Redox Signaling Regulated by Cysteine Persulfide and Protein Polysulfidation
Abstract
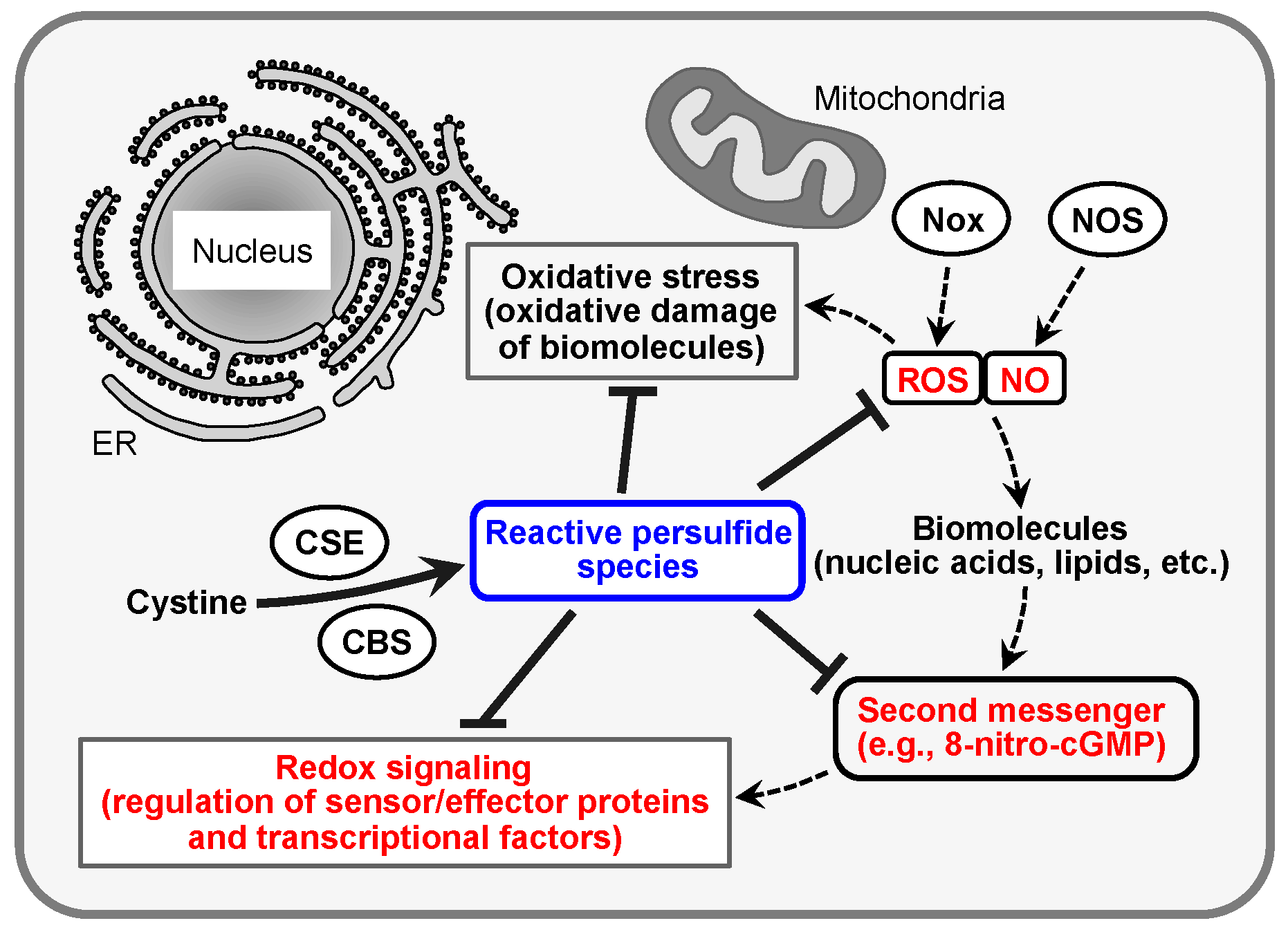
:1. Introduction
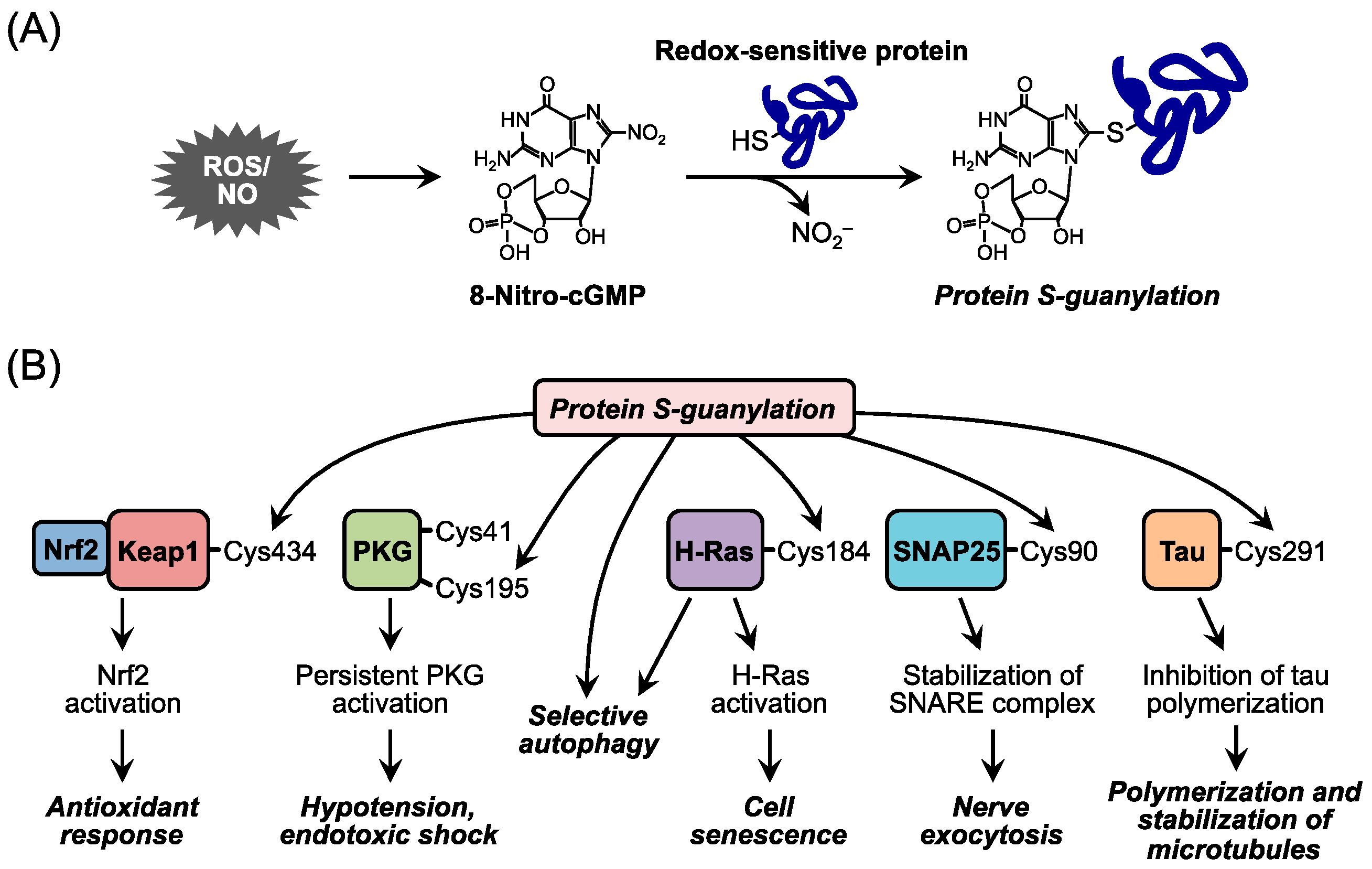
2. Redox Signaling and Its Second Messenger 8-Nitro-cGMP
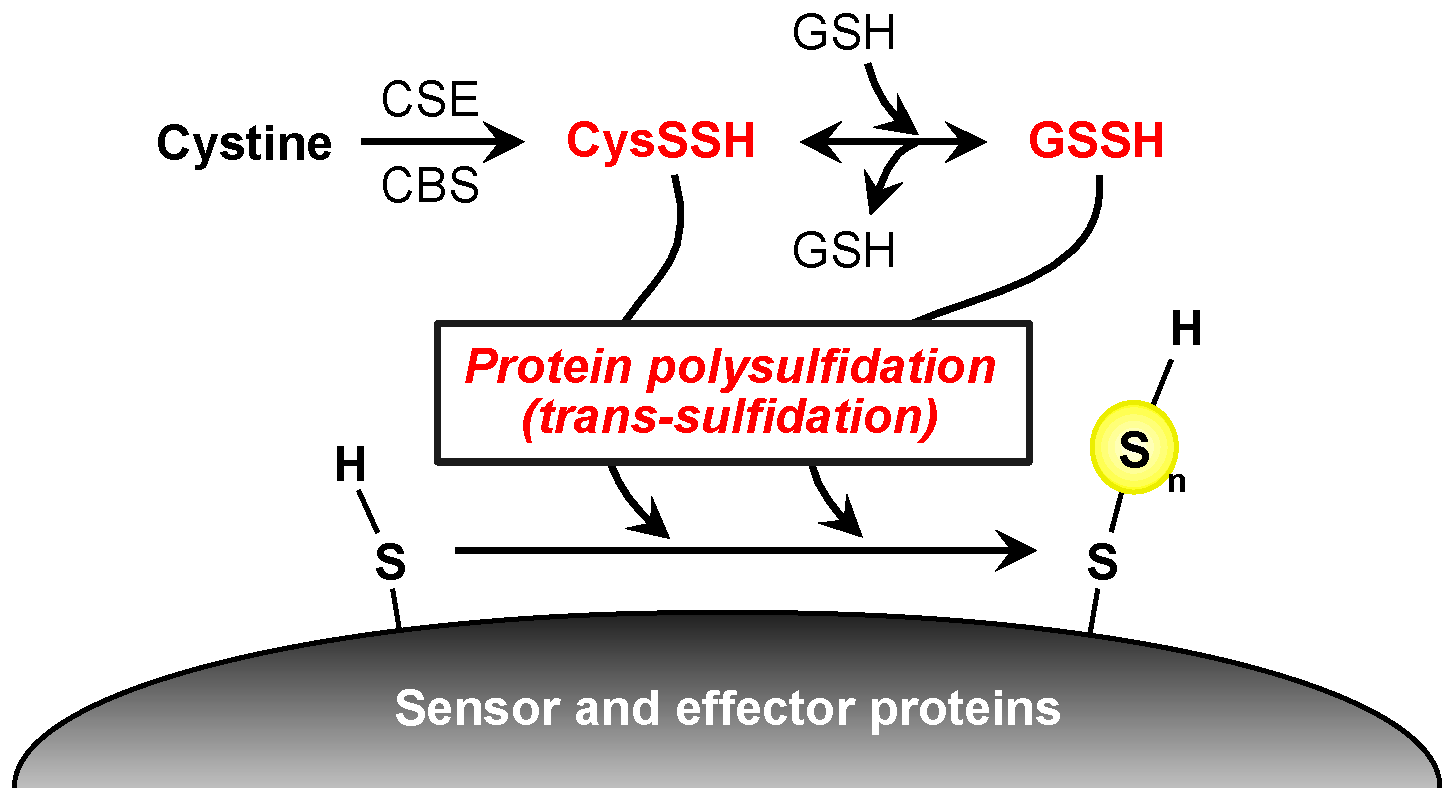
3. CysSSH and Other Reactive Persulfide Species
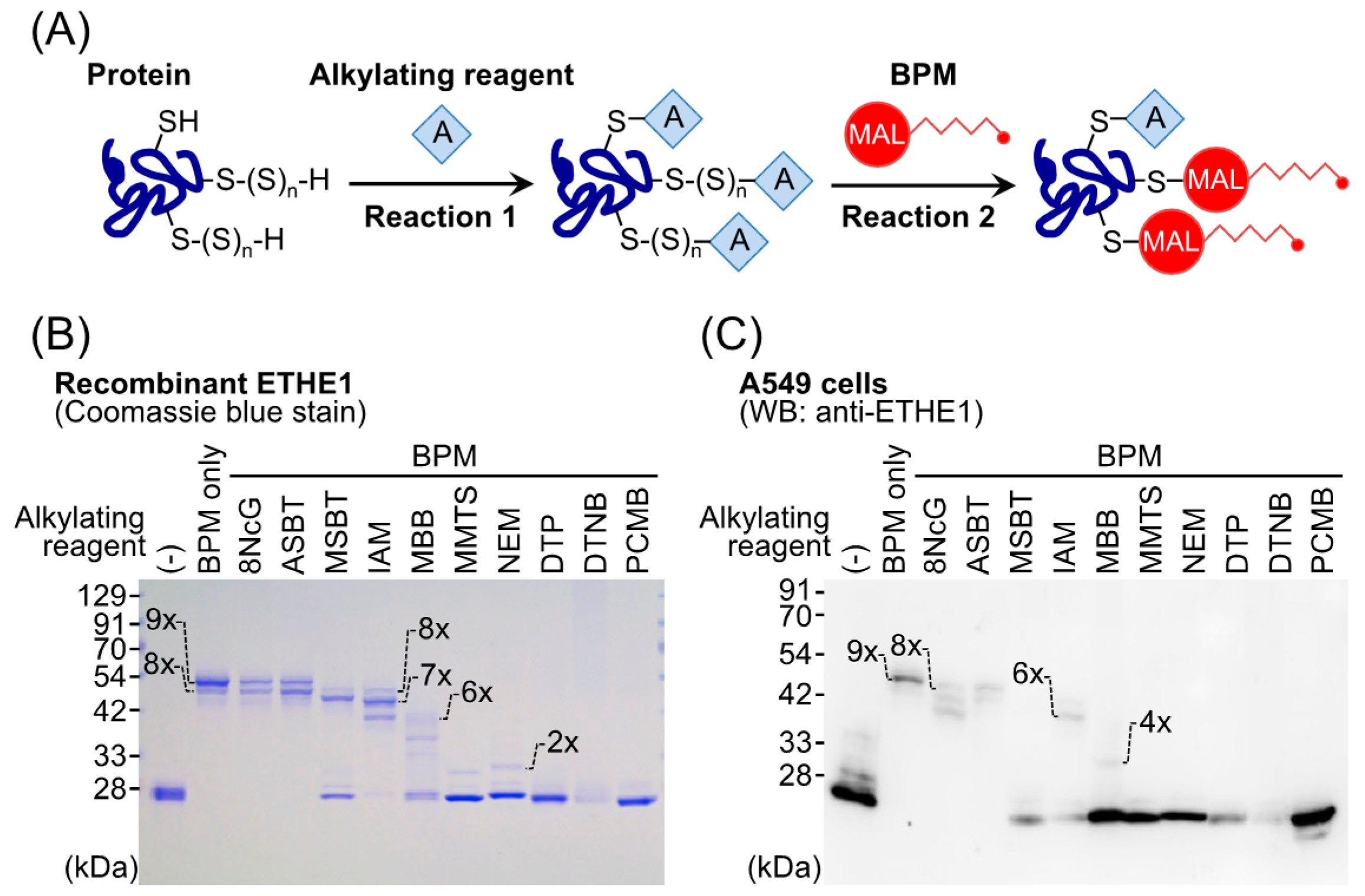
4. Protein Polysulfidation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Van der Vliet, A.; Cross, C.E. Oxidants, nitrosants, and the lung. Am. J. Med. 2000, 109, 398–421. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Fukuto, J.M.; Torres, M. Redox signaling: Thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am. J. Physiol. Cell Physiol. 2004, 287, C246–C256. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Zaki, M.H.; Okamoto, T.; Akuta, T.; Tokutomi, Y.; Kim-Mitsuyama, S.; Ihara, H.; Kobayashi, A.; Yamamoto, M.; Fujii, S.; et al. Protein S-guanylation by the biological signal 8-nitroguanosine 3′,5′-cyclic monophosphate. Nat. Chem. Biol. 2007, 3, 727–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, K.; Shibata, T. 15-Deoxy-Δ12,14-prostaglandin J2: An electrophilic trigger of cellular responses. Chem. Res. Toxicol. 2008, 21, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.H.; Fujii, S.; Okamoto, T.; Islam, S.; Khan, S.; Ahmed, K.A.; Sawa, T.; Akaike, T. Cytoprotective function of heme oxygenase 1 induced by a nitrated cyclic nucleotide formed during murine salmonellosis. J. Immunol. 2009, 182, 3746–3756. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Sawa, T.; Ihara, H.; Tong, K.I.; Ida, T.; Okamoto, T.; Ahtesham, K.A.; Ishima, Y.; Motohashi, H.; Yamamoto, M.; et al. The critical role of nitric oxide signaling, via protein S-guanylation and nitrated cyclic GMP, in the antioxidant adaptive response. J. Biol. Chem. 2010, 285, 23970–23984. [Google Scholar] [CrossRef] [PubMed]
