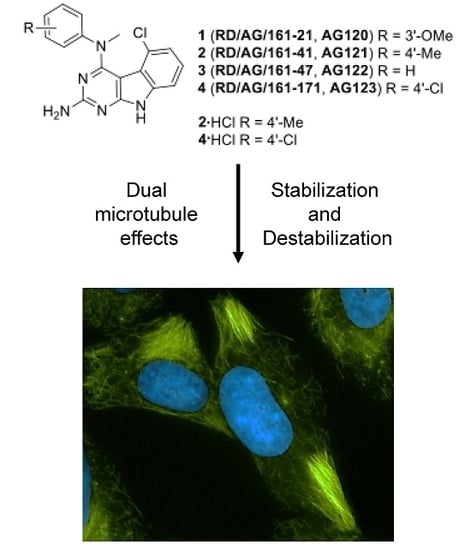
Janus Compounds, 5-Chloro-N4-methyl-N4-aryl-9H-pyrimido[4,5-b]indole-2,4-diamines, Cause Both Microtubule Depolymerizing and Stabilizing Effects
Abstract
:1. Introduction
2. Results
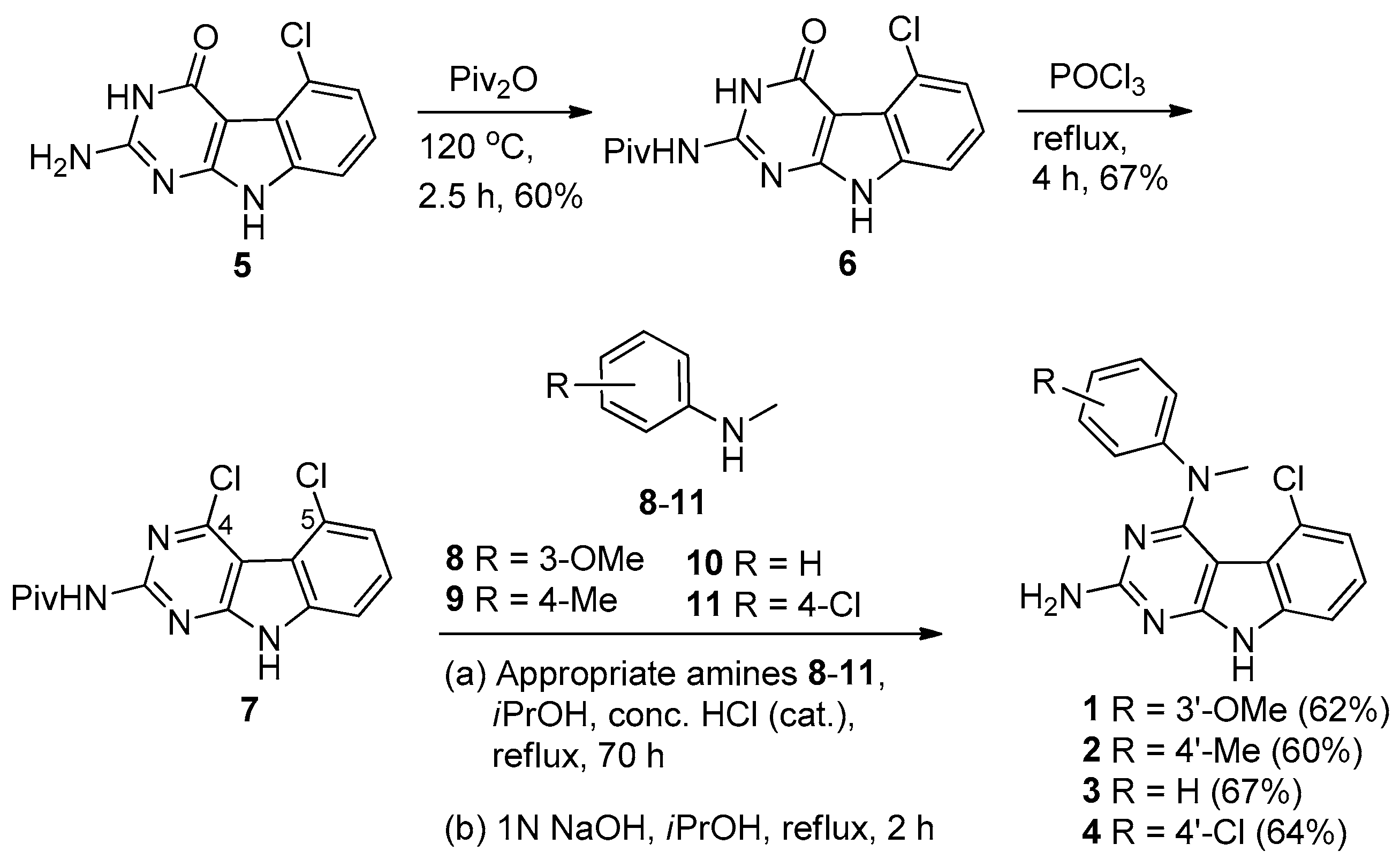
2.1. Chemical Synthesis
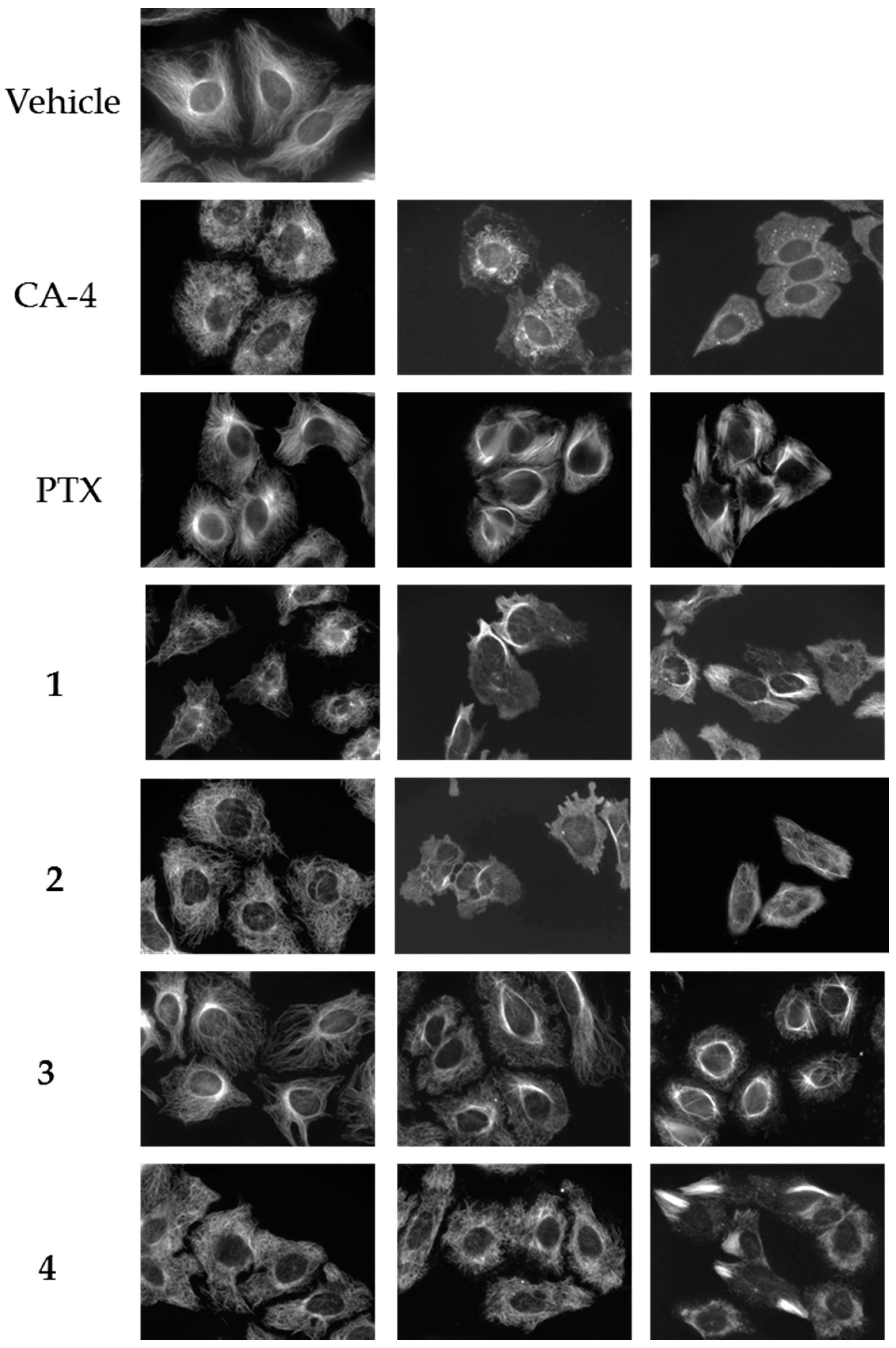
2.2. Effects of Compounds 1–4 on Cellular Microtubules
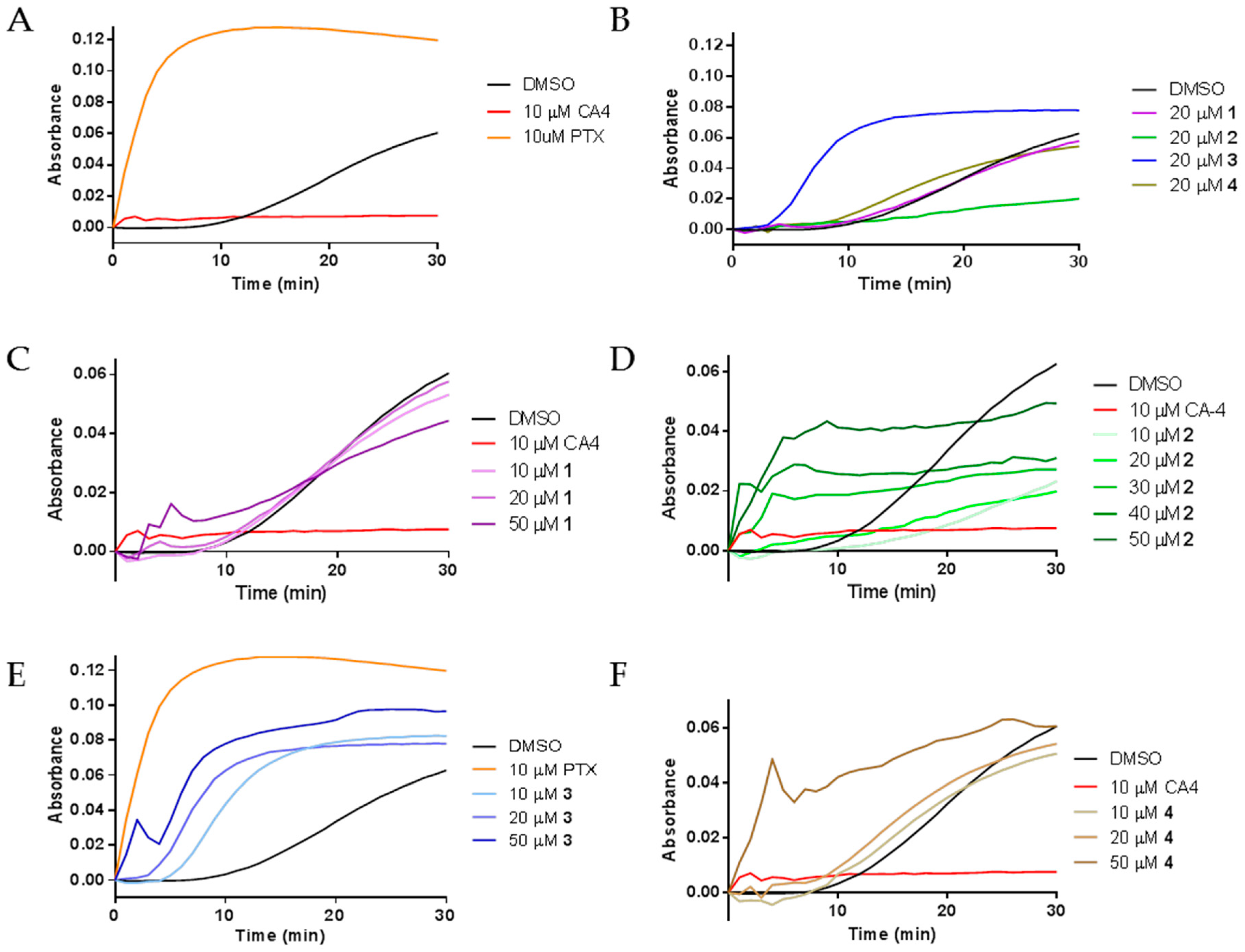
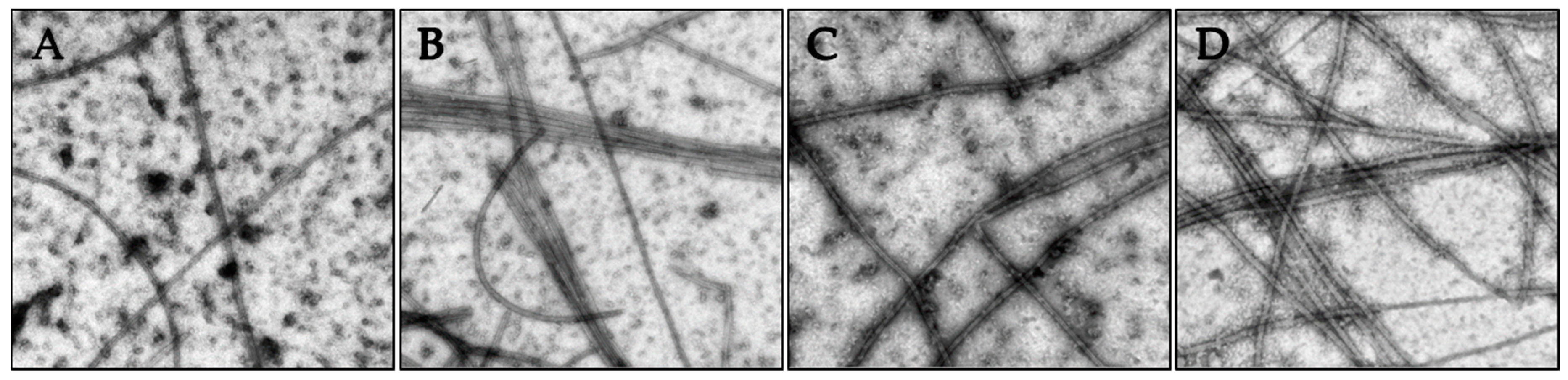
2.3. Effects of Janus Compounds on Tubulin Polymerization
2.4. Effects of Janus Compounds on Drug Sensitive and Multidrug Resistant Cancer Cell Lines
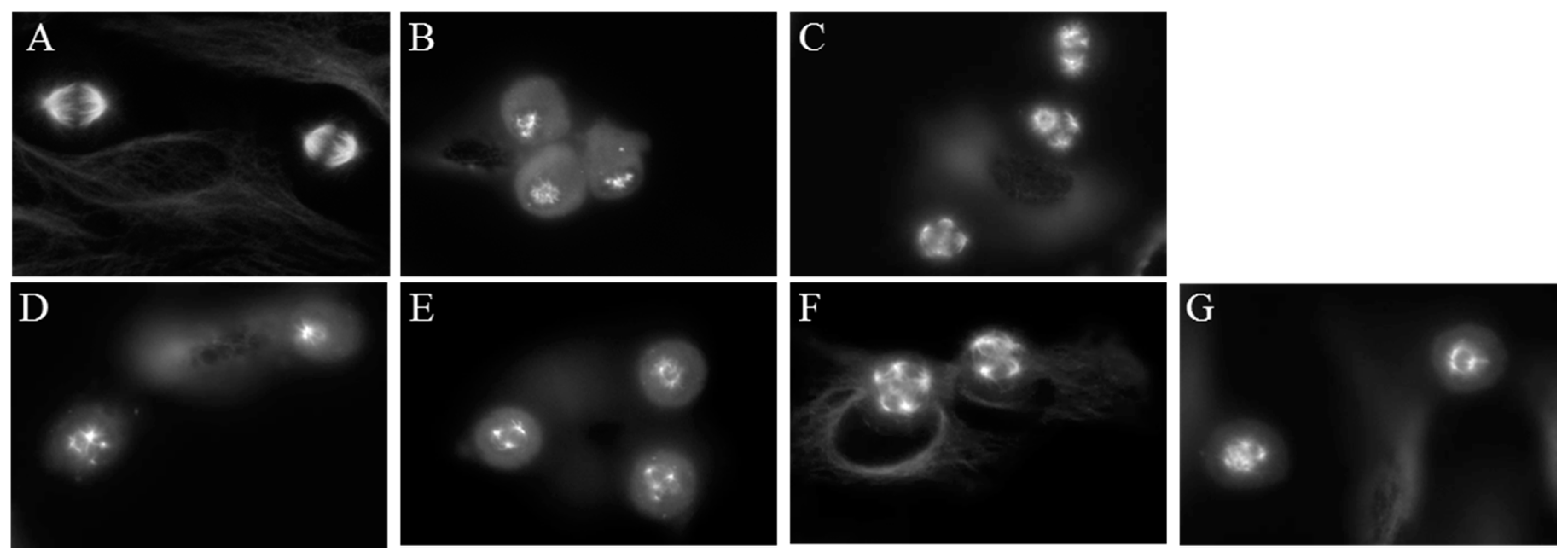
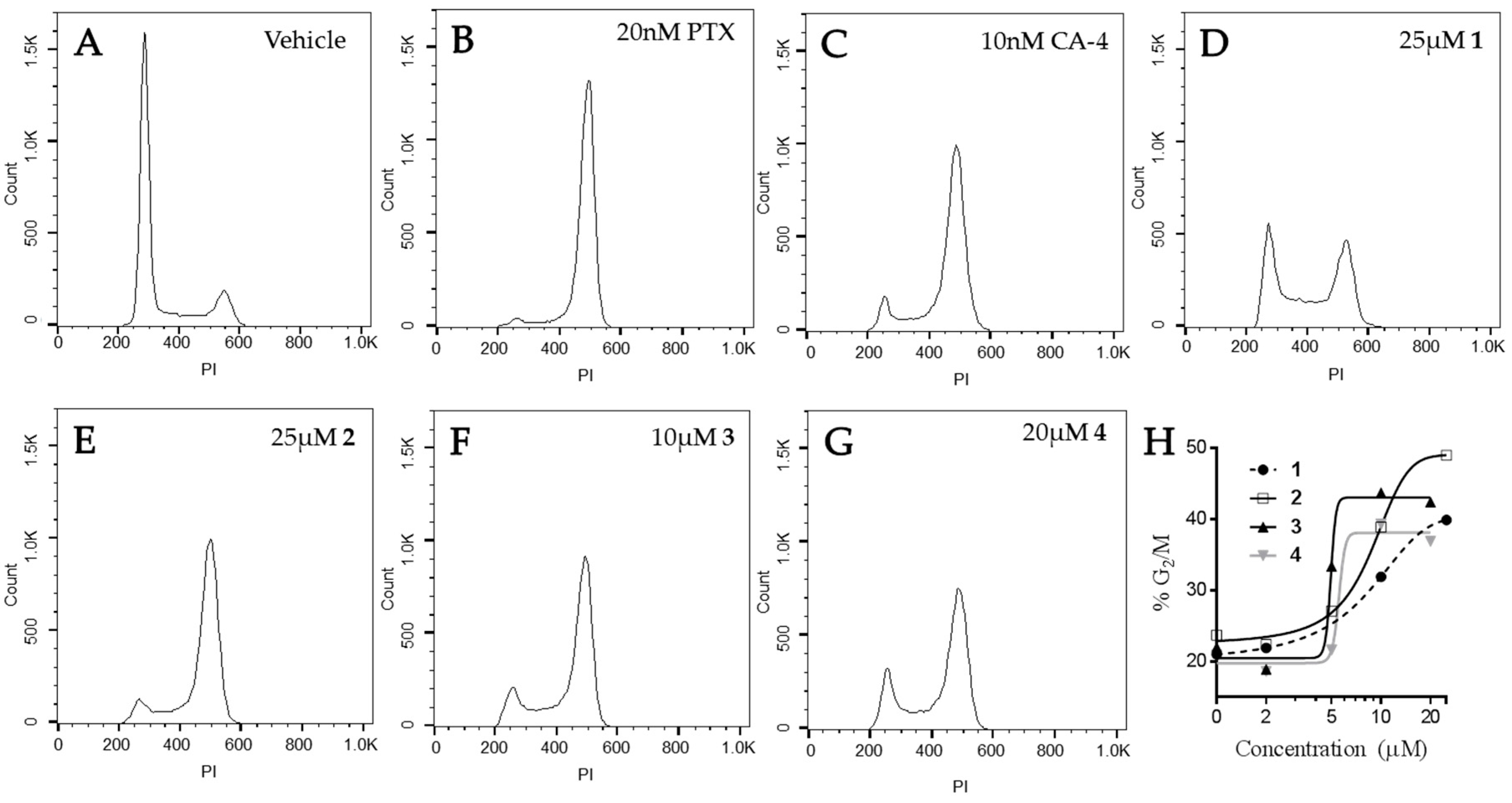
2.5. Effects of Janus Compounds on Mitotic Spindles and Cell Cycle Progression
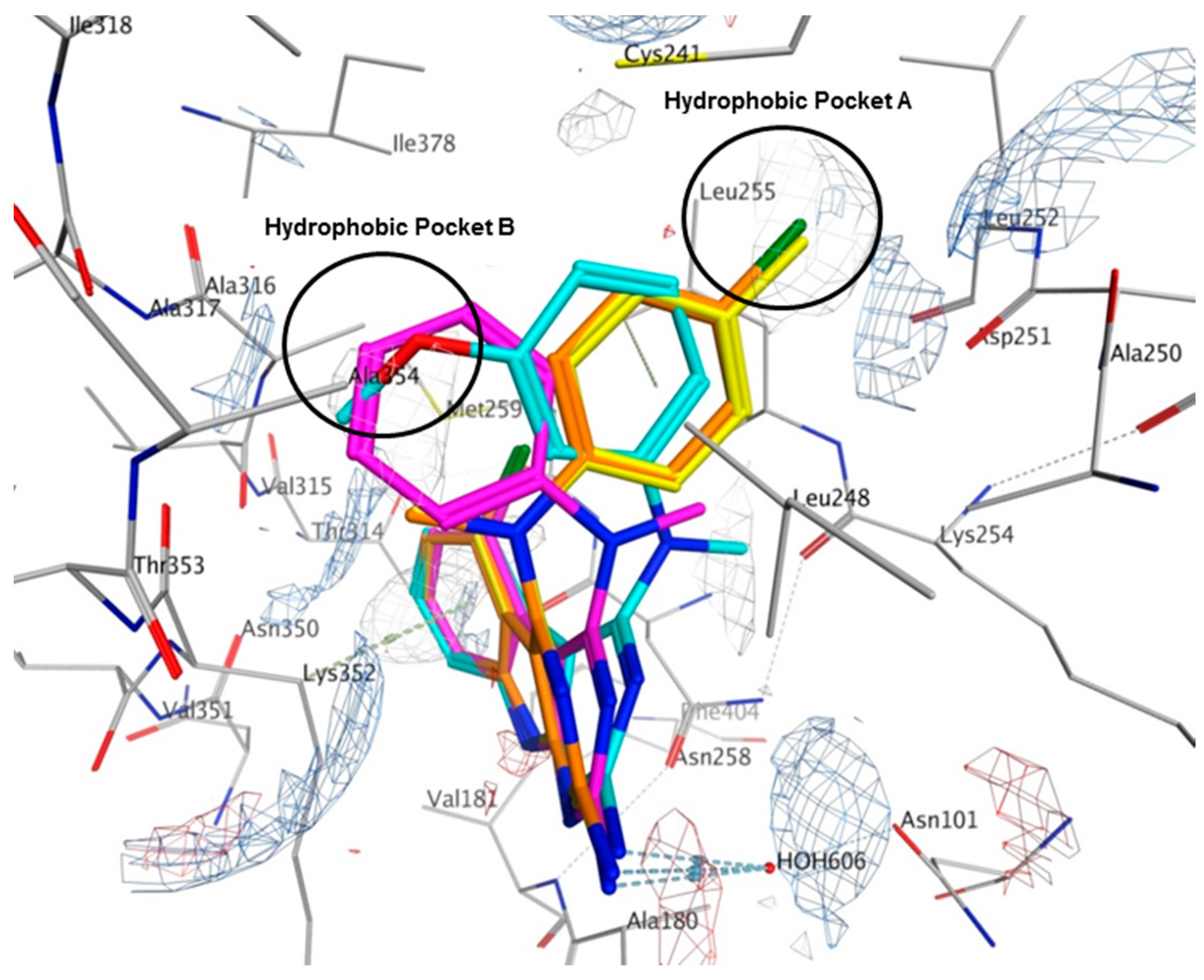
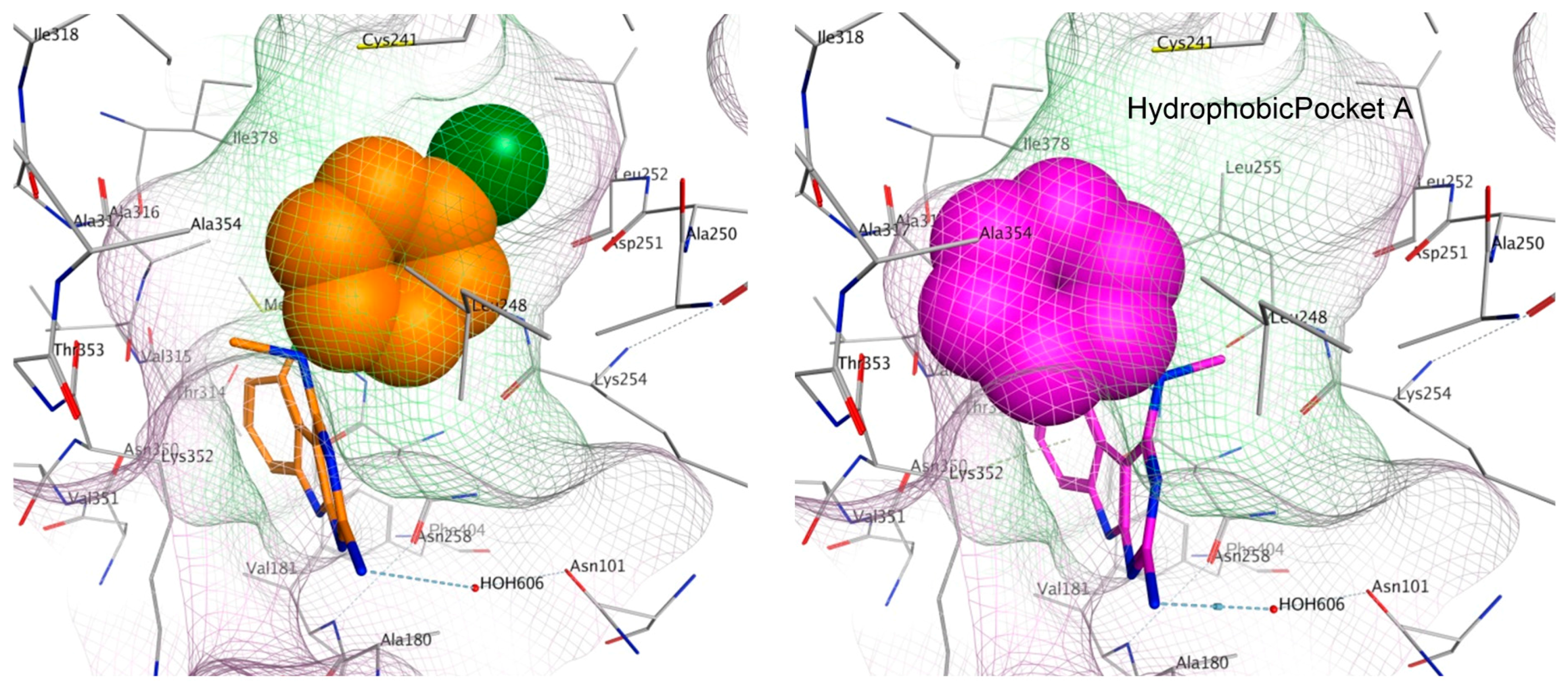
2.6. Molecular Modeling of Compounds in Colchicine Site of Tubulin
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. General Procedure for the Synthesis of 5-Chloro-N4-methyl-N4-aryl-9H-pyrimido [4,5-b]indole-2,4-diamines 1–4
4.3. Molecular Modeling
