossRef]
- Kamal, A.; Tamboli, J.R.; Vishnuvardhan, M.V.; Adil, S.F.; Nayak, V.L.; Ramakrishna, S. Synthesis and anticancer activity of heteroaromatic linked 4β-amido podophyllotoxins as apoptotic inducing agents. Bioorg. Med. Chem. Lett. 2013, 23, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hong, D.; Zhou, Y.; Zhang, Y.; Shen, Q.; Li, J.Y.; Hu, L.H.; Li, J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim. Biophys. Acta 2006, 1760, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 15~30 are available from the authors.


| Compound | X | R1 | IC50 (μM) a |
|---|---|---|---|
| 15 |  | H | 20.7 ± 3.83 |
| 16 |  | H | 16.2 ± 4.52 |
| 17 |  | H | 15.7 ± 2.15 |
| 18 |  | H | 6.33 ± 1.02 |
| 18d |  | Me | 23.7 ± 3.62 |
| Oleanolic acid b | 2.96 ± 0.35 |
| Compound | R | IC50 (μM) a | TCPTP/PTP1B b | |
|---|---|---|---|---|
| PTP1B | TCPTP | |||
| 18 | H | 6.33 ± 1.33 | 9.58 ± 0.33 | 1.5 |
| 19 | 2-Cl | 1.75 ± 0.82 | 36.15 ± 3.82 | 20.7 |
| 20 | 2-OMe | 7.66 ± 1.05 | >40 | 5.3 |
| 21 | 3-Cl | 5.37 ± 1.53 | 7.58 ± 1.32 | 1.4 |
| 22 | 3-F | >20 | >40 | - c |
| 23 | 3-Br | 9.85 ± 2.04 | >40 | 4.1 |
| 24 | 3-Me | 3.68 ± 1.12 | 18.91 ± 1.18 | 5.1 |
| 25 | 3-NO2 | 2.05 ± 0.54 | 13.25 ± 1.70 | 6.5 |
| 26 | 4-Me | 3.56 ± 0.28 | 7.67 ± 0.35 | 2.2 |
| 27 | 4-OMe | 2.23 ± 0.51 | 8.02 ± 0.99 | 3.6 |
| 28 | 4-F | 3.34 ± 0.81 | 5.19 ± 0.31 | 1.6 |
| 29 | 3,4-Cl2 | 0.91 ± 0.33 | 3.78 ± 0.22 | 4.2 |
| 30 | 3,4-F2 | 1.22 ± 0.48 | 6.02 ± 1.13 | 4.9 |
| Lithocholic acid | 12.54 ± 2.51 | 20.95 ± 3.66 | 1.7 | |
| Oleanolic acid d | 2.71 ± 0.19 | 6.12 ± 0.15 | 2.3 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Ge, Y.; Dai, H.; Cui, S.; Ye, F.; Jin, J.; Shi, Y. Design, Synthesis and Biological Evaluation of Stilbene Derivatives as Novel Inhibitors of Protein Tyrosine Phosphatase 1B. Molecules 2016, 21, 1722. https://doi.org/10.3390/molecules21121722
He H, Ge Y, Dai H, Cui S, Ye F, Jin J, Shi Y. Design, Synthesis and Biological Evaluation of Stilbene Derivatives as Novel Inhibitors of Protein Tyrosine Phosphatase 1B. Molecules. 2016; 21(12):1722. https://doi.org/10.3390/molecules21121722
Chicago/Turabian StyleHe, Haibing, Yinghua Ge, Hong Dai, Song Cui, Fei Ye, Jia Jin, and Yujun Shi. 2016. "Design, Synthesis and Biological Evaluation of Stilbene Derivatives as Novel Inhibitors of Protein Tyrosine Phosphatase 1B" Molecules 21, no. 12: 1722. https://doi.org/10.3390/molecules21121722
APA StyleHe, H., Ge, Y., Dai, H., Cui, S., Ye, F., Jin, J., & Shi, Y. (2016). Design, Synthesis and Biological Evaluation of Stilbene Derivatives as Novel Inhibitors of Protein Tyrosine Phosphatase 1B. Molecules, 21(12), 1722. https://doi.org/10.3390/molecules21121722