- Akaike, T.; Fujii, S.; Sawa, T.; Ihara, H. Cell signaling mediated by nitrated cyclic guanine nucleotide. Nitric Oxide 2010, 23, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, T.K.; Freeman, B.A. Transduction of redox signaling by electrophile-protein reactions. Sci. Signal. 2009, 2, re7. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, F.J.; Cipollina, C.; Freeman, B.A. Formation and signaling actions of electrophilic lipids. Chem. Rev. 2011, 111, 5997–6021. [Google Scholar] [CrossRef] [PubMed]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Akaike, T.; Sawa, T.; Kumagai, Y.; Wink, D.A.; Tantillo, D.J.; Hobbs, A.J.; Nagy, P.; Xian, M.; Lin, J.; et al. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: Implications of their possible biological activity and utility. Free Radic. Biol. Med. 2014, 77, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [PubMed]
- De Beus, M.D.; Chung, J.; Colón, W. Modification of cysteine 111 in Cu/Zn superoxide dismutase results in altered spectroscopic and biophysical properties. Protein Sci. 2004, 13, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Vandiver, M.S.; Paul, B.D.; Xu, R.; Karuppagounder, S.; Rao, F.; Snowman, A.M.; Ko, H.S.; Lee, Y.I.; Dawson, V.L.; Dawson, T.M.; et al. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013, 4, 1626. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Qu, C.; Zhou, Y.; Konkel, J.E.; Shi, S.; Liu, Y.; Chen, C.; Liu, S.; Liu, D.; Chen, Y.; et al. Hydrogen sulfide promotes Tet1- and Tet2-mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity 2015, 43, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Krokowski, D.; Guan, B.J.; Bederman, I.; Majumder, M.; Parisien, M.; Diatchenko, L.; Kabil, O.; Willard, B.; Banerjee, R.; et al. Quantitative H2S-mediated protein sulfhydration reveals metabolic reprogramming during the integrated stress response. eLife 2015, 4, e10067. [Google Scholar] [CrossRef] [PubMed]
- Dóka, É.; Pader, I.; Bíró, A.; Johansson, K.; Cheng, Q.; Ballagó, K.; Prigge, J.R.; Pastor-Flores, D.; Dick, T.P.; Schmidt, E.E.; et al. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016, 2, e1500968. [Google Scholar] [CrossRef] [PubMed]
- Sumimoto, H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008, 275, 3249–3277. [Google Scholar] [CrossRef] [PubMed]
- Van der Vliet, A. NADPH oxidases in lung biology and pathology: Host defense enzymes, and more. Free Radic. Biol. Med. 2008, 44, 938–955. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Chance, B. The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett. 1970, 11, 172–176. [Google Scholar] [CrossRef]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [PubMed]
- Sies, H.; Summer, K.H. Hydroperoxide-metabolizing systems in rat liver. Eur. J. Biochem. 1975, 57, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Sawa, T.; Ihara, H.; Kasamatsu, S.; Yoshitake, J.; Rahaman, M.M.; Okamoto, T.; Fujii, S.; Akaike, T. Regulation by mitochondrial superoxide and NADPH oxidase of cellular formation of nitrated cyclic GMP: Potential implications for ROS signalling. Biochem. J. 2012, 441, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Tsutsuki, H.; Jung, M.; Zhang, T.; Ono, K.; Ida, T.; Kunieda, K.; Ihara, H.; Akaike, T.; Sawa, T. Endogenous occurrence of protein S-guanylation in Escherichia coli: Target identification and genetic regulation. Biochem. Biophys. Res. Commun. 