4.4. Biological Evaluations
4.5. Fluorescence Microscopy
4.6. Inhibition of Cellular Proliferation
4.7. Cell Cycle Analysis
4.8. Tubulin Polymerization and Electron Microscopy
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rohena, C.C.; Mooberry, S.L. Recent progress with microtubule stabilizers: New compounds, binding modes and cellular activities. Nat. Prod. Rep. 2014, 31, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Field, J.J.; Kanakkanthara, A.; Miller, J.H. Microtubule-targeting agents are clinically successful due to both mitotic and interphase impairment of microtubule function. Bioorg. Med. Chem. 2014, 22, 5050–5059. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Mu, X.; Du, G. Microtubule-stabilizing agents: New drug discovery and cancer therapy. Pharmacol. Ther. 2016, 162, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Emami, S. Recent advances of cytotoxic chalconoids targeting tubulin polymerization: Synthesis and biological activity. Eur. J. Med. Chem. 2016, 121, 610–639. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Eastman, A. Microtubule destabilizing agents: Far more than just anti-mitotic anti-cancer drugs. Br. J. Clin. Pharmacol. 2016. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Q.; Li, W. Recent advances in heterocyclic tubulin inhibitors targeting the colchicine binding site. Anticancer Agents Med. Chem. 2016, 16, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Zhao, Y.; Lin, L.; Raghavan, S.; Roberts, E.G.; Risinger, A.L.; Hamel, E.; Mooberry, S.L. Synthesis and discovery of water-soluble microtubule targeting agents that bind to the colchicine site on tubulin and circumvent pgp mediated resistance. J. Med. Chem. 2010, 53, 8116–8128. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Zhao, Y.; Hamel, E.; Westbrook, C.; Mooberry, S.L. Synthesis and biological activities of (R)- and (S)-N-(4-methoxyphenyl)-N,2,6-trimethyl-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-aminium chloride as potent cytotoxic antitubulin agents. J. Med. Chem. 2011, 54, 6151–6155. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Pavana, R.K.; Li, W.; Hamel, E.; Westbrook, C.; Mooberry, S.L. Novel water-soluble substituted pyrrolo[3,2-d]pyrimidines: Design, synthesis, and biological evaluation as antitubulin antitumor agents. Pharm. Res. 2012, 29, 3033–3039. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Zaware, N.; Devambatla, R.K.V.; Raghavan, S.; Westbrook, C.D.; Dybdal-Hargreaves, N.F.; Hamel, E.; Mooberry, S.L. Synthesis of N4-(substituted phenyl)-N4-alkyl/desalkyl-9H-pyrimido[4,5-b]indole-2,4-diamines and identification of new microtubule disrupting compounds that are effective against multidrug resistant cells. Bioorg. Med. Chem. 2013, 21, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Zhao, Y.; Raghavan, S.; Rohena, C.C.; Mooberry, S.L.; Hamel, E. Structure-activity relationship and in vitro and in vivo evaluation of the potent cytotoxic anti-microtubule agent N-(4-methoxyphenyl)-N,2,6-trimethyl-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-aminium chloride and its analogues as antitumor agents. J. Med. Chem. 2013, 56, 6829–6844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Raghavan, S.; Ihnat, M.; Thorpe, J.E.; Disch, B.C.; Bastian, A.; Bailey-Downs, L.C.; Dybdal-Hargreaves, N.F.; Rohena, C.C.; Hamel, E.; et al. The design and discovery of water soluble 4-substituted-2,6-dimethylfuro[2,3-d]pyrimidines as multitargeted receptor tyrosine kinase inhibitors and microtubule targeting antitumor agents. Bioorg. Med. Chem. 2014, 22, 3753–3772. [Google Scholar] [CrossRef] [PubMed]
- Devambatla, R.K.V.; Namjoshi, O.A.; Choudhary, S.; Hamel, E.; Shaffer, C.V.; Rohena, C.C.; Mooberry, S.L.; Gangjee, A. Design, synthesis, and preclinical evaluation of 4-substituted-5-methyl-furo[2,3-d]pyrimidines as microtubule targeting agents that are effective against multidrug resistant cancer cells. J. Med. Chem. 2016, 59, 5752–5765. [Google Scholar] [CrossRef] [PubMed]
- Gangjee, A.; Zaware, N.; Raghavan, S.; Ihnat, M.; Shenoy, S.; Kisliuk, R.L. Single agents with designed combination chemotherapy potential: Synthesis and evaluation of substituted pyrimido[4,5-b]indoles as receptor tyrosine kinase and thymidylate synthase inhibitors and as antitumor agents. J. Med. Chem. 2010, 53, 1563–1578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Raghavan, S.; Ihnat, M.; Hamel, E.; Zammiello, C.; Bastian, A.; Mooberry, S.L.; Gangjee, A. The