2016, 478, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Sawa, T.; Kitajima, N.; Ono, K.; Inoue, H.; Ihara, H.; Motohashi, H.; Yamamoto, M.; Suematsu, M.; Kurose, H.; et al. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat. Chem. Biol. 2012, 8, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Saito, Y.; Nozawa, T.; Fujii, S.; Sawa, T.; Inoue, H.; Matsunaga, T.; Khan, S.; Akashi, S.; Hashimoto, R.; et al. Endogenous nitrated nucleotide is a key mediator of autophagy and innate defense against bacteria. Mol. Cell 2013, 52, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Kunieda, K.; Tsutsuki, H.; Ida, T.; Kishimoto, Y.; Kasamatsu, S.; Sawa, T.; Goshima, N.; Itakura, M.; Takahashi, M.; Akaike, T.; et al. 8-Nitro-cGMP enhances SNARE complex formation through S-guanylation of Cys90 in SNAP25. ACS Chem. Neurosci. 2015, 6, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Akashi, S.; Ahmed, K.A.; Sawa, T.; Ono, K.; Tsutsuki, H.; Burgoyne, J.R.; Ida, T.; Horio, E.; Prysyazhna, O.; Oike, Y.; et al. Persistent activation of cGMP-dependent protein kinase by a nitrated cyclic nucleotide via site specific protein S-guanylation. Biochemistry 2016, 55, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Yoshitake, J.; Soeda, Y.; Ida, T.; Sumioka, A.; Yoshikawa, M.; Matsushita, K.; Akaike, T.; Takashima, A. Modification of tau by 8-nitro-cGMP: Effects of nitric oxide-linked chemical modification on tau aggregation. J. Biol. Chem. 2016, 291, 22714–22720. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.M.; Sawa, T.; Ahtesham, K.A.; Khan, S.; Inoue, H.; Irie, A.; Fujii, S.; Akaike, T. S-Guanylation proteomics for redox-based mitochondrial signaling. Antioxid. Redox Signal. 2014, 20, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, D.; de Marco, C.; Mondovi, B. Cleavage of cystine by a pyridoxal model. Arch. Biochem. Biophys. 1960, 87, 281–288. [Google Scholar] [CrossRef]
- Cooper, A.J.L.; Pinto, J.T. Aminotransferase, l-amino acid oxidase and β-lyase reactions involving l-cysteine S-conjugates found in allium extracts: Relevance to biological activity? Biochem. Pharmacol. 2005, 69, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.T.; Krasnikov, B.F.; Cooper, A.J.L. Redox-sensitive proteins are potential targets of garlic-derived mercaptocysteine derivatives. J. Nutr. 2006, 136, 835–841. [Google Scholar]
- Toohey, J.I. Sulfur signaling : Is the agent sulfide or sulfane? Anal. Biochem. 2011, 413, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I.; Cooper, A.J.L. Thiosulfoxide (sulfane) sulfur: New chemistry and new regulatory roles in biology. Molecules 2014, 19, 12789–12813. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.O.; Pearson, R.G. The factors determining nucleophilic reactivities. J. Am. Chem. Soc. 1962, 84, 16–24. [Google Scholar] [CrossRef]
- Honda, K.; Yamada, N.; Yoshida, R.; Ihara, H.; Sawa, T.; Akaike, T.; Iwai, S. 8-Mercapto-cyclic GMP mediates hydrogen sulfide-induced stomatal closure in Arabidopsis. Plant Cell Physiol. 2015, 56, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Ihara, H.; Ida, T.; Fujii, S.; Nishida, M.; Akaike, T. Formation, signaling function6s, and metabolisms of nitrated cyclic nucleotide. Nitric Oxide 2013, 34, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.; Toyama, T.; Shinkai, Y.; Sawa, T.; Akaike, T.; Kumagai, Y. Detoxification of methylmercury by hydrogen sulfide-producing enzyme in mammalian cells. Chem. Res. Toxicol. 