design, synthesis and biological evaluation of conformationally restricted 4-substituted-2,6-dimethylfuro[2,3-d]pyrimidines as multi-targeted receptor tyrosine kinase and microtubule inhibitors as potential antitumor agents. Bioorg. Med. Chem. 2015, 23, 2408–2423. [Google Scholar] [CrossRef] [PubMed]
- Haskins, K.M.; Donoso, J.A.; Himes, R.H. Spirals and paracrystals induced by vinca alkaloids: Evidence that microtubule-associated proteins act as polycations. J. Cell Sci. 1981, 47, 237–247. [Google Scholar] [PubMed]
- Janssen, A.; Beerling, E.; Medema, R.; van Rheenen, J. Intravital fret imaging of tumor cell viability and mitosis during chemotherapy. PLoS ONE 2013, 8, e64029. [Google Scholar] [CrossRef] [PubMed]
- Darshan, M.S.; Loftus, M.S.; Thadani-Mulero, M.; Levy, B.P.; Escuin, D.; Zhou, X.K.; Gjyrezi, A.; Chanel-Vos, C.; Shen, R.; Tagawa, S.T.; et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011, 71, 6019–6029. [Google Scholar] [CrossRef] [PubMed]
- Poruchynsky, M.S.; Komlodi-Pasztor, E.; Trostel, S.; Wilkerson, J.; Regairaz, M.; Pommier, Y.; Zhang, X.; Kumar Maity, T.; Robey, R.; Burotto, M.; et al. Microtubule-targeting agents augment the toxicity of DNA-damaging agents by disrupting intracellular trafficking of DNA repair proteins. Proc. Natl. Acad. Sci. USA 2015, 112, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Komlodi-Pasztor, E.; Sackett, D.L.; Fojo, A.T. Inhibitors targeting mitosis: Tales of how great drugs against a promising target were brought down by a flawed rationale. Clin. Cancer Res. 2012, 18, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Komlodi-Pasztor, E.; Sackett, D.; Wilkerson, J.; Fojo, T. Mitosis is not a key target of microtubule agents in patient tumors. Nat. Rev. Clin. Oncol. 2011, 8, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Prota, A.E.; Danel, F.; Bachmann, F.; Bargsten, K.; Buey, R.M.; Pohlmann, J.; Reinelt, S.; Lane, H.; Steinmetz, M.O. The novel microtubule-destabilizing drug bal27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization. J. Mol. Biol. 2014, 426, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE), 2015.10; Chemical Computing Group Inc.: Montreal, QC, Canada, 2016.