2011, 24, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Abiko, Y.; Yoshida, E.; Ishii, I.; Fukuto, J.M.; Akaike, T.; Kumagai, Y. Involvement of reactive persulfides in biological bismethylmercury sulfide formation. Chem. Res. Toxicol. 2015, 28, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Abiko, Y.; Ishii, I.; Kamata, S.; Tsuchiya, Y.; Watanabe, Y.; Ihara, H.; Akaike, T.; Kumagai, Y. Formation of sulfur adducts of N-acetyl-p-benzoquinoneimine, an electrophilic metabolite of acetaminophen in vivo: Participation of reactive persulfides. Chem. Res. Toxicol. 2015, 28, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Kasamatsu, S.; Matsunaga, T.; Akashi, S.; Ono, K.; Nishimura, A.; Morita, M.; Hamid, H.A.; Fujii, S.; Kitamura, H.; et al. Protein polysulfidation-dependent persulfide dioxygenase activity of ethylmalonic encephalopathy protein 1. Biochem. Biophys. Res. Commun. 2016, 480, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Millikin, R.; Bianco, C.L.; White, C.; Saund, S.S.; Henriquez, S.; Sosa, V.; Akaike, T.; Kumagai, Y.; Soeda, S.; Toscano, J.P.; et al. The chemical biology of protein hydropersulfides: Studies of a possible protective function of biological hydropersulfide generation. Free Radic. Biol. Med. 2016, 97, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, A.P.; Wei, W.; Gauld, J.W.; Auld, J.; Özcan, F.; Aslan, M.; Mutus, B. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is inactivated by S-sulfuration in vitro. Free Radic. Biol. Med. 2015, 89, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.; Kayama, T.; Yamamoto, S.; Komine, D.; Tanaka, R.; Nishimoto, S.; Suzuki, T.; Kishida, A.; Ogasawara, Y. Polysulfides protect SH-SY5Y cells from methylglyoxal-induced toxicity by suppressing protein carbonylation: A possible physiological scavenger for carbonyl stress in the brain. Neurotoxicology 2016, 55, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.G.; Fee, J.A. Sulfhydryl reactivity of human erythrocyte superoxide dismutase: On the origin of the unusual spectral properties of the protein when prepared by a procedure utilizing chloroform and ethanol for the precipitation of hemoglobin. Biochim. Biophys. Acta Protein Struct. 1978, 537, 100–109. [Google Scholar] [CrossRef]
- Valentine, J.S.; Hart, P.J. Misfolded CuZnSOD and amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2003, 100, 3617–3622. [Google Scholar] [PubMed]


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasamatsu, S.; Nishimura, A.; Morita, M.; Matsunaga, T.; Abdul Hamid, H.; Akaike, T. Redox Signaling Regulated by Cysteine Persulfide and Protein Polysulfidation. Molecules 2016, 21, 1721. https://doi.org/10.3390/molecules21121721
Kasamatsu S, Nishimura A, Morita M, Matsunaga T, Abdul Hamid H, Akaike T. Redox Signaling Regulated by Cysteine Persulfide and Protein Polysulfidation. Molecules. 2016; 21(12):1721. https://doi.org/10.3390/molecules21121721
Chicago/Turabian StyleKasamatsu, Shingo, Akira Nishimura, Masanobu Morita, Tetsuro Matsunaga, Hisyam Abdul Hamid, and Takaaki Akaike. 2016. "Redox Signaling Regulated by Cysteine Persulfide and Protein Polysulfidation" Molecules 21, no. 12: 1721. https://doi.org/10.3390/molecules21121721
APA StyleKasamatsu, S., Nishimura, A., Morita, M., Matsunaga, T., Abdul Hamid, H., & Akaike, T. (2016). Redox Signaling Regulated by Cysteine Persulfide and Protein Polysulfidation. Molecules, 21(12), 1721. https://doi.org/10.3390/molecules21121721