- Risinger, A.L.; Jackson, E.M.; Polin, L.A.; Helms, G.L.; LeBoeuf, D.A.; Joe, P.A.; Hopper-Borge, E.; Luduena, R.F.; Kruh, G.D.; Mooberry, S.L. The taccalonolides: Microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Cancer Res. 2008, 68, 8881–8888. [Google Scholar] [CrossRef] [PubMed]
- Krishan, A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J. Cell Biol. 1975, 66, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Rohena, C.C.; Telang, N.S.; Da, C.; Risinger, A.L.; Sikorski, J.A.; Kellogg, G.E.; Gupton, J.T.; Mooberry, S.L. Biological characterization of an improved pyrrole-based colchicine site agent identified through structure-based design. Mol. Pharmacol. 2016, 89, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available by request from the authors.


| Compound (μM) | MDA-MB-435 | HeLa | WT βIII | Rr | SK-OV-3 | SK-OV-3-MDR-1/M6/6 | Rr |
|---|---|---|---|---|---|---|---|
| 1 | 6.5 ± 0.3 | 1.4 ± 0.2 | 5.4 ± 0.2 | 3.9 | 5.4 ± 0.8 | 7.4 ± 1.0 | 1.4 |
| 2 | 3.0 ± 0.3 | 2.4 ± 0.1 | 2.7 ± 0.2 | 1.1 | 2.5 ± 0.1 | 3.1 ± 0.2 | 1.2 |
| 3 | 4.2 ± 0.5 | 2.4 ± 0.4 | 4.6 ± 0.1 | 1.9 | 5.0 ± 0.4 | 5.4 ± 0.03 | 1.1 |
| 4 | 4.2 ± 0.5 | 3.5 ± 0.1 | 3.4 ± 0.1 | 1.0 | 4.1 ± 1.0 | 4.7 ± 0.5 | 1.1 |
| PTX (nM) | 4.5 ± 0.5 | 2.8 ± 0.4 | 24 ± 3.0 | 8.6 | 5.0 ± 0.6 | 1200 ± 60 | 240 |
| CA-4 (nM) | 4.4 ± 0.5 | 3.3 ± 0.4 | 3.3 ± 0.3 | 1.0 | 5.5 ± 0.5 | 7.2 ± 1.1 | 1.3 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohena, C.C.; Risinger, A.L.; Devambatla, R.K.V.; Dybdal-Hargreaves, N.F.; Kaul, R.; Choudhary, S.; Gangjee, A.; Mooberry, S.L. Janus Compounds, 5-Chloro-N4-methyl-N4-aryl-9H-pyrimido[4,5-b]indole-2,4-diamines, Cause Both Microtubule Depolymerizing and Stabilizing Effects. Molecules 2016, 21, 1661. https://doi.org/10.3390/molecules21121661
Rohena CC, Risinger AL, Devambatla RKV, Dybdal-Hargreaves NF, Kaul R, Choudhary S, Gangjee A, Mooberry SL. Janus Compounds, 5-Chloro-N4-methyl-N4-aryl-9H-pyrimido[4,5-b]indole-2,4-diamines, Cause Both Microtubule Depolymerizing and Stabilizing Effects. Molecules. 2016; 21(12):1661. https://doi.org/10.3390/molecules21121661
Chicago/Turabian StyleRohena, Cristina C., April L. Risinger, Ravi Kumar Vyas Devambatla, Nicholas F. Dybdal-Hargreaves, Roma Kaul, Shruti Choudhary, Aleem Gangjee, and Susan L. Mooberry. 2016. "Janus Compounds, 5-Chloro-N4-methyl-N4-aryl-9H-pyrimido[4,5-b]indole-2,4-diamines, Cause Both Microtubule Depolymerizing and Stabilizing Effects" Molecules 21, no. 12: 1661. https://doi.org/10.3390/molecules21121661